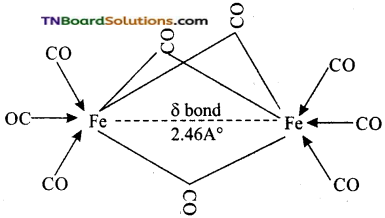
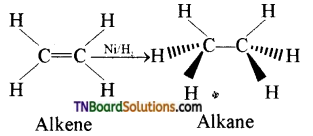
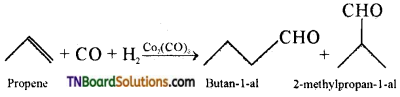
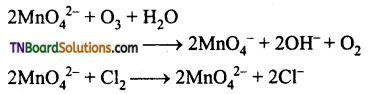
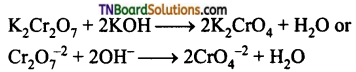
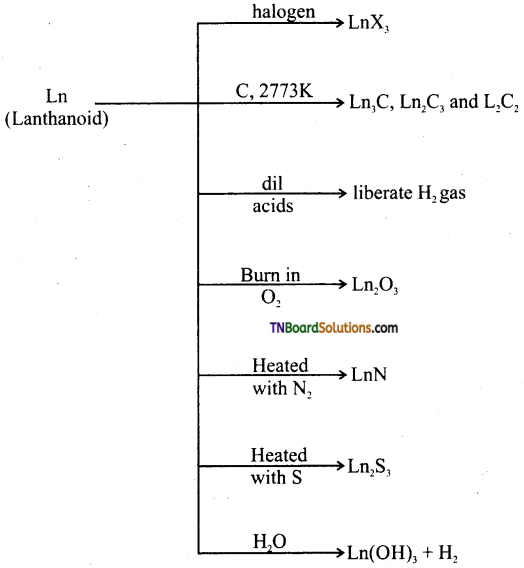
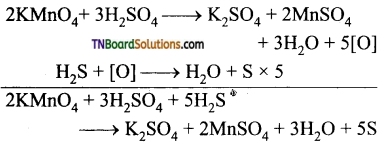
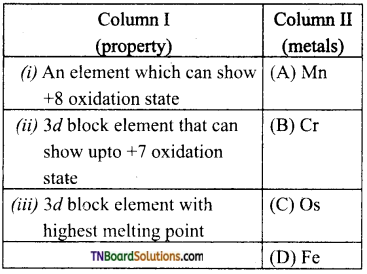
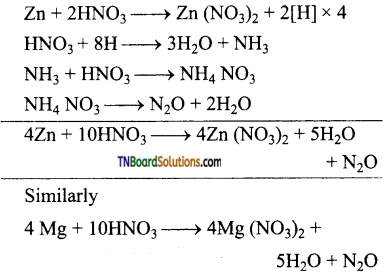
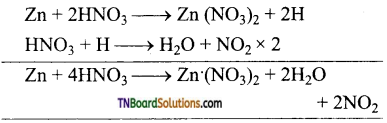
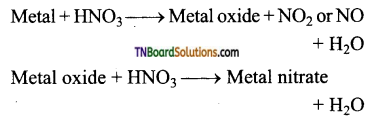
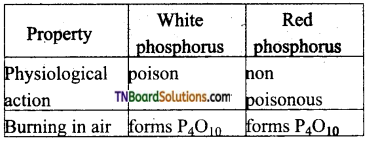
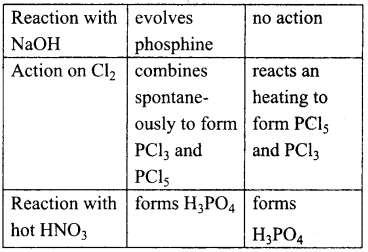
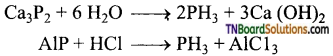
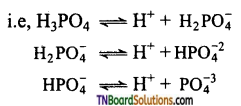
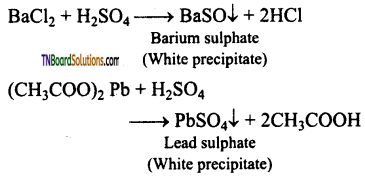
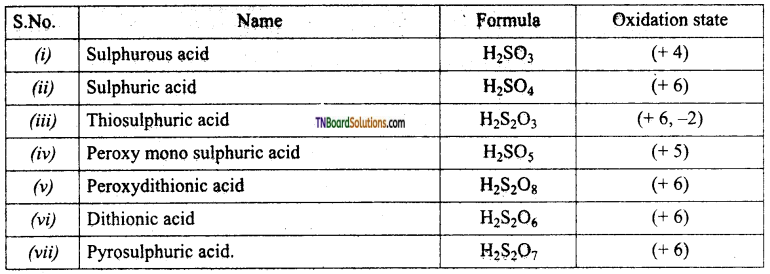
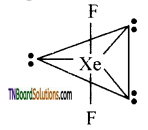
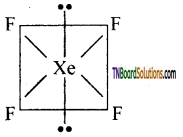
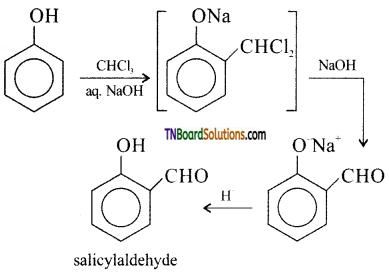
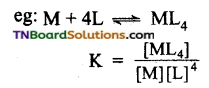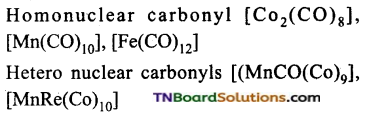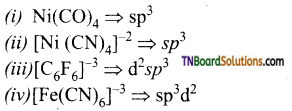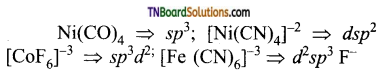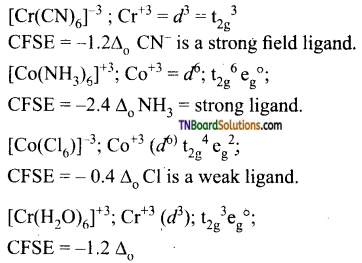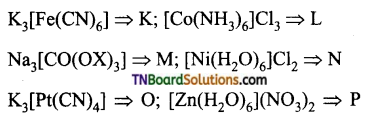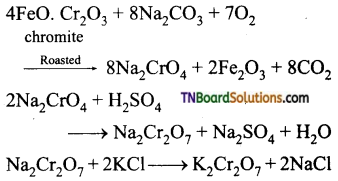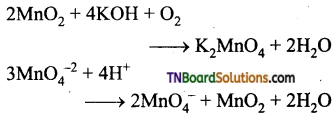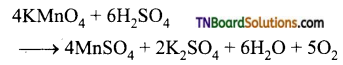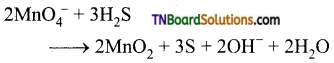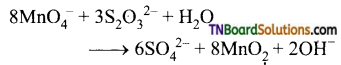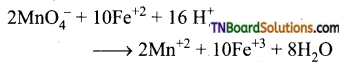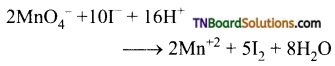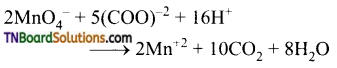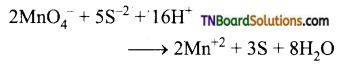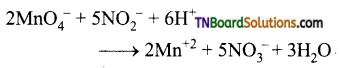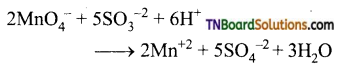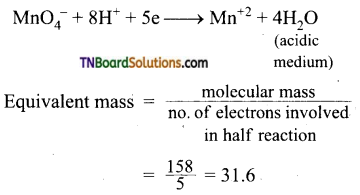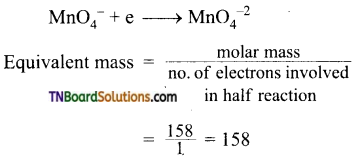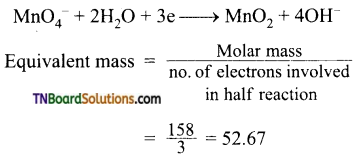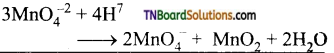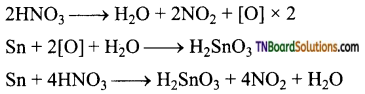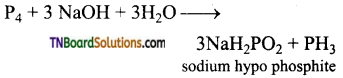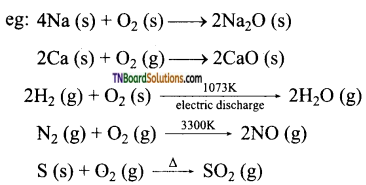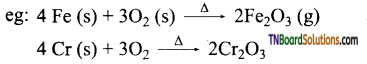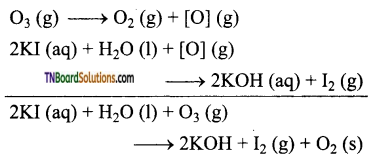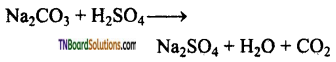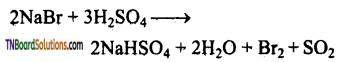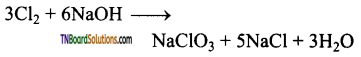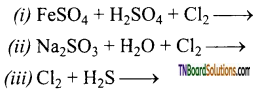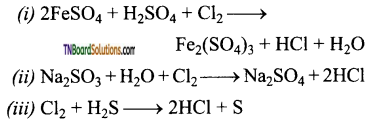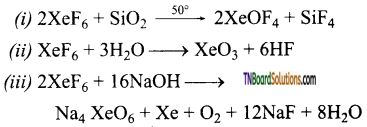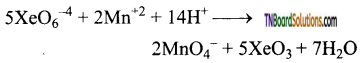Students get through the TN Board 12th Chemistry Important Questions Chapter 12 Carbonyl Compounds and Carboxylic Acids which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 12 Carbonyl Compounds and Carboxylic Acids
Answer the following questions.
Question 1.
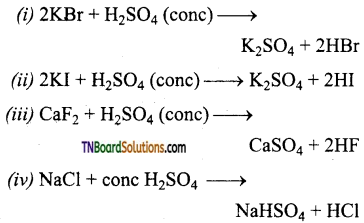
Write the IUPAC names of the following:
Answer:
(i) 3-phenyl prop-2-en-l-al
(ii) cyclohexanecarbaldehyde
(iii) 3-oxopentan-l-al
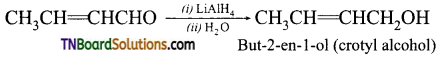
(iv) But-2-en-l-al
Question 2.
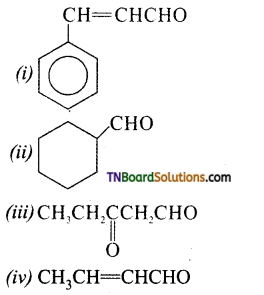
Give the structure of the following compounds.
(i) 4 – Nitropropiophenone
(ii) 2 – Hydroxycyclopentane carbaldehyde
(iii) phenyl acetaldehyde
Answer:
![]()
Question 3.
Write the IUPAC names of
(i) Diacetone alcohol
(ii) Crotonaldehyde
Answer:
(i) 4-Hydroxy-4-methylpentan-2-ol
(ii) But-2-en-l-al
Question 4.
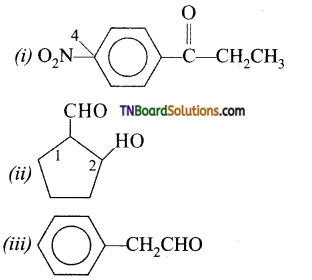
Write the structure of
(i) 3 – oxopentanal
(ii) 1 – phenylpentan – 1 – one
Answer:
Question 5.
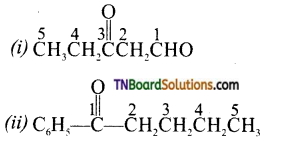
Name the following compounds according to IUPAC system of nomenclature.
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2CO(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO(P)
Answer:
(i) 4-methyl pentanal
(ii) 6-chloro-4-ethylhexan-3 -one
(iii) But-2-en-l-al
(iv) Pentane 2,4, dione
(v) 3, 3, 5 Trimethylhexan – 2 – one
(vi) 3, 3, Dimethyl butanoic acid
(vii) Benzene 1,4, dicarbaldehyde
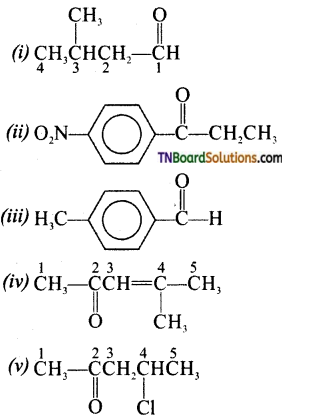
Question 6.
Draw the structures of the following compounds.
(i) 3-methyl butanal
(ii) 4-nitro propiophenone
(iii) p-methyl benzaldehyde
(iv) 4-methylpent-3-en-2-one
(v) 4-chloropentan-2-one
(vi) 3-Bromo-4-phenyl pentanoic acid
(vii) p, p’ dihydroxy benzo phenone
(viii) Hex-2-en-4 yonic acid
Answer:
![]()
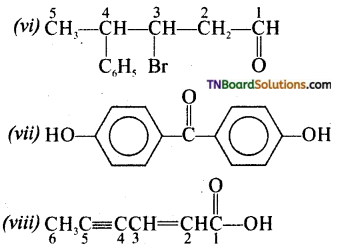
Question 7.
Write IUPAC names of the following aldehydes and ketones. Wherever possible give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CH(Br)CH2CH(CH3)CHO
(iii) CH3(CH2)4CHO
(iv) Ph—CH=CH.CHO
(v)

(vi) PhCOPh
Answer:
Question 8.
Give the product of ozonolysis of the following alkenes.
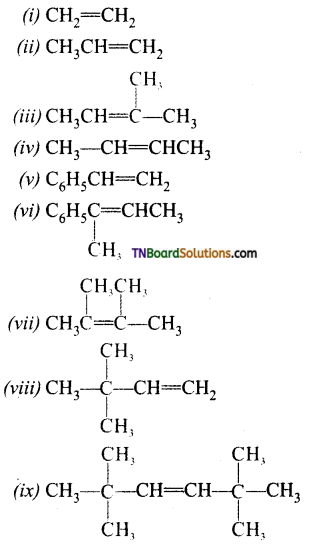
Answer:
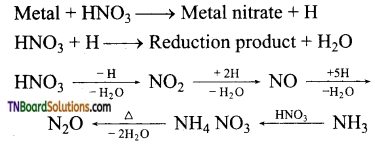
To identify the product of ozonolysis, draw a line in between C=C bond and ‘O’ on either side of the double bond
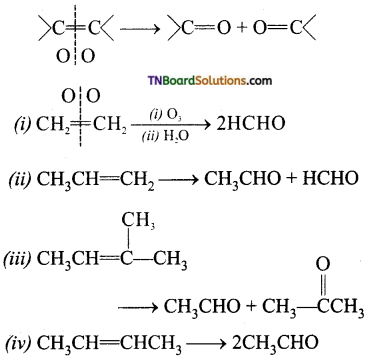
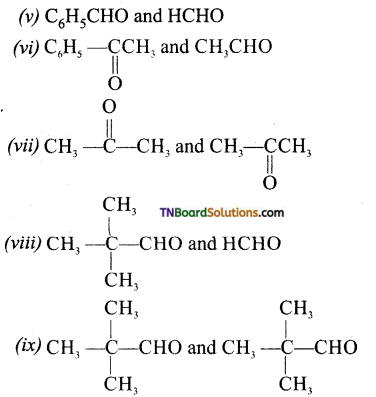
![]()
Question 9.
Identify the products of hydration of the following:
(i) ethyne
(ii) prop-1-yne
(iii) Hex-1-yne
(iv) Diphenyl acetylene
Answer:
Hydration means addition of water. The reagent used is mercuric sulphate and dilute sulphuric acid at 333K. Ketones are formed on hydration of alkynes. MarkovnikofFs mle is followed in water addition.
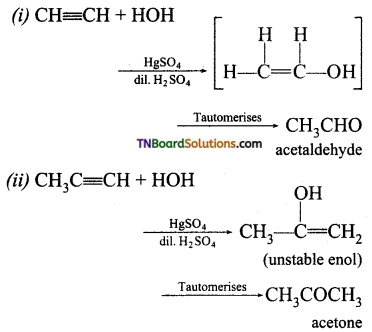
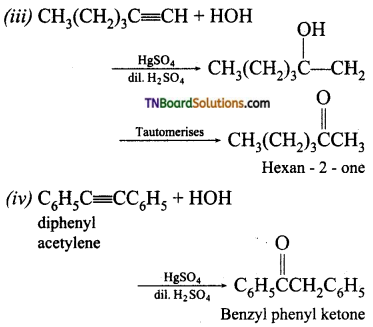
Question 10.
How are the following compounds formed by distillation of calcium salts of their carboxylic acids?
(i) Formaldehyde,
(ii) Acetaldehyde,
(iii) Acetone,
(iv) Butan-2-one,
(v) Cyclopentanone,
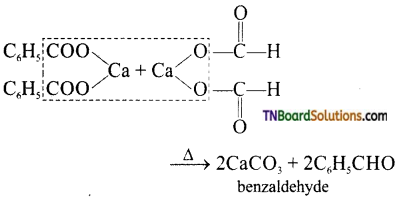
(vi) Benzaldehyde
Answer:
(i) Calcium formate alone is heated.
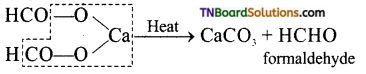
(ii) By dry distillation of calcium acetate and calcium formate.
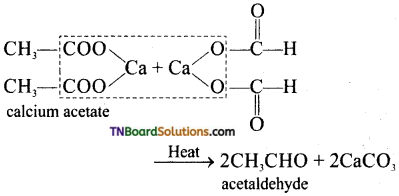
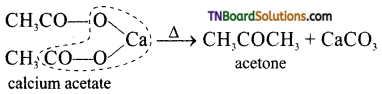
(iii) Dry distillation of calcium acetate alone.
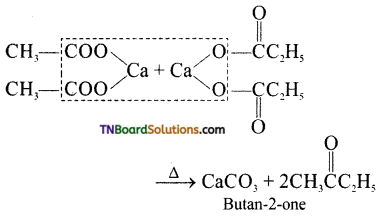
(iv) Calcium acetate and calcium propionate are distilled together.
(v) Cyclic ketones are formed when calcium salts of dibasic acids are heated.
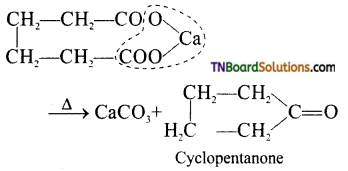
(vi) By dry distillation of a mixture of calcium benzoate and calcium formate.
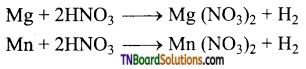
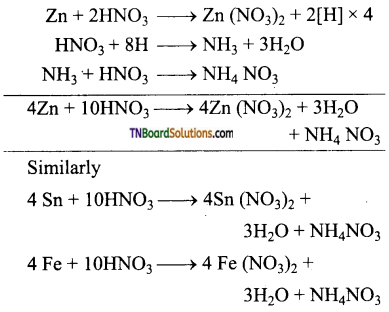
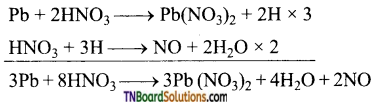
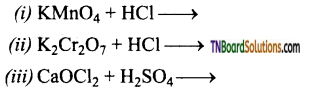
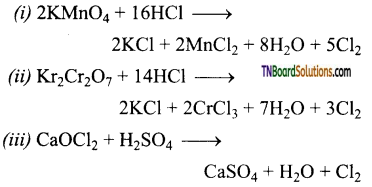
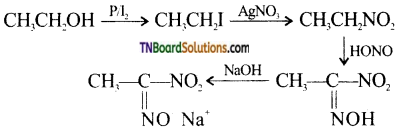
Question 11.
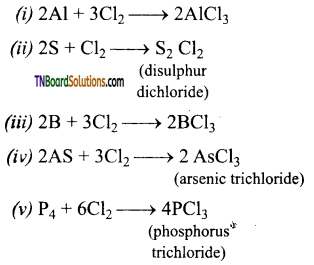
Complete the following equations:
Answer:

![]()
Question 12.
![]()
(i) Identify the product.
(ii) Name the reaction.
(iii) What is the intermediate formed in the reaction.
Answer:
(i) CH3CHO (acetaldehyde)
(ii) Stephen’s reaction
(iii) CH3—CH=NH (an imine)
Complete reaction:
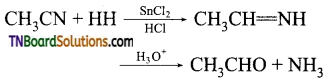
Question 13.
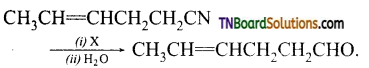
(i) Name the reagent i.e., X used for the above reduction.
(ii) Why other reducing agents like H2, in the presence of a catalyst are not used.
(iii) Write the IUPAC name of the product formed.
Answer:
(i) Diisobutyl aluminium hydride (DIBAL – H) is used as a reducing agent.
X = H2O
(ii) It selectively reduces alkyl cyanides to imines, which on hydrolysis gives aldehydes. The double bond in the nitrile is not reduced. The other reducing agents reduce the double bond also.
(iii) hex-4-enal is the IUPAC name of the product.
Question 14.
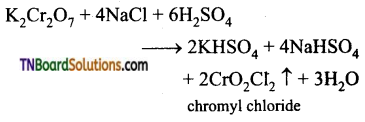
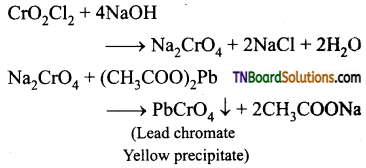
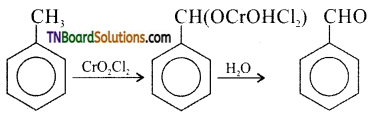
How is benzaldehyde prepared from (i) methyl benzene, (ii) benzene, (iii) benzyl alcohol. Give equations.
Answer:
(i) Oxidation of methyl benzene, using chromyl chloride as oxidising agent gives benzaldehyde. Acetic anhydride and CrO3 can also be used for reaction.
(ii) Benzene is treated with CO and HCl Benzaldehyde is formed.
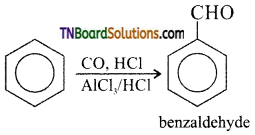
This reaction is known as Gattermann- koch reaction.
(iii) Oxidation of benzyl alcohol using PCC (pyridinium chromo chromate) gives benzaldehyde.
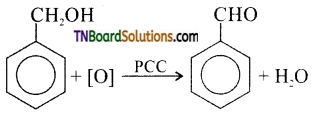
Question 15.
Identify the product of the following reactions. Write the complete equation.
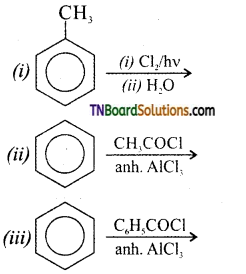
Answer:
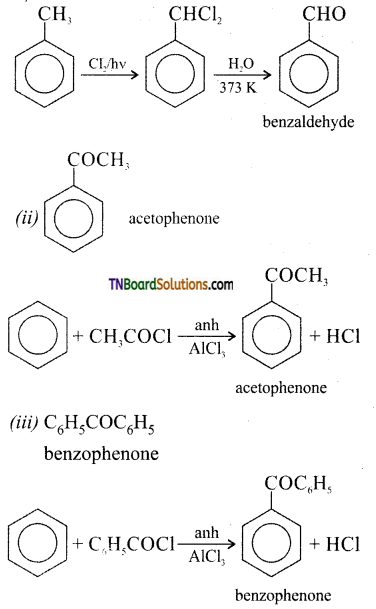
Question 16.
Give examples for Friedel-crafts acetylation reaction.
Answer:
Refer Question 15 (ii) and (iii). These reaction are called Friedel-crafts reaction. When acetyl chloride is used it is known as acetylation reaction. When benzoyl chloride is used, it is called benzoylation reaction.
![]()
Question 17.
The boiling points of aldehydes and ketones are high compared to hydrocarbons and ethers of comparable molecular mass. Explain why?
Answer:
It is due to the weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
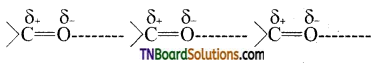
Question 18.
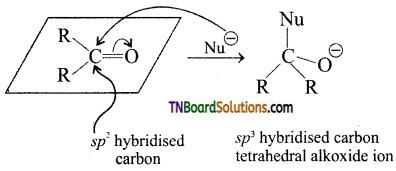
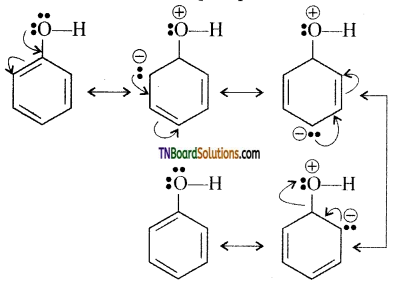
Explain nucleophilic addition reaction with an example.
Answer:
The carbonyl carbon carries a small degree of positive charge. Nucleophile such as CN– can attack the carbonyl carbon and uses its lone pair to form a new carbon – nucleophile ‘σ‘ bond, at the same time two electrons from the carbon – oxygen double bond move to the most electronegative oxygen atom. This results in the formation of an alkoxide ion. In this process, the hybridisation of carbon changes from sp2 to sp3.
The tetrahedral intermediate can be protonated by water or an acid to form an alcohol.
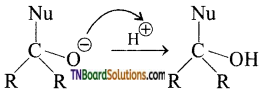
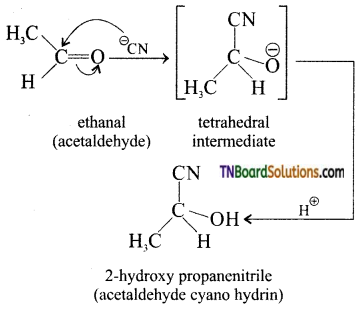
eg: Addition of HCN
Attack of CN– on carbonyl carbon followed by protonation gives cyanohydrins.
Question 19.
Explain why the carbonyl group in aldehydes and ketones is polar.
Answer:
The carbonyl group of aldehydes and ketones contains a double bond between carbon and oxygen. Oxygen is more electronegative than carbon and it attracts the shared pair of electron which makes the carbonyl group as polar and hence aldehydes and ketones have high dipole moments.

Question 20.
What is meant by the following terms? Give an example in each case.
(i) Cyanohydrin,
(ii) Acetal,
(iii) Semicarbazone,
(iv) Aldol
(v) Hemiacetal,
(vi) Oxime
(vii) Ketal,
(viii) Imine,
(ix) 2,4, DNP derivative
(x) Schiff’s base
Answer:
(i) gem – Hydroxy nitriles i.e., compounds containing hydroxyl and cyano groups on the same carbon atom are called cyano hydrins. These are produced by the addition of HCN to aldehydes and ketones in weakly basic medium.

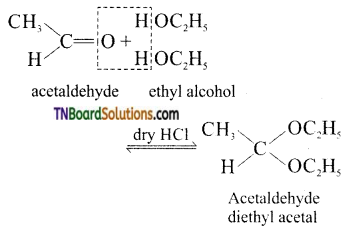
(ii) gem – dialkoxy alkanes in which two alkoxy groups attached to the terminal carbon atom are called acetals. They are produced by the action of an aldehyde with two equivalent of monohydric alcohol in the presence of dry HCl gas.
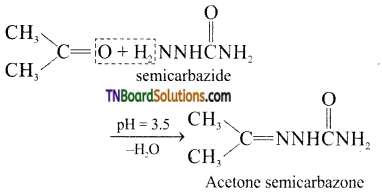
(iii) Semi carbazones are derivatives of aldehydes and ketones and are produced by the action of semicarbazides on them in a weakly acidic medium.
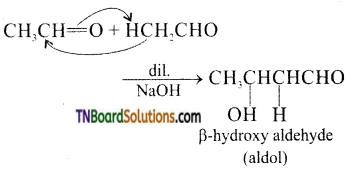
(iv) Aldols are β – hydroxy aldehydes or ketones and are produced by the condensation of two molecules of the same or one molecule each of two different aldehydes or ketones (containing an alpha hydrogen atom) in the presence of dilute, aqueous NaOH.
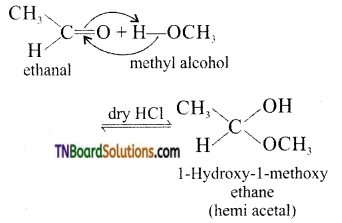
(v) α – alkoxy alcohols are called hemi acetals. They are produced by the addition of one molecule of a mono hydric alcohol to an aldehyde in the presence of dry HCl gas.
(vi) Oximes are produced when aldehydes or ketones react with hydroxyl amine in weakly acidic medium.
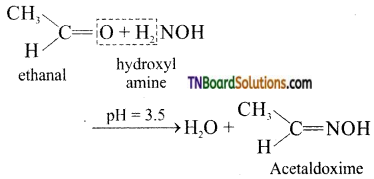
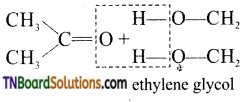
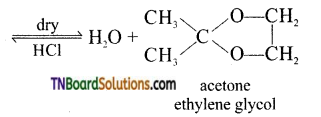
(vii) gem-dialkoxy alkanes in which two alkoxy groups present on the same carbon atom are called ketals.
They are produced when a ketone is heated with ethylene glycol in the presence of dry HCl gas.
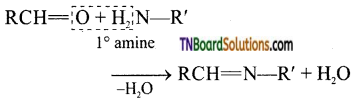
(viii) Compounds containing >C=N group are called imines. These are produced when aldehydes and ketones react with ammonia and its derivatives.
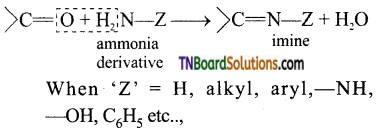
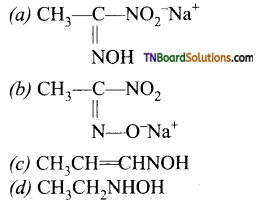
When ‘Z’ = H, alkyl, aryl,—NH, —OH, C6H5 etc..,
(ix) 2, 4, dinitro phenyl hydrazones (i.e., 2, 4, DNP derivative) are produced when aldehydes or ketones react with 2,4 dinitro phenyl hydrazine in weakly acidic medium.
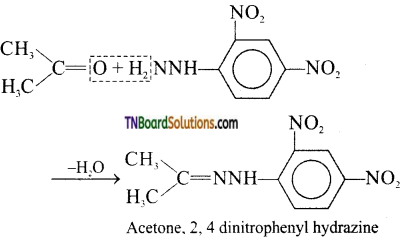
(x) Aldehydes and ketones react with primary aliphatic or aromatic amines in the presence of acid form azomethenes or schifTs bases. Schiffs bases may also be regarded as imines.
![]()
Question 21.
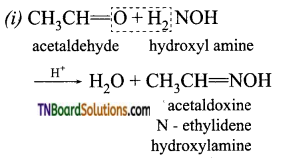
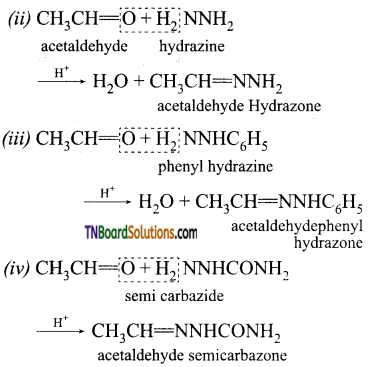
How does acetaldehyde react with (i) hydroxyl amine, (ii) hydrazine, (iii) phenyl hydrazine, (iv) semicarbazide? Give equations.
Answer:
Question 22.
Give equations for the reaction between
(i) acetaldehyde and sodium bisulphite
(ii) acetone and sodium bisulphite
Answer:
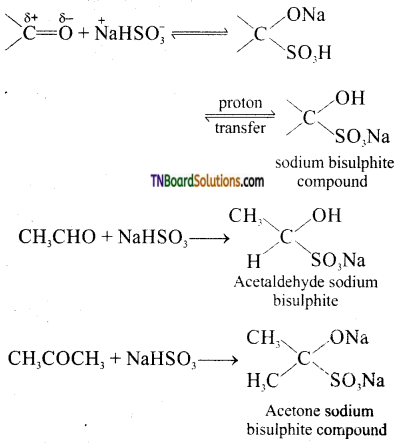
Question 23.
Write the structure of the prodcuts formed when acetone reacts with (i) hydrazine, (ii) phenyl hydrazine, (iii) semicarbazide.
Answer:
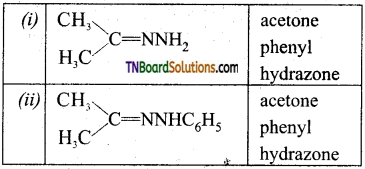

Question 24.
What is urotropine? How it is formed? Write its structure.
Answer:
Hexamethelenetetramine is called urotropine. It is formed when formaldehyde reacts with ammonia.
6HCHO + 4NH3 → (CH2)6N4 + 6H2O
Structure:
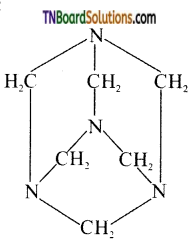
Question 25.
Mention the uses of urotropine.
Answer:
- Urotropine is used as a medicine to treat urinary infection.
- Nitration of Urotropine under controlled condition gives an explosive RDX (Research and development explosive). It is also called cyclonite or cyclotri methylenetrinitramine.
![]()
Question 26.
What is popoffs rule? How it is used to predict the oxidation products of unsymmetrical ketones.
Answer:
It states that during the oxidation of an unsymmetrical ketone, a (C—CO) bond is cleaved in such a way that the keto group stays with the smaller alkyl group.
Eg:
Question 27.
How will you prepare
(i) diacetone amine from acetone.
(ii) aldimine from acetaldehyde.
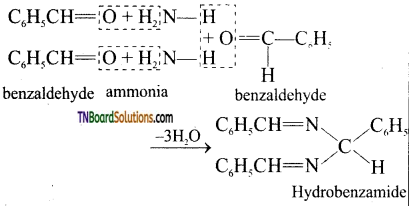
(iii) hydrobenzamide from benzaldehyde.
Answer:
(i)
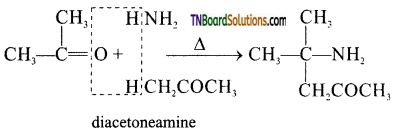
Two molecules of acetone, combine with ammonia to form diacetoneamine.
(ii)
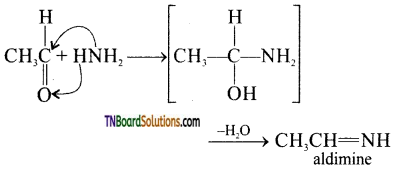
(iii) With ammonia, benzaldehyde forms a complex condensation product called hydrobenzamide.
Question 28.
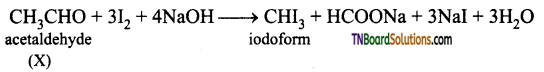
What is a haloform reaction? Explain with suitable example.
Answer:
Aldehydes and ketones containing CH3CO group (methyl carbonyl group), on treatment with excess halogen in the presence of an alkali produce a haloform (chloro form, bromo form, iodoform). This reaction is known as haloform reaction.
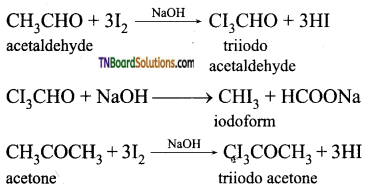
Question 29.
What is iodoform test? Explain.
Answer:
Compounds containing CH3CO (methyl carbonyl) group or compounds on oxidation give compounds containing CH3CO group, when treated with iodine and NaOH gives a yellow precipitate is called iodoform. This is known as iodoform test.
![]()
Question 30.
How will you distinguish between by means of a chemical test.
(i) Propanal and propanone
(ii) acetaldehyde and benzaldehyde
(iii) Ethanal and propanal
Answer:
All these pairs of compounds can be distinguished by iodoform test.
(i) Propanone (CH3 CO CH3) will answer iodoform test. Propanal will not undergo iodo form test.
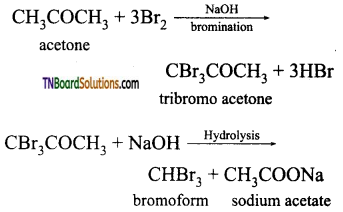
(ii) Acetaldehyde (CH3CHO) will answer iodoform test, whereas benzaldehyde does not answer iodoform test
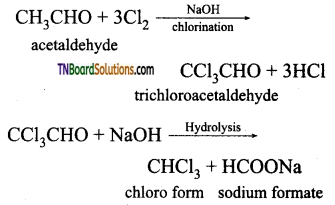
(iii) Ethanal (acetaldehyde) will answer iodoform test, but not propanal acetaldehyde
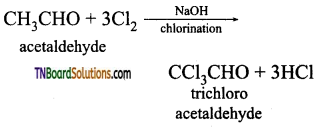
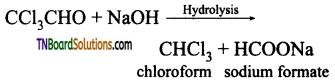
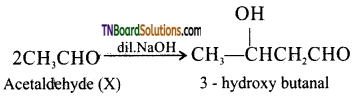
Question 31.
Write a short note an aldol condensation.
Answer:
The carbon attached to carbonyl carbon is called α – carbon and the hydrogen atom attached to α – carbon is called α – hydrogen. In presence of dilute base NaOH, or KOH, two molecules of an aldehyde or ketone having α – hydrogen add together to give β – hydroxyl aldehyde (aldol) or β – hydroxyl ketone (ketol). The reaction is called aldol condensation reaction. The aldol or ketol readily loses water to give α, β – unsaturated compounds which are aldol condensation products.
Acetaldehyde when warmed with dil.NaOH gives β – hydroxyl butraldehyde (acetaldol)
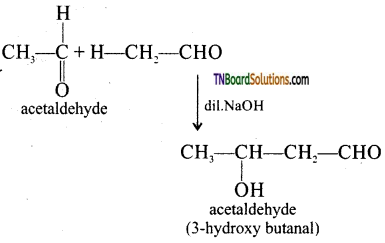
Question 32.
Give the mechanism of aldol condensation.
Answer:
Mechanism: The mechanism of aldol condensation of acetaldehyde takes place in three steps.
Step 1: The carbanion is formed as the α – hydrogen atom is removed as a proton by the base.

Step 2: The carbanion attacks the carbonyl carbon of another unionized aldehyde to form an alkoxide ion.
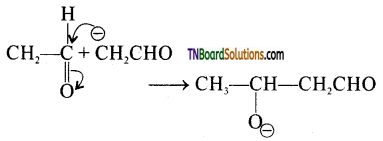
Step 3: The alkoxide ion formed is protonated by water to form aldol.
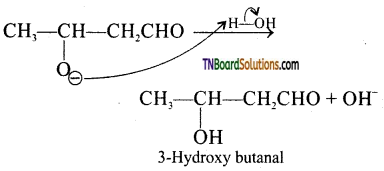
The aldol rapidly undergoes dehydration on heating with acid to form α – β unsaturated aldehyde.
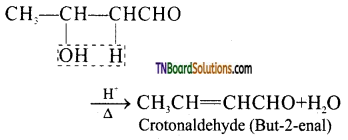
![]()
Question 33.
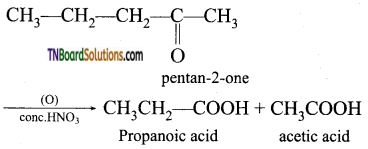
Give the oxidation products obtained when pentan-2-one is oxidised by (conc.HNO3).
Answer:
During these oxidations, rupture of the carbon-carbon bonds occur on either side of the keto group giving a mixture of carboxylic acid, each containing less a number of carbon atom. Than the original ketone.
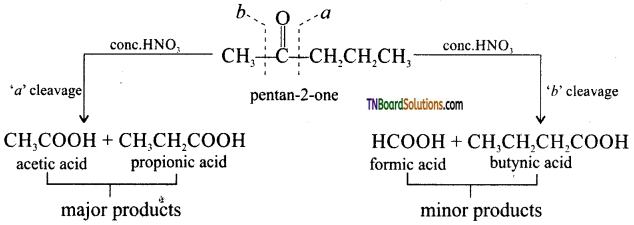
Question 34.
What is crossed aldol condensation? Give an example.
Answer:
Aldol condensation can also takes place between two different aldehydes or ketones or between one aldehyde and one ketone such an aldol condensation is called crossed or mixed aldol condensation. This reaction is not very useful as the product is usually a mixture of all possible condensation products and cannot be separated easily.
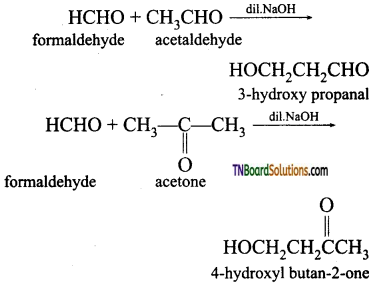
Question 35.
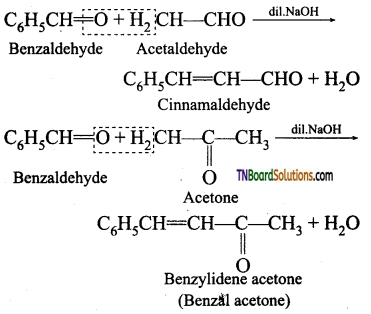
What happens when benzaldehyde reacts with (i) acetaldehyde in the presence of dilute NaOH (ii) acetone in the presence of dilute NaOH.
[OR] Explain with examples Claisen – Schmidt condensation.
Answer:
Benzaldehyde condenses with aliphatic aldehyde or methyl ketone in the presence of dil. alkali at room temperature to form unsaturated aldehyde or ketone. This type of reaction is called Claisen – Schmidt condensation.
Question 36.
Write a short note on Cannizaro reaction.
Answer:
In the presence of concentrated aqueous or alcoholic alkali, aldehydes which do not have α – hydrogen atom under go self oxidation and reduction (disproportionation) to give a mixture of alcohol and a salt of carboxylic acid. This reaction is called Cannizaro reaction.
Benzaldehyde on treatment with concentrated NaOH (50%) gives benzyl alcohol and sodium benzoate.
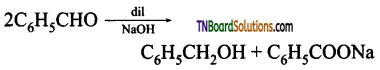
This reaction is an example of disproportion reaction.
![]()
Question 37.
Write the mechanism of Cannizaro reaction.
Answer:
Cannizaro reaction involves three steps.
Step 1: Attack of OH– on the carbonyl carbons.
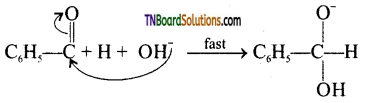
Step 2: Hydride ion transfer
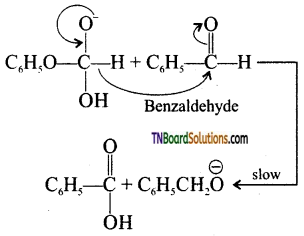
Step 3: Acid – base reaction
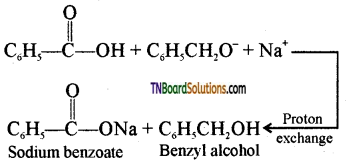
Cannizaro reaction is a characteristic of aldehyde having no α – hydrogen.
Question 38.
Give an example for crossed Cannizaro reaction.
Answer:
When Cannizaro reaction takes place between two different aldehyde (neither containing an α hydrogen atom), the reaction is called as cross cannizaro reaction.
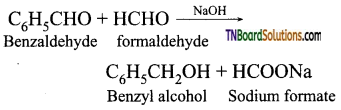
In crossed cannizaro reaction more reactive aldehyde is oxidized and less reactive aldehyde is reduced.
Question 39.
Give an example for benzoin condensation.
Answer:
Benzaldehyde reacts with alcoholic KCN to form benzoin
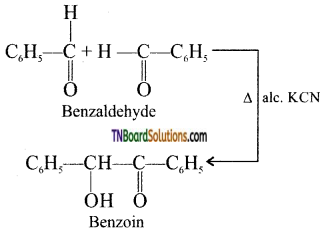
![]()
Question 40.
Complete the following equation.
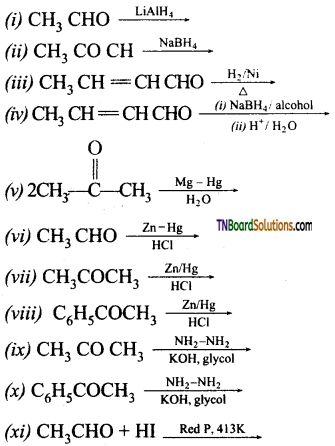
![]()
Answer:
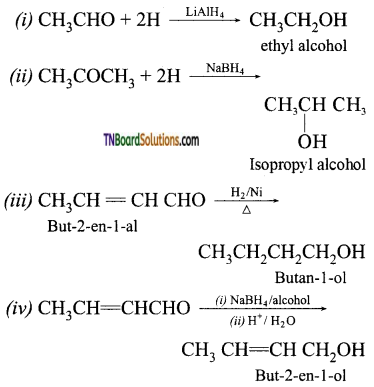
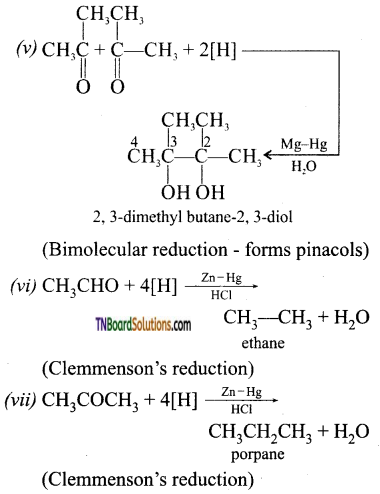
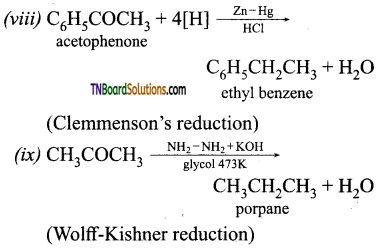
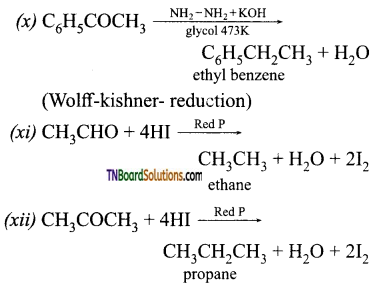
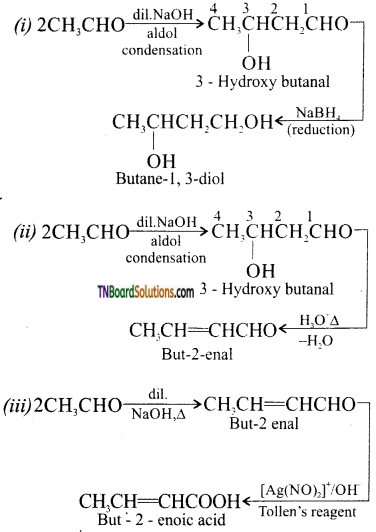
Question 41.
How will you convert ethanal into following compounds?
(i) Butane 1, 3 diol
(ii) But -2-enal
(iii) But-2-enoic acid.
Answer:
![]()
Question 42.
Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Acetophenone and benzophenone
(ii) Phenol and benzoic acid
(iii) Pentan -2- one and pentan – 3-one
(iv) Benzaldehyde and acetophenone
Answer:
(i) Acetophenone and benzophenone: Acetophenone being a methyl ketone, when treated with I2 / NaOH (NaOI) gives a yellow precipitate of iodoform but benzophenone does not give a yellow precipitate.
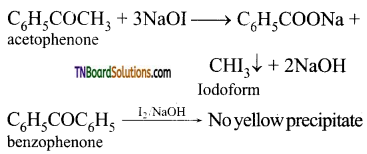
(ii) Phenol and benzoic acid: NaHCO3 test: Benzoic acid being a stronger acid than carbonic acid (H2CO3) decomposes NaHCO3 to evolve CO2, but phenol being weaker than carbonic acid does not.

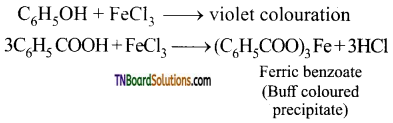
FeCl3 Test: Phenol gives a violet colouration with neutral ferric chloride solution, while benzoic acid gives a buff coloured precipitate of ferric benzoate.
(iii) Pentan-2-one and pentan-3-one:
Sodium bisulphite test: 2-Pentanone being a methyl ketone when treated with a saturated solution of sodium bisulphite gives a white precipitate of 2 – pentanone sodium bisulphite addition compound where as 3 – pentanone does not.
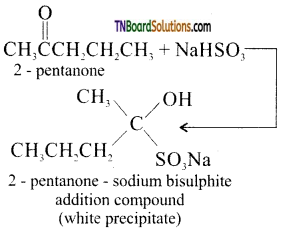

(Note: Sterically hindered ketones, do not undergo this reaction, eg: acetophenone)
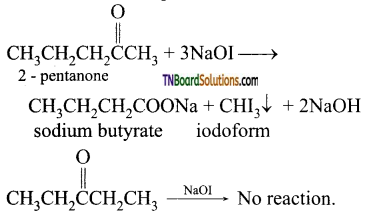
Iodoform Test: 2 – pentanone, being a methyl ketone, when treated NaOI (I2 / NaOH) gives a yellow precipitate of iodoform but 3-pentanone does not.
(iv) Benzaldehyde and acetophenone:
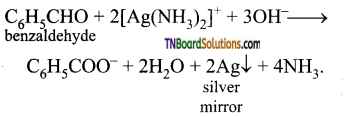
AgNO3 test: Benzaldehye reduces Tollen’s reagent to give a silver mirror (Ag) but acetophenone does not reduce Tollen’s reagent.
Iodoform test: Acetophenone gives a yellow precipitate when treated with I2 / NaOH but benzaldehyde does not.
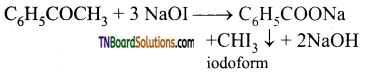
![]()
Question 43.
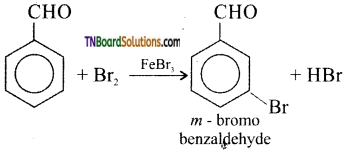
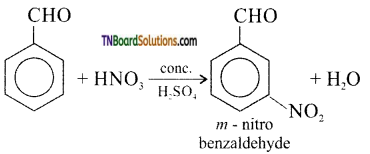
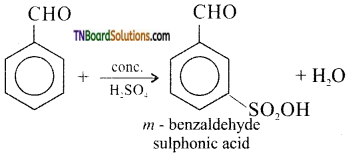
What happens when benzaldehyde is treated with
(i) Br2 in the presence of FeBr3
(ii) a mixture of conc.H2SO4 and conc.HNO3
(iii) conc.H2SO4
(iv) chlorine
(v) chlorine in the presence of FeCl3
(vi) methyl bromide in the presence of anhydrous AlCl3.
Answer:
(i) m-bromobenzaldehyde is formed.
(ii) m-nitrobenzaldehyde is formed.
(iii) m- benzaldehyde sulphonic acid is formed:
(iv) Benzoyl chloride is formed:
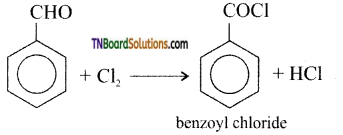
(v) m-chloro benzaldehyde is formed:
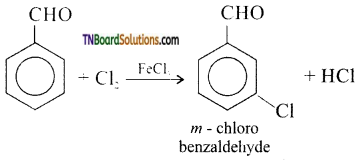
Question 44.
Mention the uses of (i) formaldehyde (ii) urotropine (iii) acetaldehyde (iv) acetone (v) benzaldehyde (vi) acetophenone and benzophenone.
Answer:
Formaldehyde:
- 40% aqueous solution of formaldehyde is called formalin. It is used for preserving biological specimens.
- Formalin has hardening effect, hence it is used for tanning.
- Formalin is used in the production of thermosetting plastic known as bakelite, which is obtained by heating phenol with formalin.
Urotropine:
- Urotropine is used as a medicine to treat urinary infection.
- Nitration of Urotropine under controlled condition gives an explosive RDX (Research and development explosive). It is also called cyclonite or cyclotri methylene trinitramine.
Acetaldehye:
- Acetaldehyde is used for silvering of mirrors.
- Paraldehyde is used in medicine as a hypnotic.
- Acetaldehyde is used in the commercial preparation of number of organic compounds like acetic acid, ethyl acetate etc.,
Acetone:
- Acetone is used as a solvent, in the manufacture of smokeless powder (cordite).
- It is used as a nail polish remover.
- It is used in the preparation of sulphonal, a hypnotic.
- It is used in the manufacture of thermosoftening plastic Perspex.
Benzaldehyde is used:
- as a flavoring agent
- in perfumes
- in dye intermediates
- as starting material for the synthesis of several other organic compounds like cinnamaldehyde, cinnamic acid, benzyl chloride etc.
Aromatic Ketones:
- Acetophenone has been used in perfumery and as a hypnotic under the name hyphone.
- Benzophenone is used in perfumery and in the preparation of benzhydrol drop.
![]()
Question 45.
Give equation for the following reactions.
(i) Nitration of acetophenone
(ii) Bromination of benzophenone
(iii) Friedel – crafts alkylation of benzo phenone
Answer:
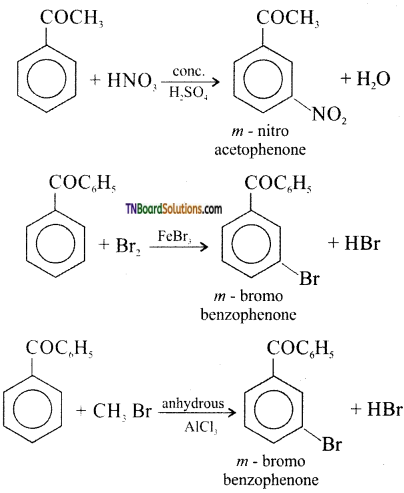
Question 46.
Explain the structure of carboxylic acid group.
Answer:
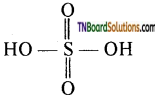
A carboxyl group is made up of a carboxyl group joined to a hydroxyl group.

In the carboxyl group, the carbon atom is attached to two carbon atoms: one by a double bond and the other by a single bond which in turn linked to a hydrogen atom by a single bond. The remaining free valency of the carbon atom of the carboxyl is satisfied by a H atom or an alkyl group. Thus, the structure of carboxylic acids.

where ‘R’ is ‘H’ or alkyl group.
The carbon atom and the two oxygen atoms are sp2 hybridised. The two sp2 hybridesed orbitals of the carboxyl carbon overlap with one sp2 hybridised orbital of each oxygen atom while the third sp2 hybridised orbital of carbon overlaps with either a ‘s’ orbital of H – atom or a sp3 hybridised orbital of ‘C’ atom of the alkyl group to form three a bonds. Each of the two oxygen atoms are left with one unhybridised orbital which is perpendicular to the a bonding skeleton.
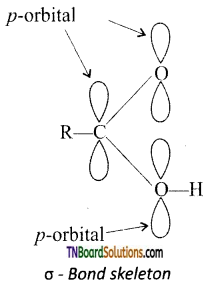
All the three ‘p’ orbitals being parallel, overlap to form n bond which is partly delocalised between carbon and oxygen atoms on one side and carbon and oxygen of the ‘OH’group on the other side.
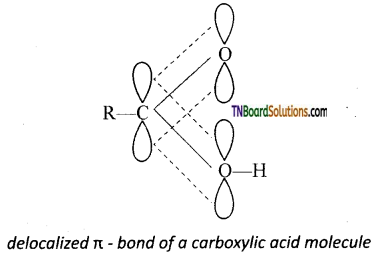
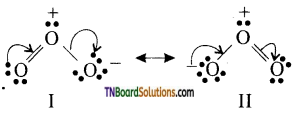
In other words, RCOOH may be represented as a resonance hybrid of the following two canonical structures.
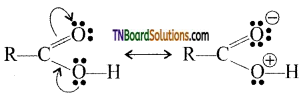
As a result of resonance (i) the C—O single bond length in carboxylic acids is shorter than the normal C—O single bond in alcohols and ethers and (ii) C=O bond length in carboxylic acids is slightly larger than the normal C=O bond length in aldehydes and ketones.
![]()
Question 47.
How is acetic acid prepared from (i) ethanol (ii) methyl cyanide (iii) ethyl acetate (iv) methyl magnesium bromide (v) acetyl chloride (vi) acetic anhydride? Give equation.
Answer:
(i) Oxidation of ethanol by acidified K2Cr2O7.
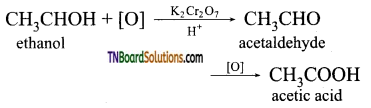
(ii) Acid hydrolysis of methyl cyanide.
![]()
(iii) Acid hydrolysis of ethylacetate
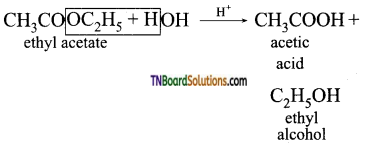
(iv) Methyl magnesium bromide reacts with carbondioxide and the product formed on hydrolysis gives acetic acid.
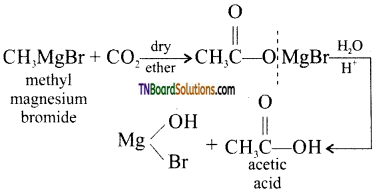
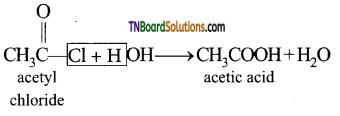
(v) Hydrolysis of acetyl chloride
(vi) Hydrolysis of acetic anhydride
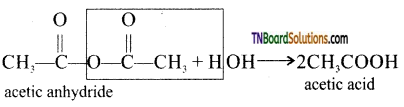
Question 48.
Identify X and Y
![]()
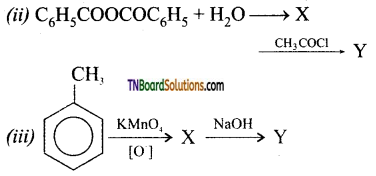
Answer:
(i) X = C6H5COOMg Br; Y = C6H5COOH
(ii) X = C6H5COOH; Y = C6H5COCl
(iii) X = C6H5COOH; Y = C6H5COONa
![]()
Question 49.
Explain why?
(i) Carboxylic acids have higher boiling point than aldehydes, ketones, or even alcohols of comparable molecular mass.
(ii) Lower aliphatic / carboxylic acid are miscible with water while higher carboxylic acids are immiscible with water.
Answer:
(i) Carboxylic acids have higher boiling point ’ than aldehydes, ketones and even alcohols of comparable molecular masses. This is due to more association of carboxylic acid molecules through intermolecular hydrogen bonding.
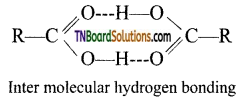
(ii) Lower aliphatic carboxylic acids (up to four carbon) are miscible with water due to the formation of hydrogen bonds with water. Higher carboxylic acid are insoluble in water due to increased hydrophobic interaction of hydrocarbon part. The simplest aromatic carboxylic acid, benzoic acid is insoluble in water.
Question 50.
How will you convert
(i) Ethyl benzene to benzoic acid
(ii) Isopropyl benzene to benzoic acid
(iii) p-nitro toluene to p-nitrpbenzoic acid
(iv) o-xylene to phthalic acid
(v) But-2-ene to ethanoic acid
(vi) Benzonitrile to benzoic acid
(vii) Ethyl magnesium iodide to propanoic acid
(viii) Ethyl benzoate to benzoic acid
(ix) Benzamide to benzoic acid
(x) Propan – 2 – one to butanoic acid.
Answer:
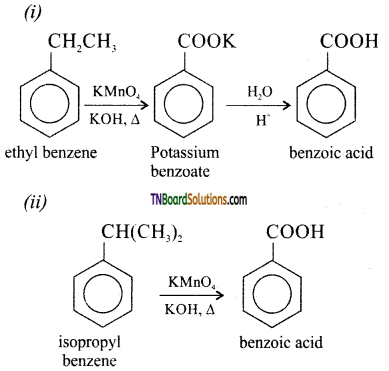
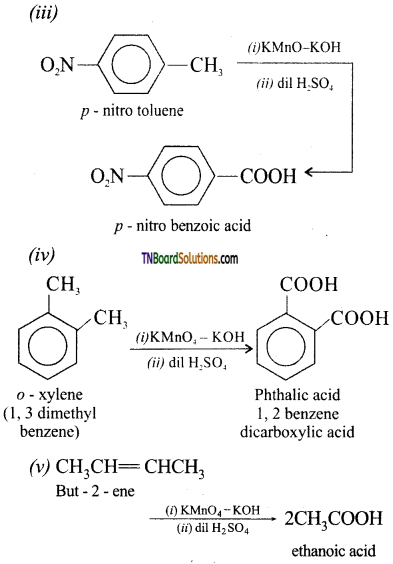
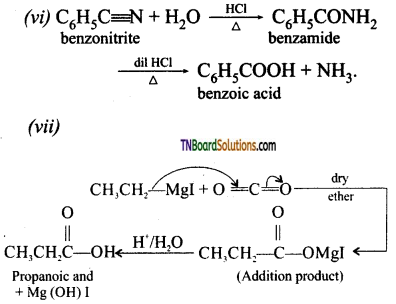
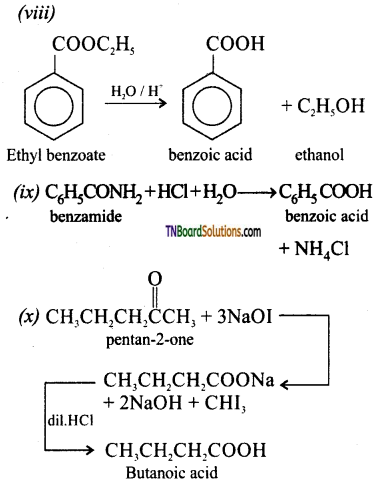
![]()
Question 51.
Which of the following compounds will undergo aldol condensation? Which the Cannizaro reaction, and which neither? Write the structure of the expected products of aldol condensation and Cannizaro reaction.
(i) Methanal
(ii) 2 – methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vii) Phenyl acetaldehyde
(viii) Butan-1-ol
(ix) 2, 2-dimethyl butanal
Answer:
I. 2 – methyl pentanal
Cyclohexanone
1 – phenyl propanone and phenyl acetaldehyde
contain one or more a hydrogen and hence undergo aldol condensation.
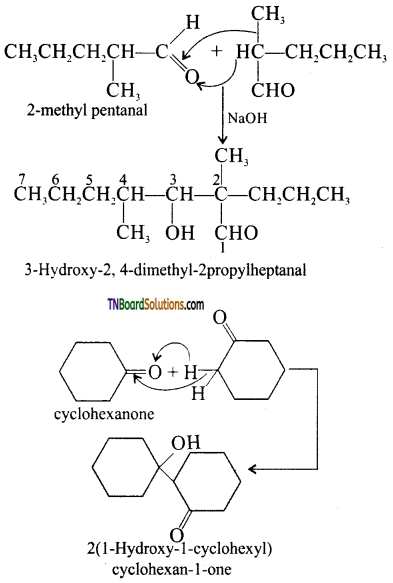
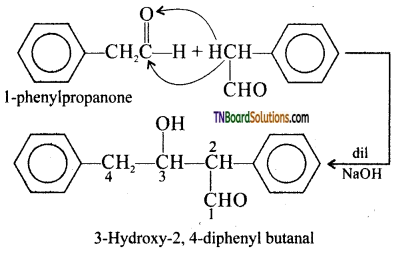
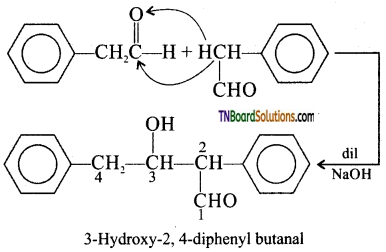
II. Methanal, benzaldehyde, and 2,2 dimethyl butanal do not contain a hydrogen atoms and hence undergo cannizaro reaction. The reaction and structures are:
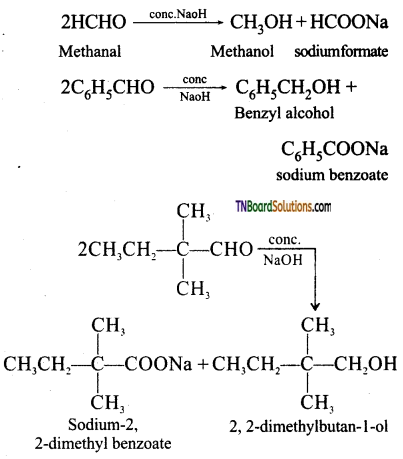
III. Benzophenone is a ketone with no a hydrogens while butan-1-ol is an alcohol. Both of these neither undergo aldol condensation nor cannizaro reaction.
Question 52.
Give equations for reactions of acetic acid with the following reagents.
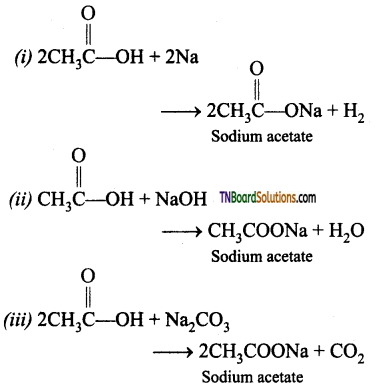
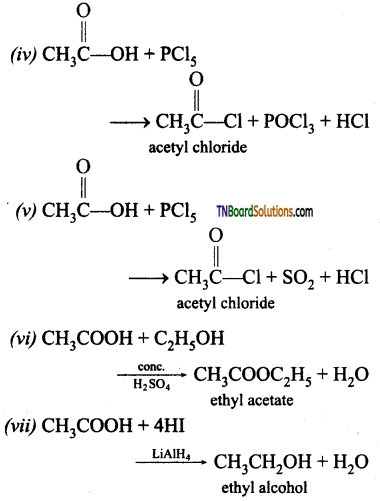
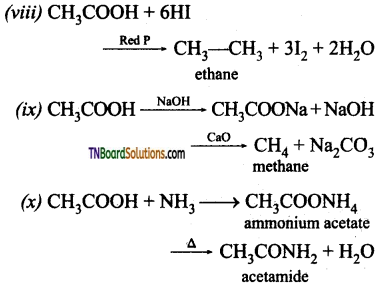
(i) Na (ii) NaOH (iii) Na2CO3 (iv) PCl5 (v) SOCl2 (vi) C2H5OH (vii) LiAlH4 (viii) Red P and HI (ix) Sodalime (x) NH3 followed by heating (xi) Cl2 / Red P.
Answer:
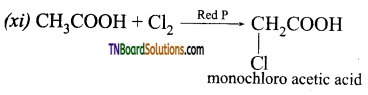
![]()
Question 53.
Show how each of the following could be converted to benzoic acid:
(i) Ethyl benzene,
(ii) acetophenone,
(iii) benzophenone,
(iv) phenyl ethene.
Answer:
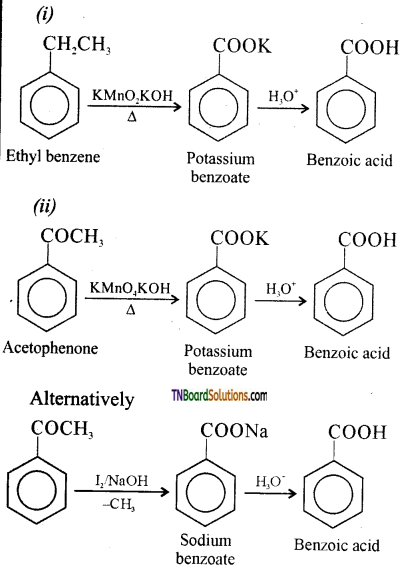
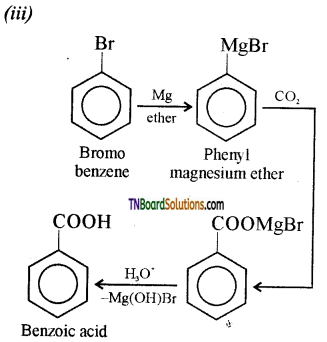
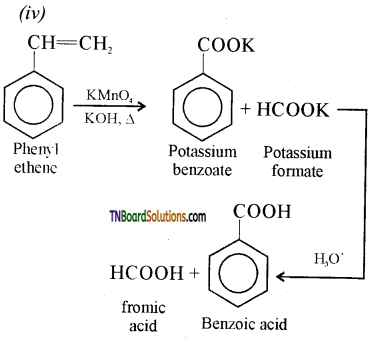
Question 54.
What is esterification? Give an example.
Answer:
The formation of an ester by the action of carboxylic acid and alcohol in the presence of an acid is called esterification.
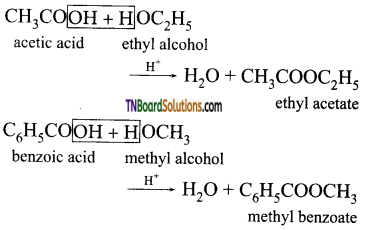
In these reaction the C—OH bond in acids is cleaved.
![]()
Question 55.
Give a brief accounts of decarboxylation reaction.
Answer:
Removal of CO2 from carboxyl group in called as decarboxylation. Carboxylic acids lose carbon di oxide to form hydrocarbon when their sodium salts are heated with soda lime (NaOH and CaO in the ratio 3:1)
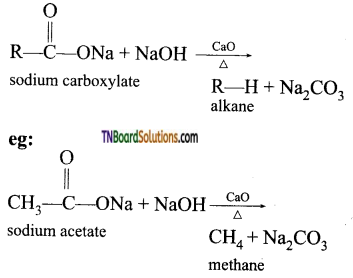
Question 56.
Suggest a suitable reagent to bring about the following conversions. Give equations.
(i) Acetic acid to acetyl chloride
(ii) Benzoic acid to ethyl benzoate
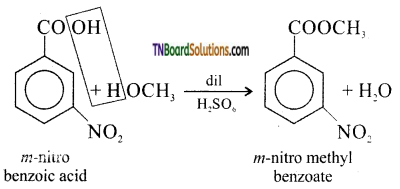
(iii) m – nitro benzoic acid to m – nitro methyl benzoate
(iv) Ethanoic acid to ethanol
(v) Acetic acid to ethane
Answer:
(i) PCl5 or SOCl2

(ii) C2H5OH in the presence of dil H2SO4
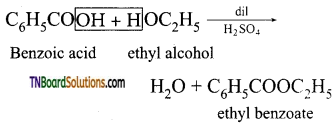
(iii) Methyl alcohol in the presence of dil. H2SO4
(iv) Lithium aluminium hydride

(v) HI in the presence of Red phosphorus
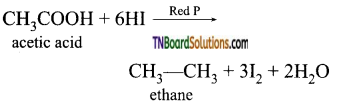
Question 57.
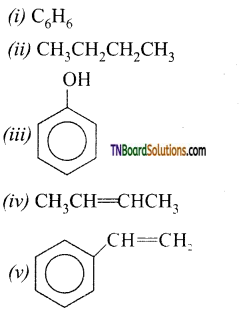
Give the products of the following:
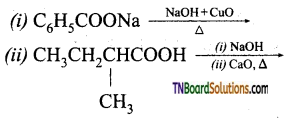
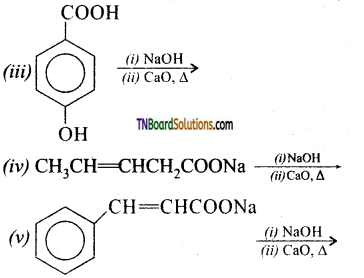
Answer:
All these reactions are decarboxylation reactions. In these reactions the COONa group is replaced by ‘H’.
![]()
Question 58.
What is HVZ reaction? Give an example.
Answer:
Carboxylic acids having an α – hydrogen are halogenated at the α – position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to form α halo carboxylic acids. This reaction is known as Hell-Volhard-Zelinsky reaction (HVZ reaction) The α-Halogenated acids are convenient starting materials for preparing α – substituted acids, eg:
Substitution reaction in the hydrocarbon part α – Halogenetion:
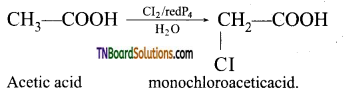
Question 59.
Explain why the ‘COOH’ group in benzoic acid in meta directing.
Answer:
The structure of benzoic acid is a resonance hybrid of the following canonical structure.
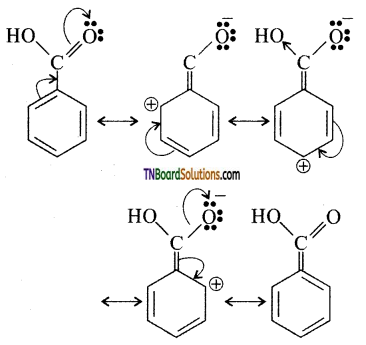
Because of -M effect of carboxylic acid, the ortho and para positions becomes less in electron density relative to the meta position, i.e., meta-position becomes rich in electron density compared to ortho and para positions. Hence, the electrophile attacks the meta position, i.e., the COOH group deactivates the benzene ring and is the meta directing group.
Question 60.
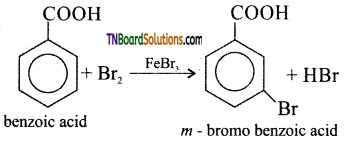
How are the following compounds prepared from benzoic acid?
(i) m- Bromo benzoic acid
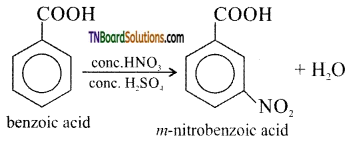
(ii) m- nitro benzoic acid
(iii) m-sulpho benzoic acid.
Answer:
(i) Bromination of benzene gives m – bromo benzoic acid.
(ii) Nitration of benzene using nitrating mixture gives m – nitro benzoic acid.
(iii) Sulphonation of benzene, using fuming sulphuric acid gives m – sulpho benzoic acid.
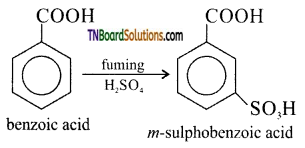
![]()
Question 61.
Formic acid is a reducing agent. Substantiate this statement with examples.
Answer:
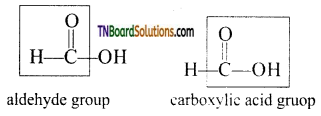
Formic acid contains both an aldehyde as well as an acid group. Hence, like other aldehydes, formic acid can easily be oxidised and therefore acts as a strong reducing agent.
(i) Formic acid reduces Tollens reagent (ammonical silver nitrate solution) to metallic silver.
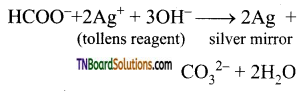
(ii) Formic acid reduces Fehlings solution. It reduces blue coloured cupric ions to red coloured cuprous ions.
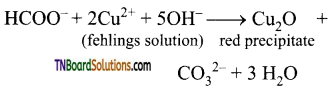
Question 62.
Give the tests for carboxylic acid group in an organic compound.
Answer:
- In an aqueous solution, carboxylic acid turns blue litmus red.
- Carboxylic acids give brisk effervescence with sodium bicarbonate due to the evolution of carbon dioxide.
- When a carboxylic acid is warmed with alcohol and conc.H2SO4 it forms an ester, which is detected by its fruity odour.
Question 63.
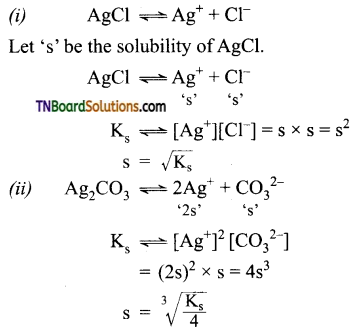
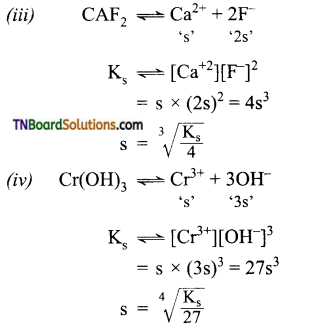
Give a brief account of the acidity of carboxylic acids.
Answer:
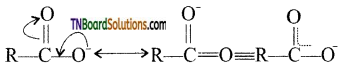
Carboxylic acids undergo ionization to produce H+ and carboxylate ions in an aqueous solution. The carboxylate anion is stabilized by resonance which makes the carboxylic acid to donate the proton easily.
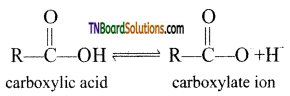
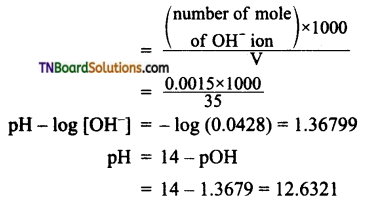
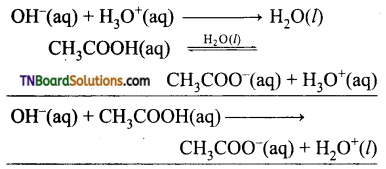
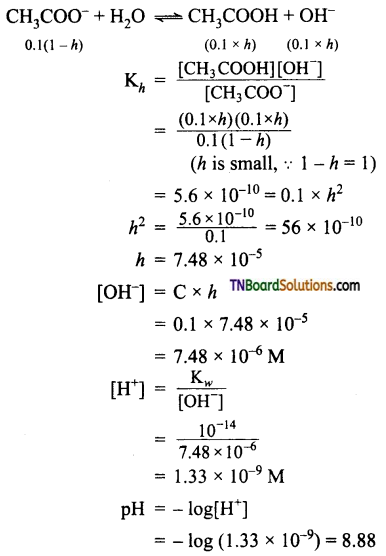
The resonance structure of carboxylate ion are given below.
The strength of carboxylic acid can be expressed in terms of the dissociation constant(Ka):
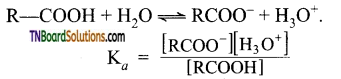
The dissociation constant is generally called acidity constant because it measures the relative strength of an acid. The stronger the acid, the large will be its Ka value.
The dissociation constant of an acid can also be expressed in terms of pKa value.
pKa = log Ka
A stronger acid will have higher Ka value but smaller pKa value, the reverse is true for weaker acids.
![]()
Question 64.
Briefly discuss the effect of substituents on the acidity of carboxylic acids.
Answer:
Effect of substituents on the acidity of carboxylic acid.
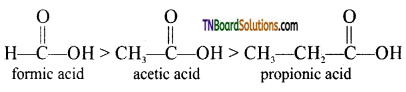
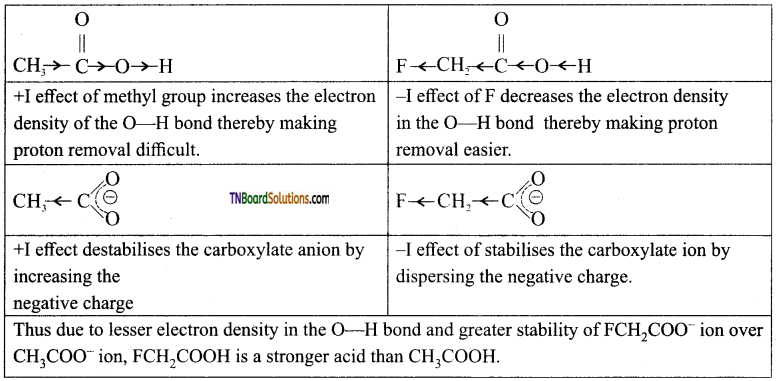
(i) Electron releasing alkyl group decreases the acidity: The electron-releasing groups (+1 groups) increase the negative charge on the carboxylate ion and destabilize it and hence the loss of proton becomes difficult. For example, formic acid is more stronger than acetic acid,
eg:
(ii) Electron withdrawing substituents increases the acidity: The electron-withdrawing substituents decrease the negative charge on the carboxylate ion and stabilize it. In such cases, the loss of proton becomes relatively easy.
Acidity increases with increasing electronegativity of the substituents. For example, the acidity of various halo acetic acids follows the order F—CH2—COOH > Cl—CH2—COOH > Br—CH2—COOH > I —CH3—COOH
Acidity increases with increasing number of electron – withdrawing substituents on the a – carbon.
For example
Cl3C—COOH > Cl2CH—COOH > ClCH2COOH > CH3COOH
The effect of various, electron withdrawing groups on the acidity of a carboxylic acid follows the order,
—NO2 > —CN >—F >—Cl >—Br >—I >Ph
The relative acidities of various organic compounds are
RCOOH > ArOH > H2O > ROH >RC ≡ CH
Question 65.
Fluorine is more electronegative than chlorine even their p- fluoro benzoic acid in weaker than p- chloro benzoic aicd. Explain.
Answer:
Since halogens are far more electronegative than carbon and also possess lone pairs of electrons, they exert both -1 and + M effects. Now, the fluorine, the lone pairs of electrons are present in ‘2p’ orbitals and in chlorine, they are present in ‘3p’ orbitals. Since ‘2p’ orbitals of fluorine and chlorine are of almost equal size, the + M effect is more pronounced in p fluoro benzoic acid than in p-chloro benzoic effect.
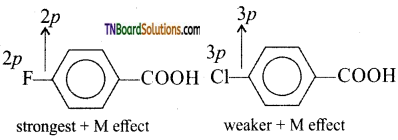
![]()
Question 66.
Explain why p – nitrobenzoic acid has a higher Ka value than benzoic acid.
Answer:
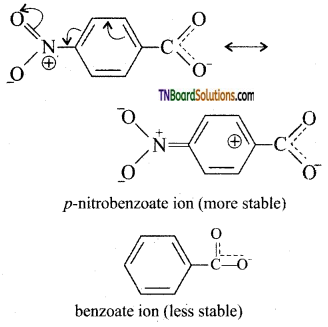
Higher the Ka value, the stronger is the acid. Thus, p-nitro benzoic acid is a stronger acid than benzoic acid. This is due to
- Because of — I and -M effect of NO2 group the electron density in O—H bond decreases. As a result, the —O—H bond becomes weak and hence, p – nitrobenzoic acid easily loses a proton than benzoic acid.
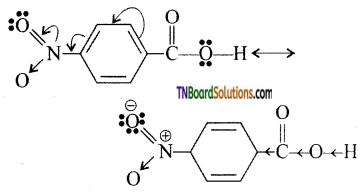
- Due to -1 and -M effect of nitrogroup dispersal of negative charge occurs and hencep -nitro benzoate ion becomes more stable than benzoate ion.
Question 67.
Give the IUPAC names of the following compounds,
(i) PhCH2CH2COOH
(ii) (CH3)2C=CHCOOH CH
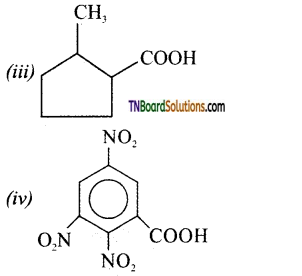
Answer:
(i) 3 -phenyl propanoic acid
(ii) 3 -methyl but – 2-enoic acid
(iii) 2 -methylcyclopentane carboxylic acid
(iv) 2, 4, 6 -trinitro benzoic acid or 2, 4, 6-trinitro benzene carboxylic acid.
![]()
Question 68.
Explain the mechanism for the reaction.
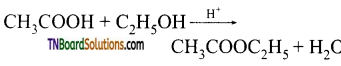
Answer:
Esterification of carboxylic acids with alcohols is a nucleophilic acyl substitution.
In such reactions the nucleophile first adds to a carbonyl group to give a tetrahedral intermediate which then readily loses the leaving group to give the substitution products. The mechanism involves the following steps.
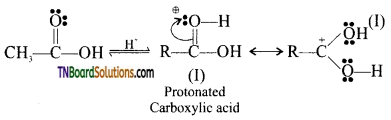
Step 1: Protonation of the carbonyl group:
In the presence of mineral acids (H2SO4 or HCl), the carbonyl group of the carboxylic acid accepts a proton to form protonated carboxylic acid.
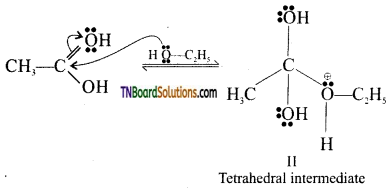
Step 2: Nucleophilic attack of the alcohol molecule. As a result of protonation, the carbonyl carbon becomes electrophilic (i.e. more electropositive) and hence readily undergoes nucleophilic attack by lone pairs of electrons on the oxygen atom of the alcohol molecule to form a tetrahedral intermediate (II).
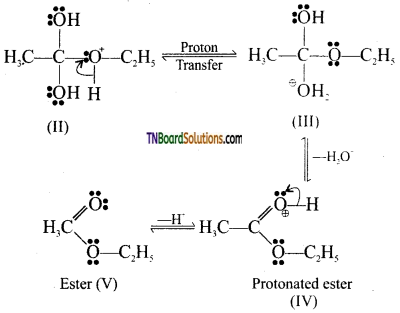
Step 3: Loss of a molecule of water and a proton. The tetrahedral intermediate (II) undergoes a proton transfer to give another tetrahedral intermediate (III). During this proton transfer, the OH group gets converted to – OH2 group which being a good leaving group is lost as a neutral water molecule. The protonation ester (IV), thus formed, finally loses a proton to give the ester (V).
Question 69.
Give the uses of (i) formic acid (ii) acetic acid (iii) benzoic acid (iv) acetyl chloride (v) acetic anhydride (vi) ethyl acetate.
Answer:
Formic acid: It is used
- for the dehydration of hides
- as a coagulating agent for rubber latex
- in medicine for the treatment of gout
- as an antiseptic in the preservation of fruit juice.
Acetic acid: It is used
- as table vinegar
- for coagulating rubber latex
- for manufacture of cellulose acetate and poly vinylacetate.
Benzoic acid: It is used
- as food preservation either in the pure form or in the form of sodium benzoate
- in medicine as an urinary antiseptic
- for manufacture of dyes.
Acetyl chloride: It is used
- as acetylating agent in organic synthesis
- in detection and estimation of —OH, — NH2 groups in organic compounds.
Acetic anhydride: It is used
- acetylating agent
- in the preparation of medicine like asprin and phenacetin
- for the manufacture plastics like cellulose acetate and poly vinyl acetate.
Ethyl acetate is used
- in the preparation of artificial fruit essences
- as a solvent for lacquers
- in the preparation of organic synthetic reagent like ethyl acetoacetate.
Uses
Acetamide is used in the preparation of primary amines.
![]()
Question 70.
A compound with molecular formula, C4H10O3 on acetylation with acetic anhydride gives a compound with molecular weight 190. Find out the number of hydroxyl groups present in the compound.
Answer:
During acetylation one ‘H’ atom (at mass = 1 amu) of the OH group is replaced by an acetyl group (CH3CO – molar mass = 43 amu)
—OH + (CH3CO)2O → —O—COCH3 + CH3COOH
In other words acetylation of each OH group increases the molecular mass by 43 – 1 = 42 amu. Now that the molecular mass of the compound C4H10O3 = 106 amu, while that of the acetylated product is 190 amu. Therefore the number of ‘OH’ groups present in the compound is \(\frac{190-106}{42}\) =2.
Question 71.
Explain (i) Perkins’ reaction (ii) Knoevenagal reaction.
Answer:
(i) Perkins’ reaction:
When an aromatic aldehyde is heated with an aliphatic acid anhydride in the presence of the sodium salt of the acid corresponding to the anhydride, condensation takes place and an α, β unsaturated acid is obtained. This reaction is known as Perkin’s reaction.
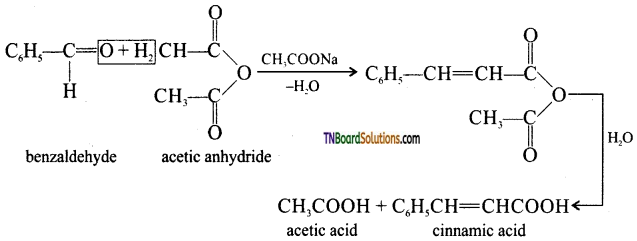
(ii) Knoevenagal reaction:
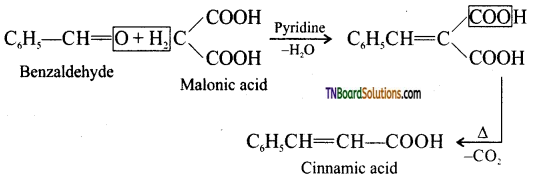
Benzaldehyde condenses with malonic acid in presence of pyridine forming cinnamic acid, Pyridine act as the basic catalyst.
Question 72.
Give names of reagents which bring about the following conversions.
(i) Hexan-1-ol to hexanal
(ii) Cyclohexanol to cyclohexanone
(iii) p-fluorotoluene to p-fluorobenzaldehyde,
(iv) Ethanenitrile to ethanal
(v) allyl alcohol to propenal
(vi) But-2-ene to ethanal
Answer:
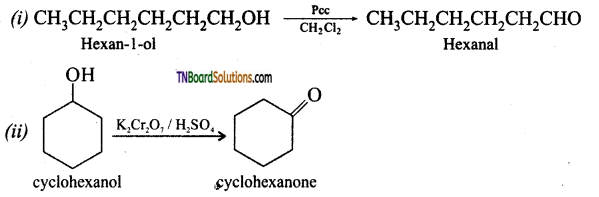
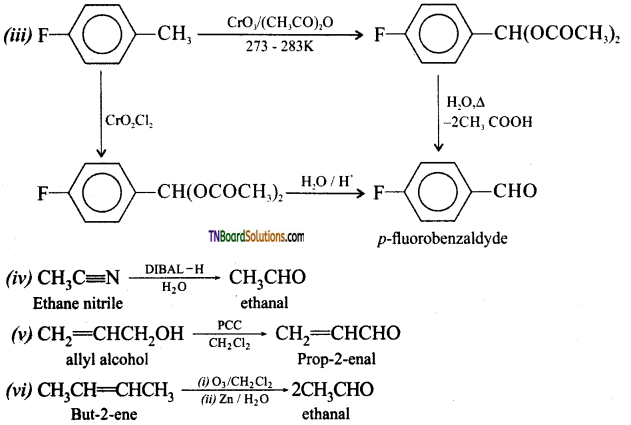
![]()
Question 73.
Bring out Che following conversions.
(i) Benzyl alcohol to phenyl ethanoic acids.
(ii) 3 – nitrobromo benzene to – 3 – nitrobromo benzoic acid
(iii) Methyl acetophenone to benzene 1, 4, dicarboxylic acid
(iv) cyclohexene to hexane 1, 6 dicarboxylic acid.
Answer:
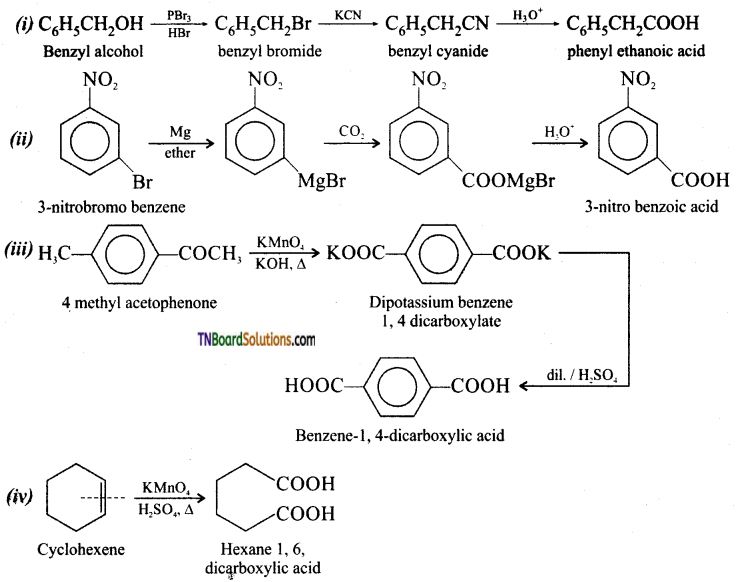
Question 74.
Identify A – E in the following reactions:
Answer:
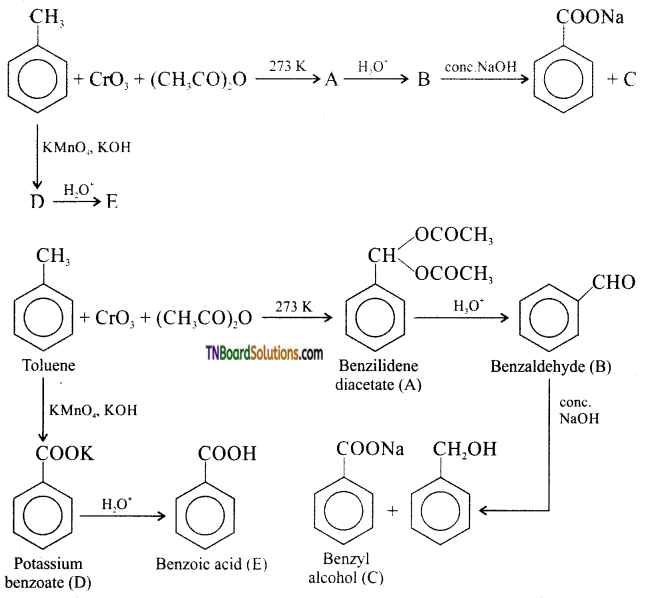
![]()
Question 75.
Write a short note on the electrophilic substitution reaction of benzaldehyde.
Answer:
The ‘CHO’ group in benzaldehyde deactivates the benzene ring due to its —M effect. As a result the electron densities in ortho and para position are decreased compared to the meta position, i.e, The meta position is electron rich compared to ortho and para position. Hence electrophilic substitution reaction occur at meta position.
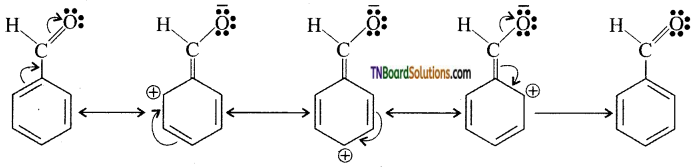
Question 76.
Which acid of each pair shown here would you expect to be stronger?
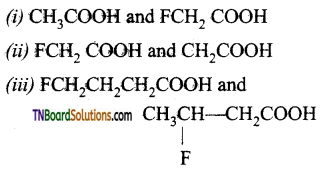
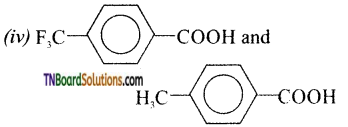
Answer:
(i)
(ii) Due to much stronger -1 effect of F over Cl FCH2 COO– ion is much more stable than ClCH2COO ion and hence FCH2COOH is a stronger acid than ClCFI2COOH.
(iii)
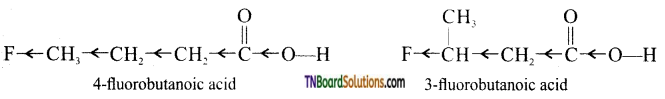
Inductive effect decreases with increasing distance, therefore, -I effect of is somewhat stronger in 3-fluorobutanoic acid than in 4-fluorobutanoic acid. In other words, CH3CHCH2COCT ion is in more stable than
FCH2CH2CH2COO– ion and hence  is a stronger acid than FCH2CH2CH2COOH.
is a stronger acid than FCH2CH2CH2COOH.
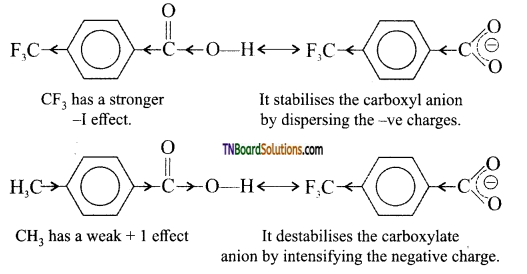
Therefore due to greater stability of CF3—C6H4—COO– (p) ion over CH3—C6H4COOH– (p) is a stronger acid than H3CC6H4—COOH (p).
(iv)
![]()
Question 77.
Identify A to E in the following reactions:
Answer:
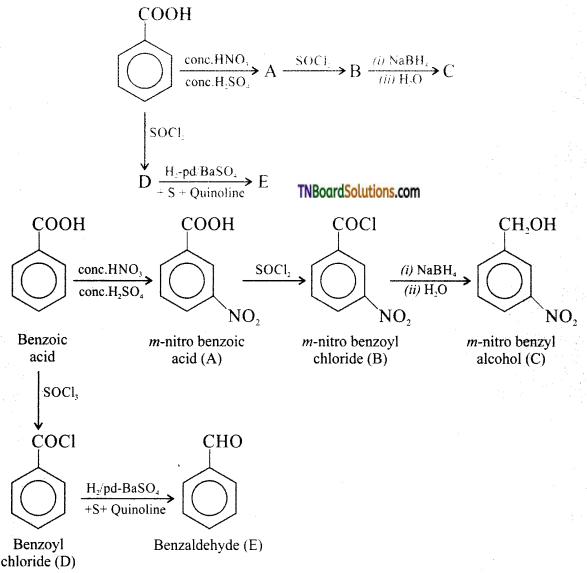
(Note: NaBH4 reduces COCl to CH2OH, but does not reduce NO2 to NH2 group.)
Question 78.
How will you bring about the following conversions in not more than two steps?
(i) Propanone to propene
(ii) Benzoic acid to benzaldehyde
(iii) Ethanol to 3 – hydroxy butanal
(iv) Benzene to m – nitrobenzene
(v) Benzaldehyde to benzophenone
(vi) bromo benzene to 1-phenyl ethanol
(vii) Benzaldehyde to 3 phenylpropan-1-ol
(viii) Benzaldehyde to a – hydroxy phenyl acetic acid
(ix) Benzoic acid to p-nitrobenzyl alcohol.
Answer:
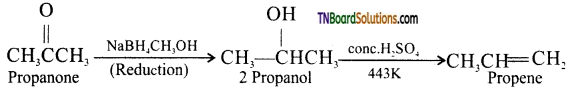
(i) Propanone to propene:
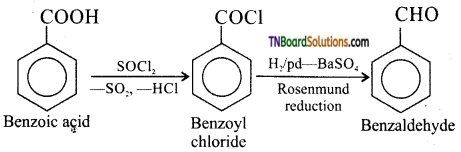
(ii) Benzoic acid to benzaldehyde
(iii) Ethanol to 3 – hydroxy butanal:

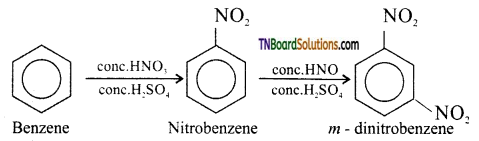
(iv) Benzene to m – dinitrobenzene:
(v) Benzaldehyde to benzophenone:
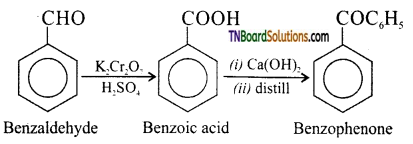
(vi) Bromobenzene to 1 – phenyl ethanol:
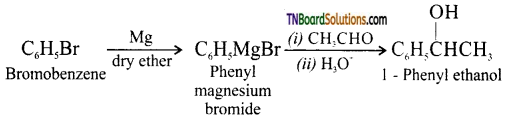
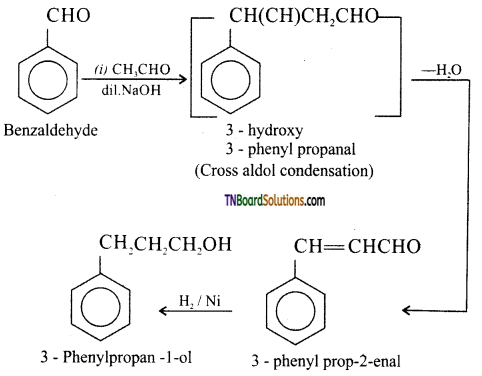
(vii) Benzaldehyde to 3 – phenylpropan – 1 – ol:
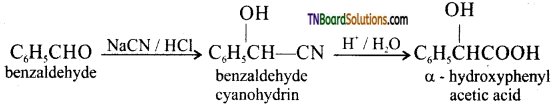
(viii) Benzaldehyde to a – hydroxyphenyl acetic acid:
(ix) Benzoic acid to m-nitrobenzyl alcohol:
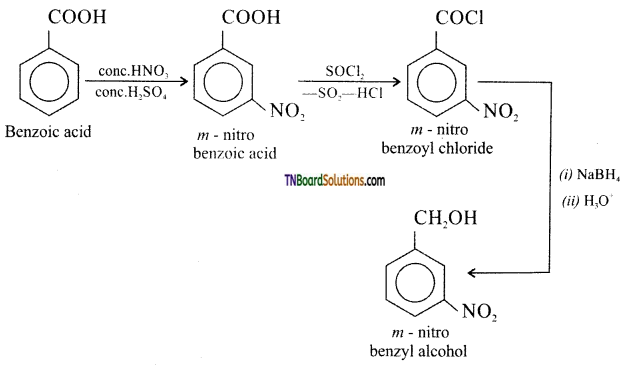
![]()
Question 79.
What is transesterification? Give an example.
Answer:
Esters of an alcohol can react with another alcohol in the presence of a mineral acid to give the ester of second alcohol. The interchange of alcohol portions of the esters is termed transesterification.
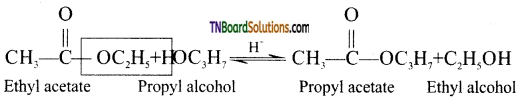
Question 80.
Give an example for Claisen condensation.
Answer:
Esters containing at least one α – hydrogen atom undergo self-condensation in the presence of a strong base such as sodium ethoxide to form β – keto ester. This reaction is known as Claisen condensation.

Question 81.
How are the following compounds prepared?
(i) acetyl chloride and ethyl chloride from ethyl acetate
(ii) acetamide from acetyl chloride
(iii) Acetamide from acetic anhydride.
Answer:
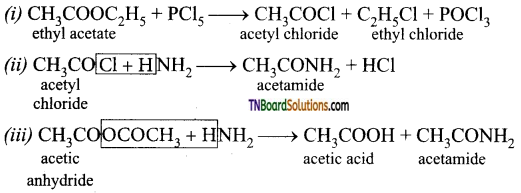
Question 82.
Complete the following equation:
(i) CH3COCl + CH3NH2 →
(ii) CH3COCl + (CH3)2 NH →
(iii) (CH3CO)2O + PCl5 →
Answer:
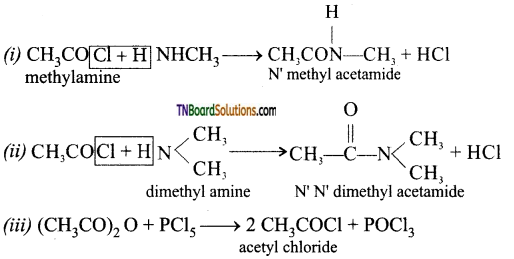
![]()
Question 83.
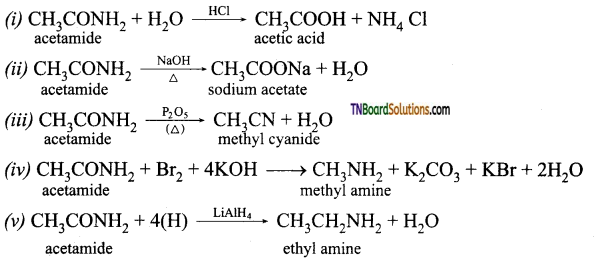
How are the following compounds prepared from acetamide?
(i) acetic acid (ii) sodium acetate (iii) methyl cyanide (iv) methyl amine (v) ethyl amine
Answer:
Question 84.
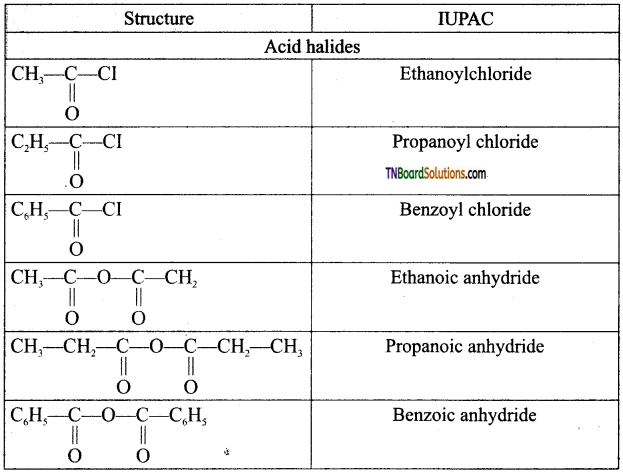
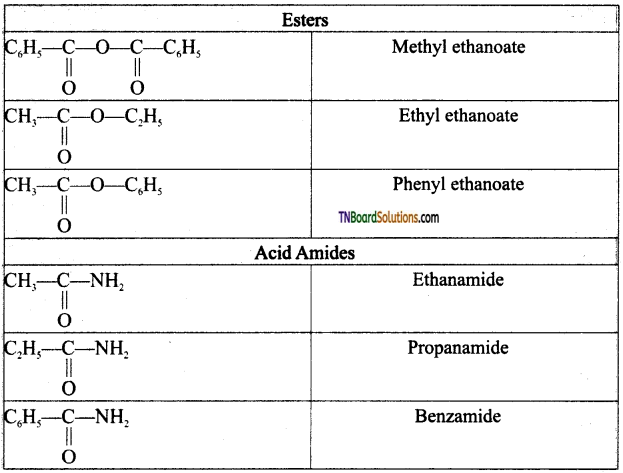
Write the structure and IUPAC names of the following acid derivatives.
(i) acetyl chloride (ii) propionyl chloride (iii) Benzoyl chloride (iv) acetic anhydride (v) propionic anhydride (vi) benzoic anhydride (vii) methyl acetate (viii) Ethyl acetate (ix) phenyl acetate (x) acetamide (xi) propionamide (xii) Benzamide.
Answer:
Question 85.
Briefly explain amphoteric nature of acid amide.
Answer:
Amides behave both as weak acid as well as weak base and thus show amphoteric character. This can be proved by the following reactions.
Acetamide (as base) reacts with hydrochloric acid to form salt.

Acetamide (as acid) reacts with sodium to form sodium salt and hydrogen gas is liberated.

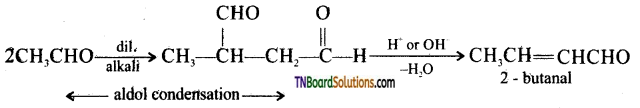
![]()
Question 86.
An unknown aldehyde (A) on reaction with alkali gives a β – hydroxy aldehyde which loses water to form an unsaturated aldehyde, 2-butanal. Another aldehyde (B) undergoes a disproportionation reaction in the presence of conc.alkali to form products (C) and (D). (C) is an aryl alcohol with formula C7H8O. Identify (A) and (B).
(ii) Write the sequence of chemical reaction involved.
(iii) Name the product, when (B) reacts with zinc amalgam and hydrochloric acid.
Answer:
Since the aldehyde (A) oft reaction With alkali gives a β – hydroxy aldehyde (i.e, an aldol), which loses water to form 2 – butanal (an unsaturated aldehyde) ‘A’ must be acetaldehyde.
Since aldehyde (B) on treatment with conc.alkali undergo disproportionation (i.e., Cannizaro reaction) to give two products (C) and (D). One of these products must be an alcohol and the other must be the corresponding acid. Further, since, the product (C) is an aryl alcohol with molecular C7H5O, (C) must be benzyl alcohol, (i.e, C6H5 aryl group) OH is alcoholic group C6H5O. C7H8O—C6H6O = CH2. i.e, C6H5CH2OH. (D) must be sodium benzoate and aldehyde (B) must be benzaldehyde.
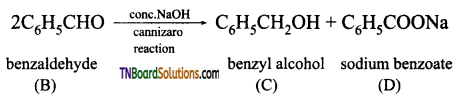
Thus aldehyde (A) is acetaldehyde, (B) is benzaldehyde.
(ii) The sequence of reaction is already explained above.
(iii) When (B) reacts with Zn—Hg / HCl, toluene obtained.

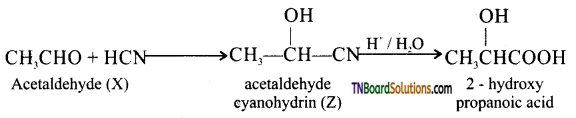
Question 87.
A compound ‘X’ (C2H4O) on oxidation gives ‘Y’ (C2H4O2). X undergoes a haloform reaction. On treatment with HCN, (X) forms (Z) which on hydrolysis gives 2 – hydroxy propanoic acid.
(i) Write down the structures of ‘X’ and ‘Z’.
(ii) Name the product when ‘X’ is treated with dilute NaOH.
(iii) Write down the equation for the reaction involved.
Answer:
(a) Since the MF C2H4O of X corresponds to the general formula CnH2nO, characteristic of aldehydes or ketones. (X) may either be an aldehyde or ketone. Since ketone must have at least ‘3’ carbon atoms but X (C2H4O) has only ‘2’ two. Therefore (X) must be an aldehyde. The only possible aldehyde is CH3CHO (MF C2H4O) (acetaldehyde).
If (X) is acetaldehyde, than the compound (Z) is acetic acid (CH3COOH).
(b) (X) is acetaldehyde is confirmed by the following reaction.
(i) It undergoes haloform reaction
(i.e.,)
(ii) On treatment with KCN, acetaldehyde forms a cyanohydrin (Z) which on hydrolysis gives 2 – hydroxy propanoic acid.
(iii) In the presence of dilute NaOH, acetaldehyde undergoes aldol condensation to form 3- hydroxy butanal commonly called aldol.
Choose the correct answer:
1. The IUPAC name of the compound is
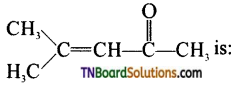
(a) Mesityl oxide
(b) 4- methyl pent – 3 – en – 2 – one
(c) 4 – methyl pent – 2 – en – 4 – one
(d) 2 – methyl pent – 4 – one
Answer:
(b)
![]()
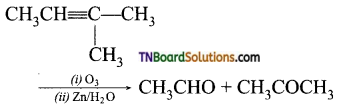
2. Ozonolysis of 2-methyl but-2-ene followed by treatment with Zn/H2O gives:
(a) ethanal
(b) propanone
(c) propanal and prop – 2 – one
(d) ethanal and propan – 2 – one
Answer:
(d)
Hint:
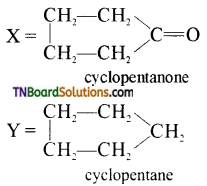
3. Identify the product ‘Y’ in the following reaction sequence.
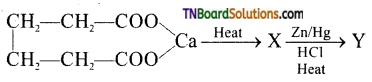
(a) pentane
(b) cyclobutane
(c) cyclopentane
(d) cyclopentanone
Answer:
(c)
Hint:
4. One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having molecular mass 44 u. The alkene is:
(a) 2 – butene
(b) ethene
(c) propene
(d) 1 – butene
Answer:
(a)
Hint: The aldehyde with molecular mass 44 u is CH3CHO (acetaldehyde). Therefore the symmetrical alkene is 2 – butene.
![]()
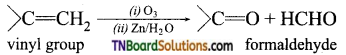
5. Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of:
(a) a vinyl group
(b) an isopropyl group
(c) an acetylene triple bond
(d) two ethylenic double bonds
Answer:
(a)
Hint:
![]()
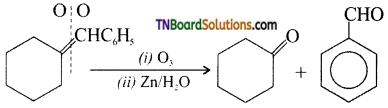
6. Identify the compound ‘X’ in the following reaction:
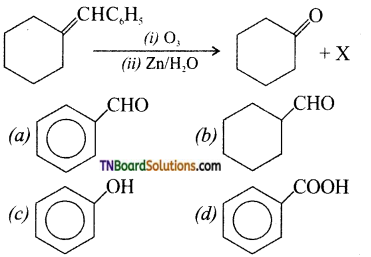
Answer: (a)
Hint:
7. The order of reactivity of phenyl magnesium bromide (PhMgBr) with the following compounds.
I. CH3CHO,
II. (CH3)2CO and
III. PhCOPh is:
(a) III > II > I (b) II > I > III
(c) I > III > II (d) I > II > III
Answer:
(d)
Hint: Reaction between RMgX with carbonyl compound is nucleophilic addition. The greater the positive charge on the carbonyl carbon, the faster is the rate of nucleophilic addition.
8. A carbonyl compound reacts with hydrogen cyanide to form a cyanohydrin which on hydrolysis forms a racemic mixture of a – hydroxy acids. The carbonyl compound is:
(a) formaldehyde
(b) acetaldehyde
(c) acetone
(d) diethyl ketone
Answer:
(b)
Hint: Acetaldehyde cyanohydrin 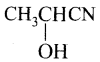 on hydrolysis gives lactic acid
on hydrolysis gives lactic acid 
This contains a chiral carbon and exists as a racemic mixture. All other compounds are symmetrical and hence, their cyanohydrins and their corresponding α – hydroxy acids are not optically active because they do not contain chiral carbon.
![]()
9. In a set of reactions, acetic acid yielded the product D
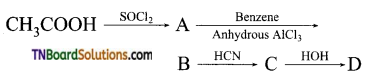
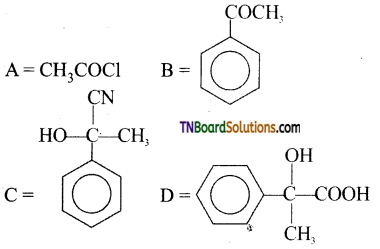
The structure of ‘D’ would be:
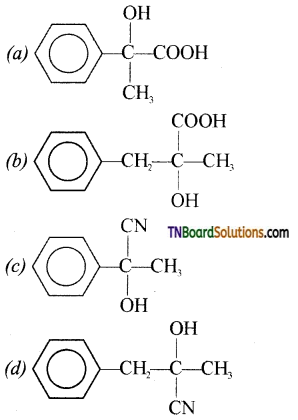
Answer:
(a)
Hint:
10. (CH3)2C=CHCOCH3 can be oxidised to (CH3)2C=CHCOOH by:
(a) Chormic acid
(b) NaOI
(c) Cu at 300°C
(d) KMnO4
Answer:
(b)
Hint:
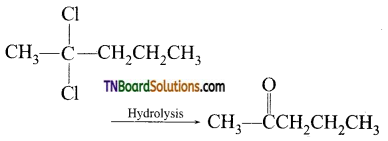
11. A compound ‘A’ (C5H10C312) on hydrolysis gives C5H10O which reacts with NH2OH, forms iodoform but does not give Fehling’s test A is:
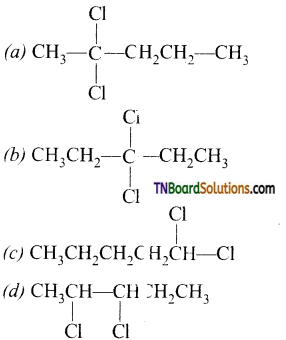
Answer:
(a)
Hint: Since the compound ‘A’ on hydrolysis gives C5H10O does i ot reduce Fehlings solution but reacts with NH2OH, forms iodoform, it must be methyl ketone.
![]()
12. Acetophenone, when reacted with a base, C2H5ONa, yields a stable compound that has the structure:
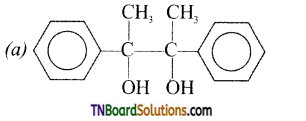
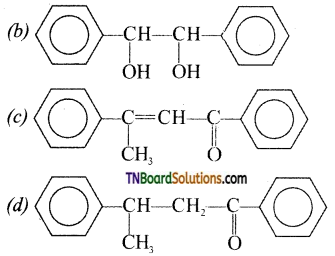
Answer:
(c)
Hint:
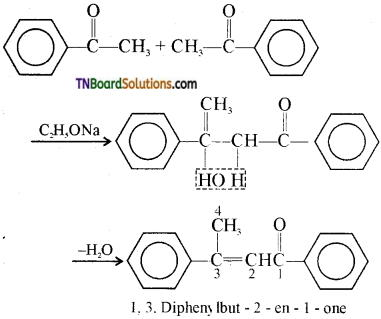
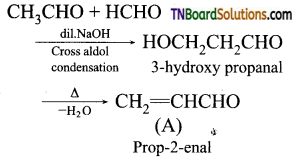
13.
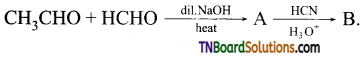
The structure of ‘B’ is:
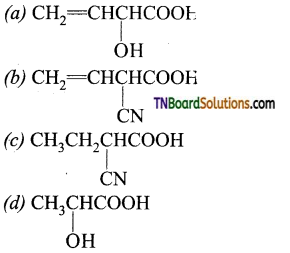
Answer: (a)
Hint:
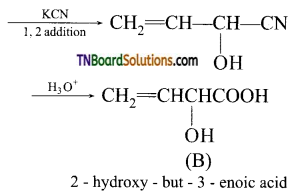
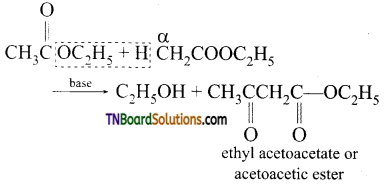
14. Self-condensation of two moles of ethyl acetate in presence of sodium ethoxide yields:
(a) ethyl propionate
(b) ethyl butyrate
(c) acetoacetic ester
(d) methyl acetoacetate
Answer:
(c)
Hint:
![]()
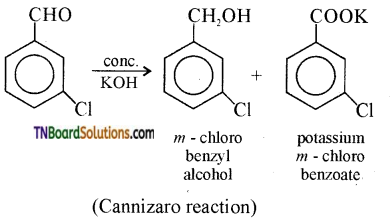
15. m- chlorobenzaldehyde on reaction with conc. KOH at room temperature gives:
(a) potassium-m-chloro benzoic acid and m-hydroxy benzaldehyde.
(b) m-hydroxy benzaldehyde and m-chloro benzyl alcohol.
(c) m-chloro benzyl alcohol and m-hydroxy benzyl alcohol.
(d) potassium-m-chloro benzoate and m-chloro benzyl alcohol.
Answer: (d)
Hint:
16. Consider the following compounds:
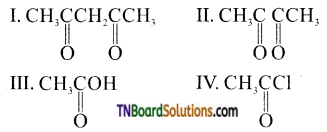
Which will give the iodoform test?
(a) only I
(b) both I and II
(c) only II
(d) all
Answer:
(c)
Hint: Although  contains a CH3CO group linked to carbon, it does not undergo iodoform test. This is because iodination occurs at the more reactive CH2 group rather than terminal CH3 which is essential for iodoform test to occur
contains a CH3CO group linked to carbon, it does not undergo iodoform test. This is because iodination occurs at the more reactive CH2 group rather than terminal CH3 which is essential for iodoform test to occur
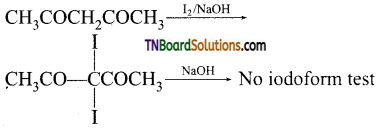
17. Fehling solution will oxidise:
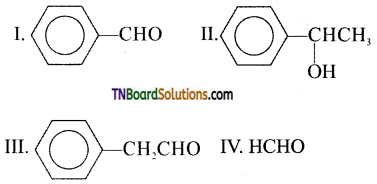
(a) All
(b) only I and IV
(c) only II and IV
(d) only III and IV
Answer:
(d)
Hint: Fehling solution oxidises only aliphatic aldehydes
![]()
18.
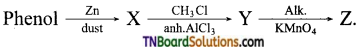
The product ‘Z’ is:
(a) benzaldehyde
(b) benzoic acid
(c) benzene
(d) toluene
Answer:
(b)
Hint:
X = Benzene
Y = Toluene
Z = Benzoic acid
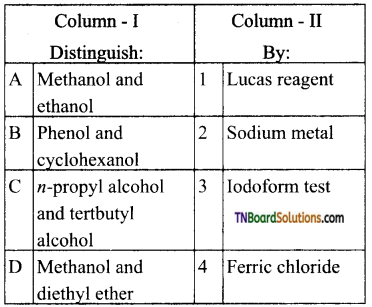
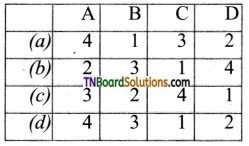
19. Match entries of column I with appropriate entries of column II.
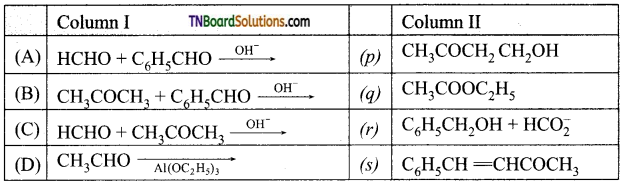
(a) (A) – (p)- (B) – (s)- (C) – (q); (D) – (r)
(b) (A) – (q); (B) – (s); (C) – (p); (D) – (r)
(c) (A) – (r); (B) – (s); (C) – (p); (D) – (q)
(d) (A) – (r); (B) – (p); (C) – (q); (D) – (s)
Answer:
(c)
20. Assertion: Fehling solution oxidizes acetaldehyde to acetic acid but not benzaldehyde to benzoic acid.
Reason: The C—H bond in benzaldehyde is stronger than acetaldehyde.
(a) Both assertion and reason are correct and the reason is the correct explanation of assertion.
(b) Both assertion and reason are correct but the reason is not the correct explanation of assertion.
(c) Assertion is true but reason is wrong.
(d) Both assertion and reason are wrong.
Answer:
(a)
![]()
21. Assertion: Carboxylic acids contain a carbonyl group but do not give characteristic reactions of the carbonyl group.
Reason: Due to resonance, the electrophilic nature of the carboxyl cation is greatly reduced as compared to carbonyl carbon in aldehydes and ketones.
(a) Both assertion and reason are correct and the reason is the correct explanation of assertion.
(b) Both assertion and reason are correct but the reason is not the correct explanation of assertion.
(c) Assertion is true but the reason is wrong.
(d) Both assertion and reason are wrong.
Answer:
(a)
22. Among the following acids which has the lowest pKa value?
(a) CH3COOH
(b) HCOOH
(c) (CH3)2CHCOOH
(d) CH3CH2COOH
Answer:
(b)
Hint: HCOOH is the strongest acid and hence the lowest pKa value.
23. Which of the following presents the correct order of the acidity in the given compounds?
(a) FCH2COOH > ClCH2COOH > BrCH2COOH > CH3COOH
(b) CH3COOH > BrCH2COOH > ClCH2COOH > FCH2COOH
(c) FCH2COOH > CH3COOH > BrCH2COOH > ClCH2COOH
(d) BrCH2COOH > ClCH2COOH > FCH2COOH > CH3COOH
Answer:
(a)
24. The correct acidity order of the following is
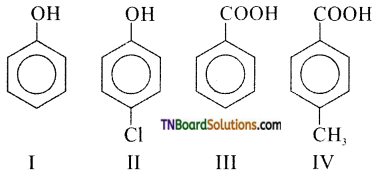
(a) III > IV > II > I
(b) IV > III > I > II
(c) III > II > I > IV
(d) II > III > IV > I
Answer: (a)
Hint: Carboxylic acids are stronger than phenols. Further, electrons donating groups decrease the acidity of phenol/carboxylic acid while electron-withdrawing groups increase the acidity of phenol/carboxylic acid. Thus, the over all order.
III > IV > II > I
![]()
25. Which one of the following pairs gives effervescence with aq.NaHCO3
I. CH3COCI
II. CH3COCH3
III. CH3COOCH3
IV. CH3COOCOCH3
(a) I and II
(b) I and IV
(c) II and III
(d) I and III
Answer:
(b)
Hint: Both CH3COCl and CH3COOCOCH3 react with water to produce acids and hence give effervescence with NaHCO3.
26. Propionic acid with Br2/P yields a dibromo product. Its structure would be:
(a) CH(Br)2—CH2COOH
(b) CH2(Br)CH2COBr
(c) CH3C(Br)2COOH
(d) CH2(Br)CH(Br)COOH
Answer:
(c)
Hint:Bromination occurs at a position.
27. When benzoic acid is treated with LiAlH4, it forms:
(a) benzaldehyde
(b) benzyl alcohol
(c) benzene
(d) toluene
Answer:
(b)
28. Sodium ethoxide reacts with ethanoyl chloride. The compound that is produced in the above reaction is:
(a) 2-butanone
(b) ethyl chloride
(c) ethyl ethanoate
(d) diethyl ether
Answer:
(c)
Hint: CH3COCl + C2H5ONa → CH3COOC2H5 + NaCl
![]()
29. Consider the following:
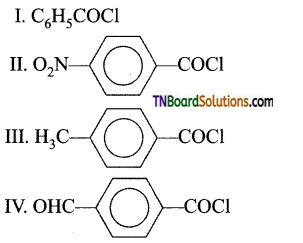
The correct decreasing order of their reactivity towards hydrolysis is:
(a) II > IV > I > III
(b) II > IV > III > I
(c) I > II > III > IV
(d) IV > II > I > III
Answer:
(a)
Hint: Electron donating groups decrease while electron-withdrawing groups increase the reactivity of acid chlorides towards hydrolysis.
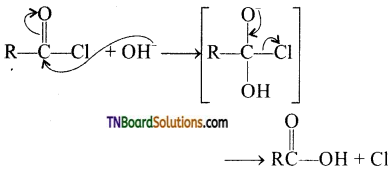
The reactivity depends on the positive charge on the cabonyl carbon.
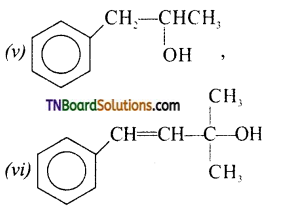
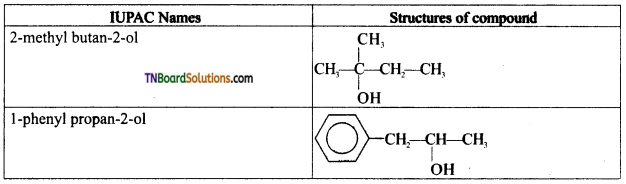
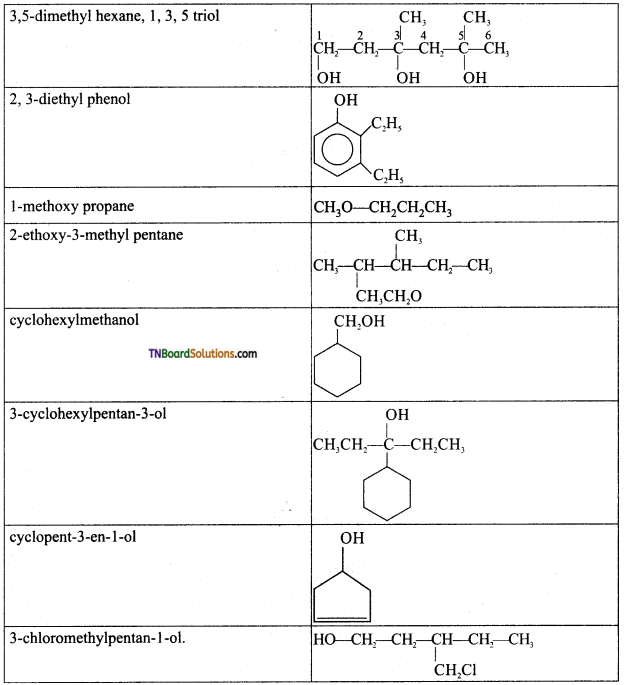
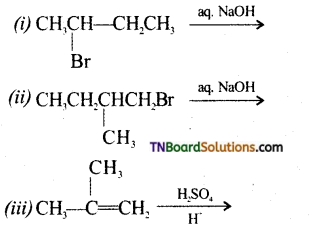
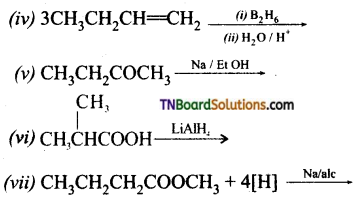
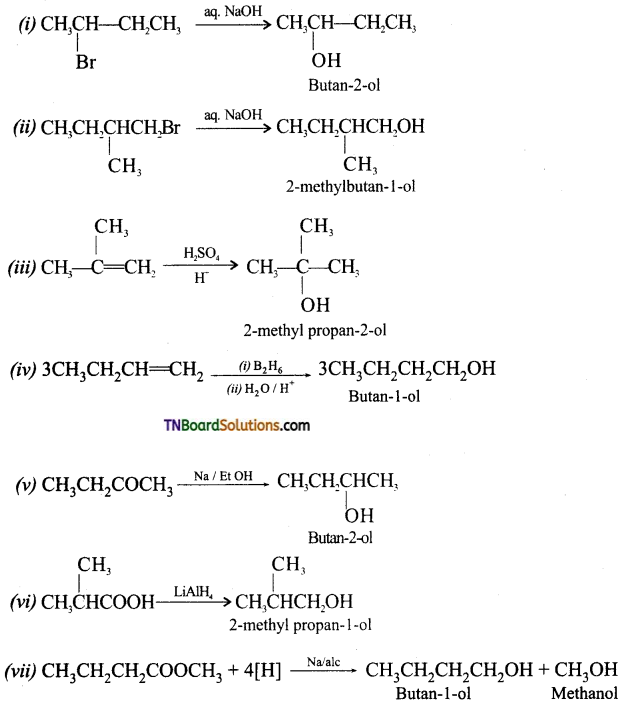

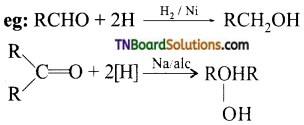
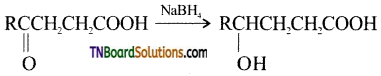
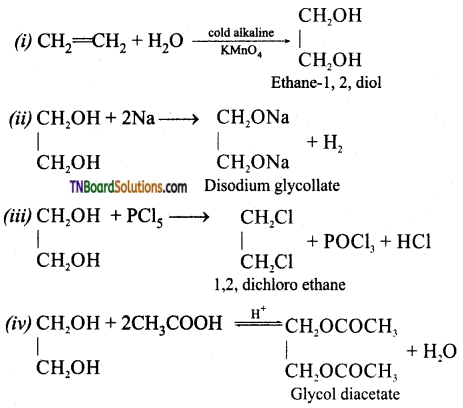
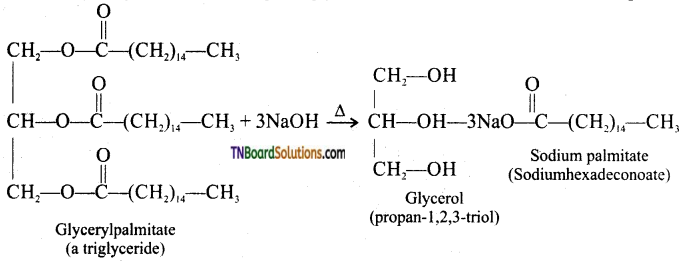

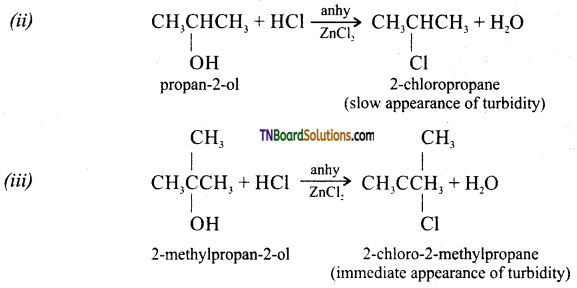
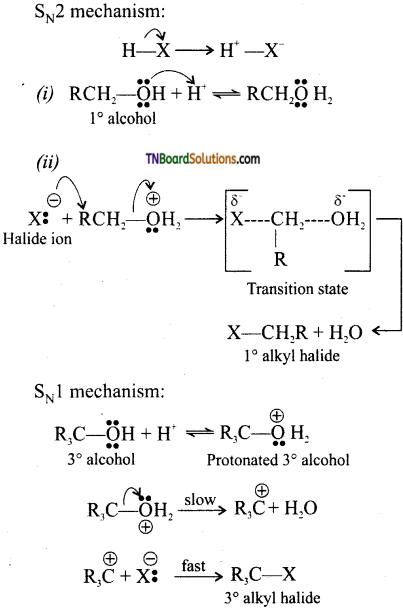

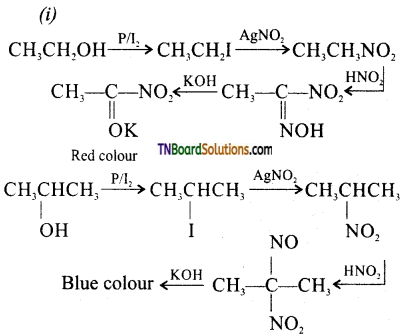
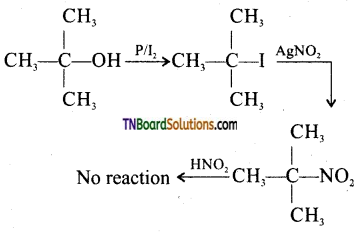
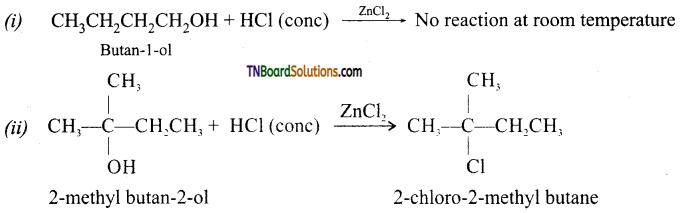
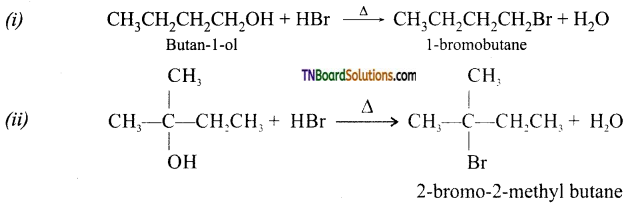
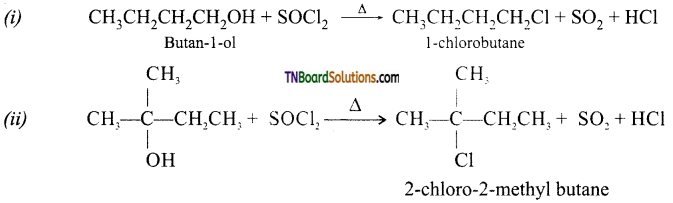
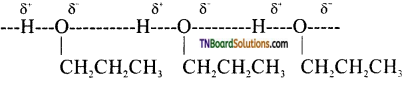

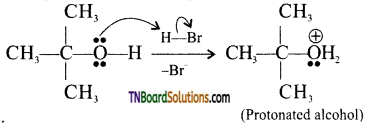
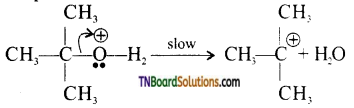
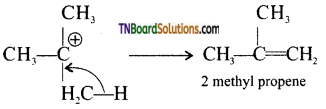
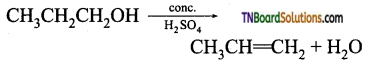


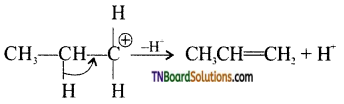
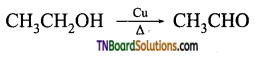
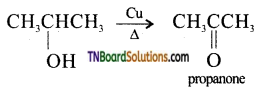
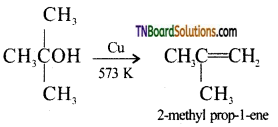
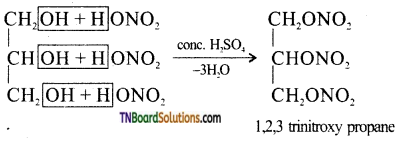
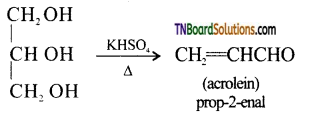
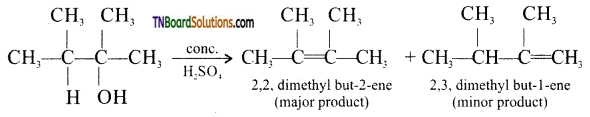
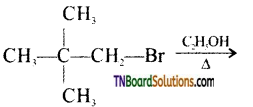
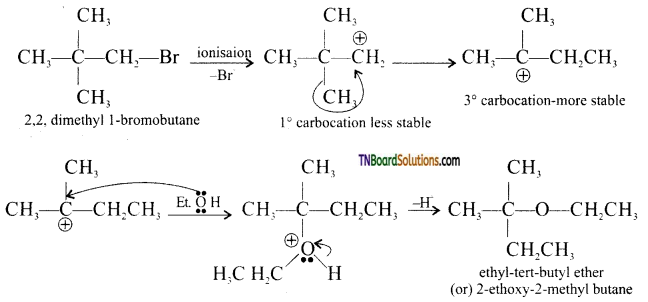
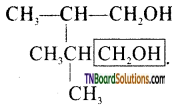
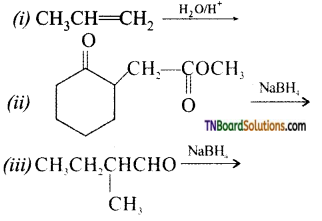
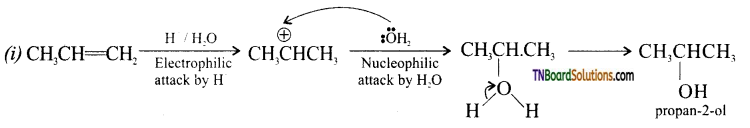
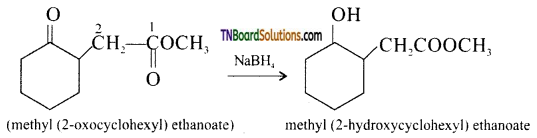
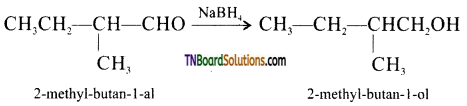
 from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.
from an alcohol and an organic acid in the presence of a catalyst is known as esterification. In the reaction, the ‘OH’ of the carboxylic acid and ‘H’ of the alcoholic group are removed as water.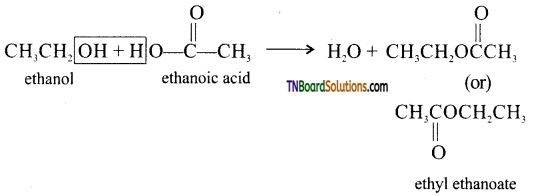
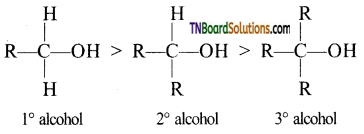
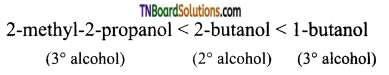
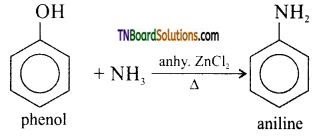
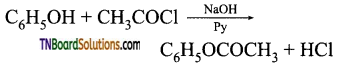
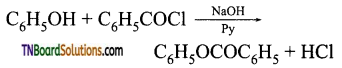
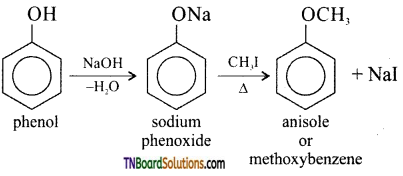
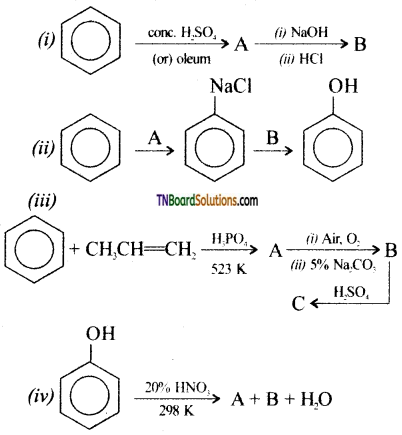
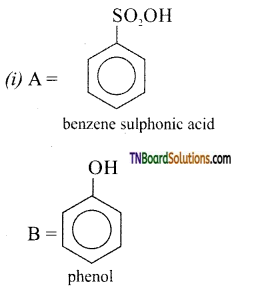
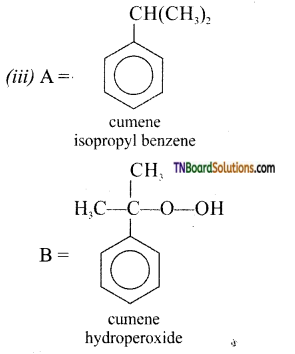
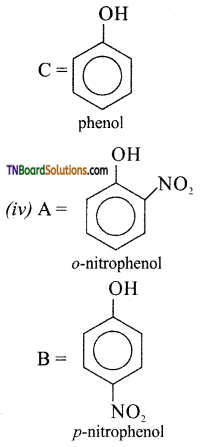
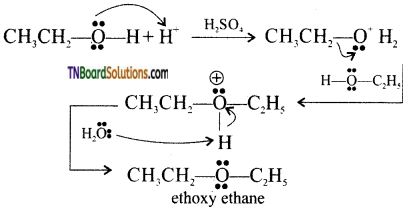
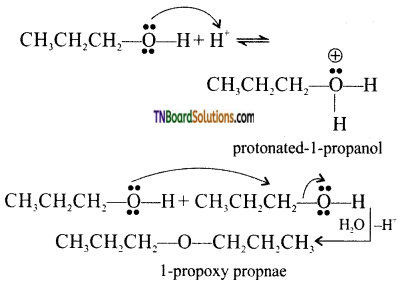
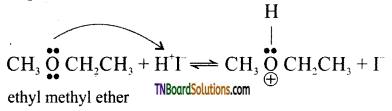
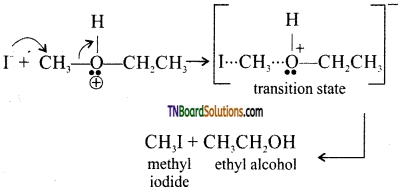
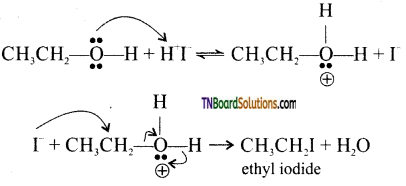
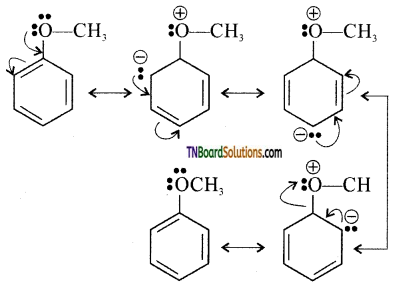


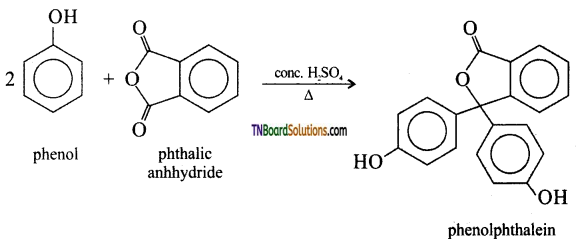
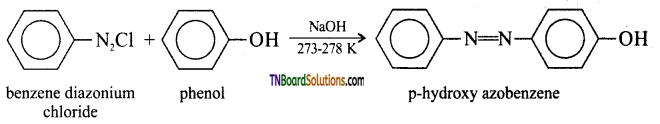


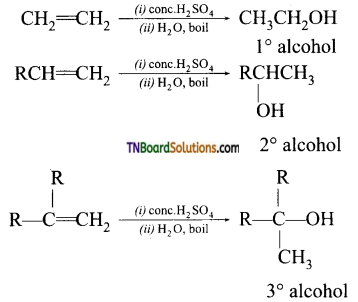
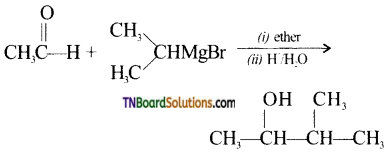
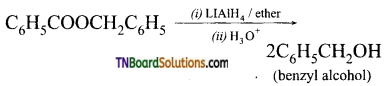
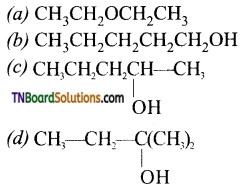
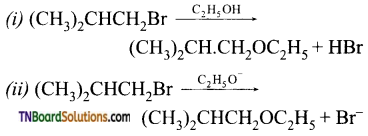

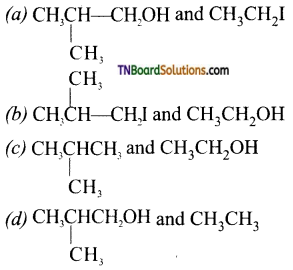
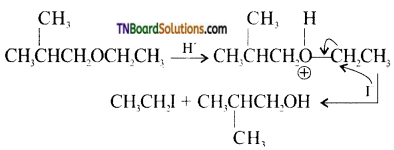
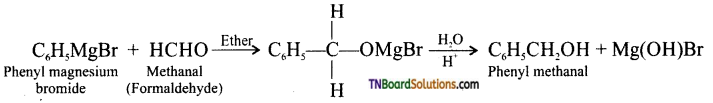
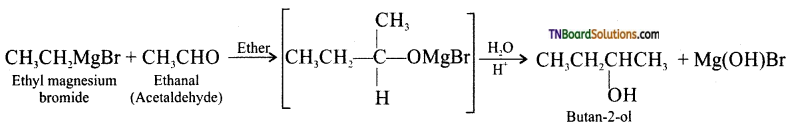
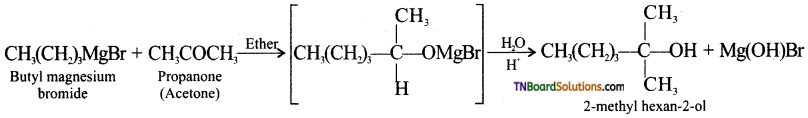
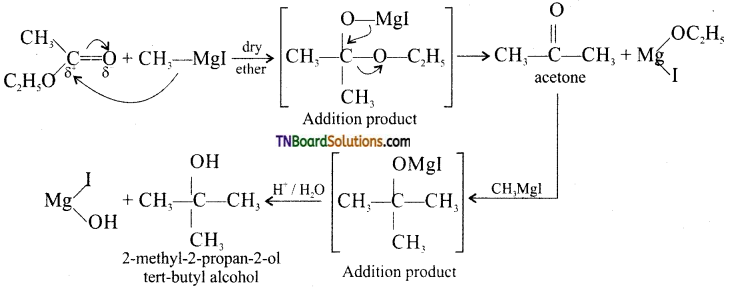
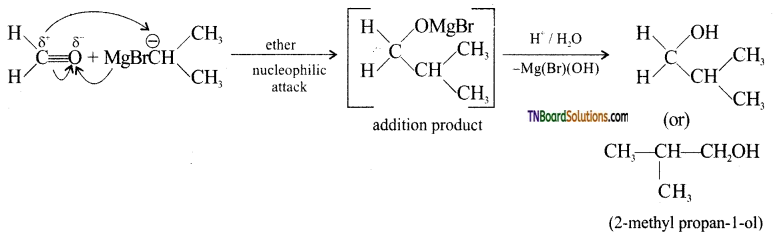
 with RMgX leads to the formation of:
with RMgX leads to the formation of:
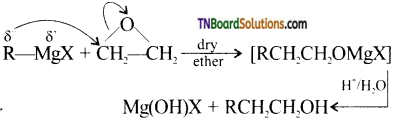
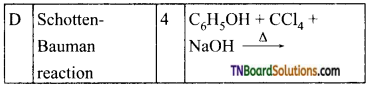



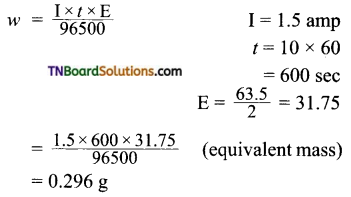
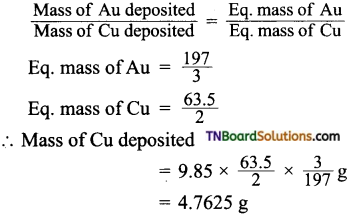
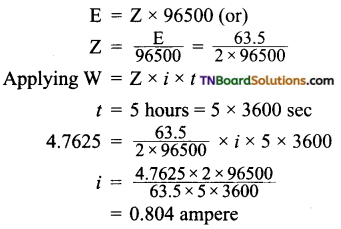
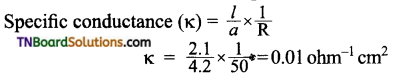
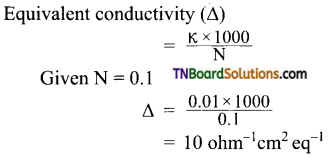
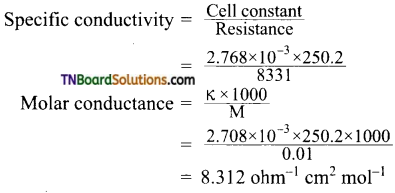
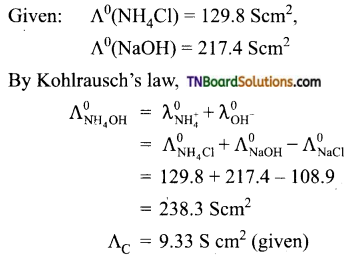
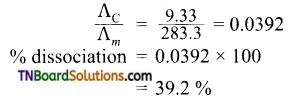





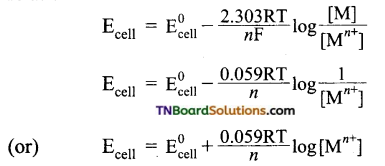
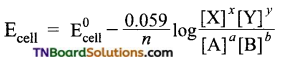


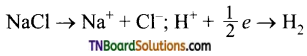
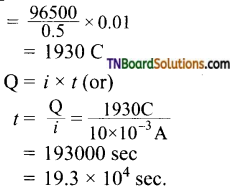
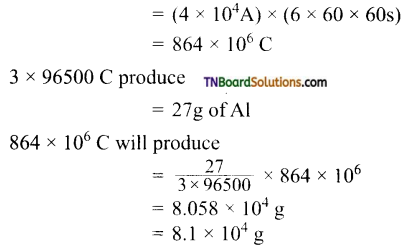
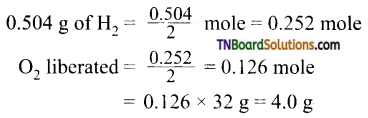

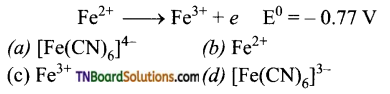


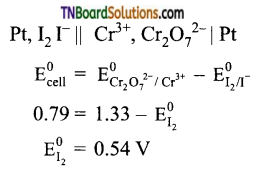
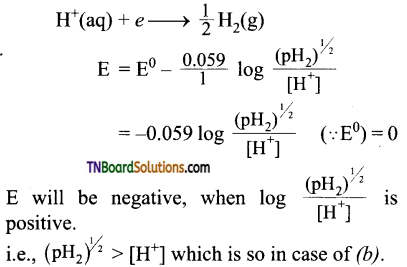
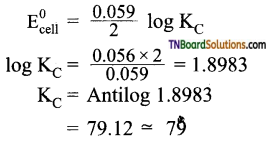
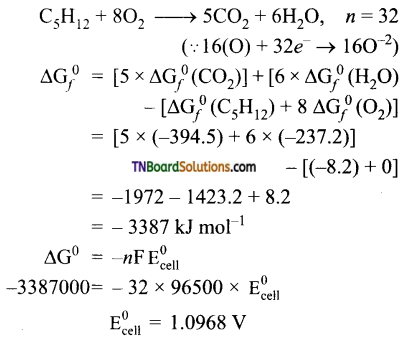
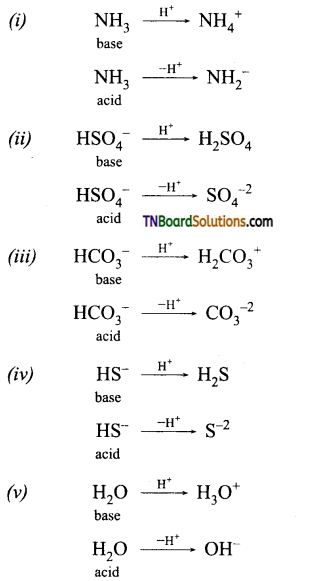
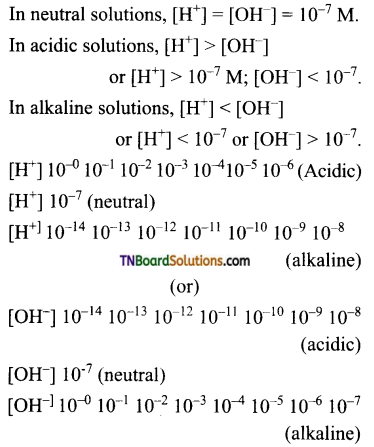
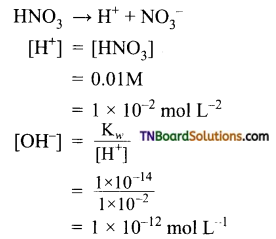
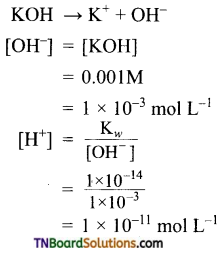


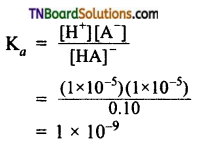
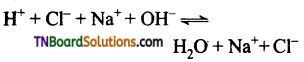
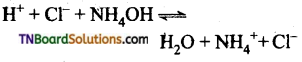
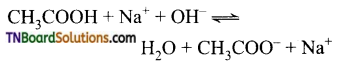
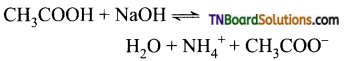
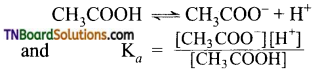
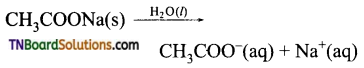
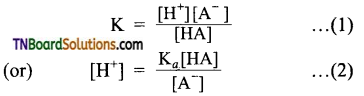
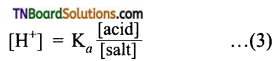
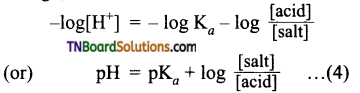
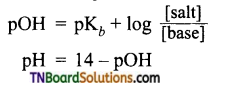

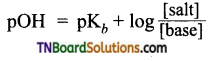

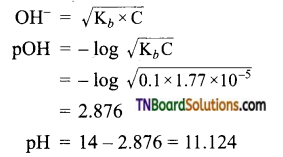




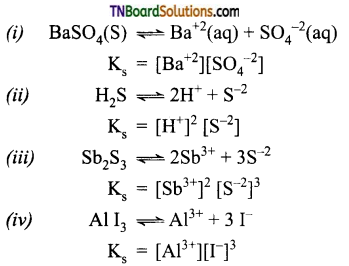




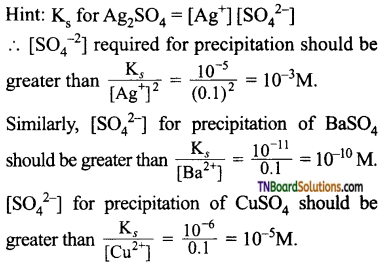
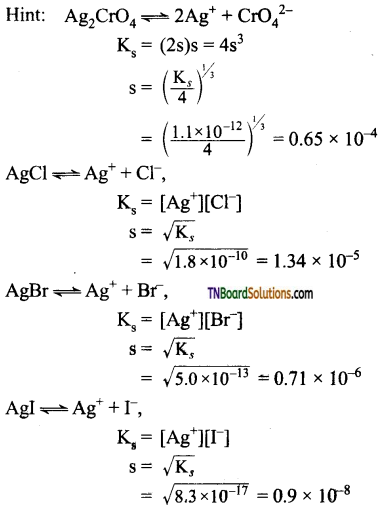
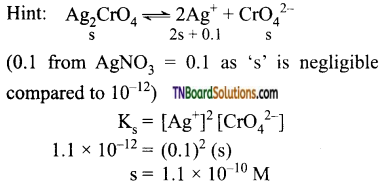


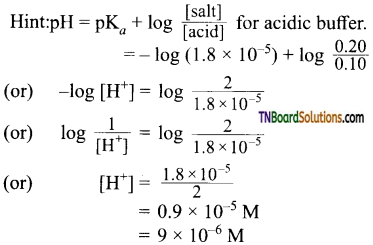

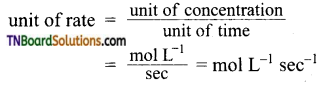


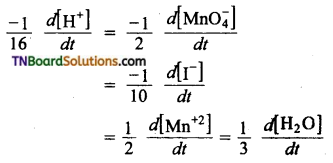
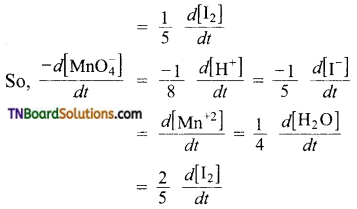
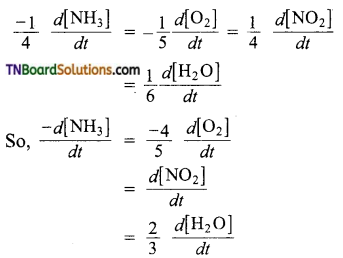

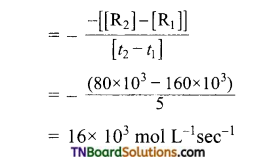

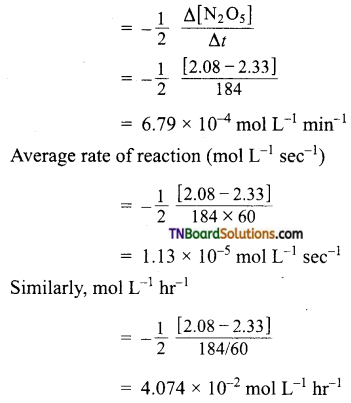
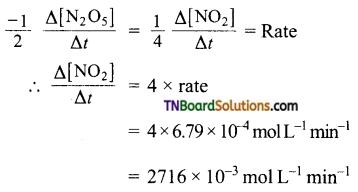
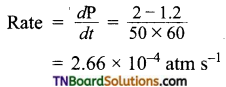
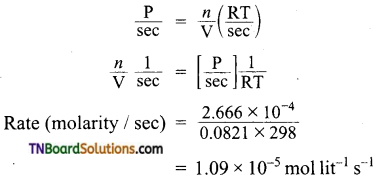

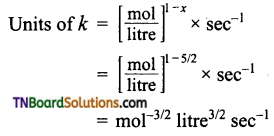
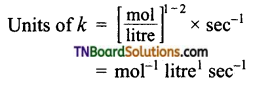
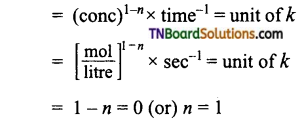
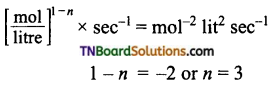
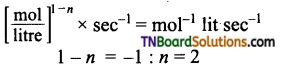
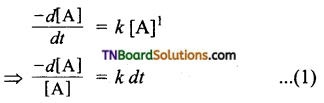
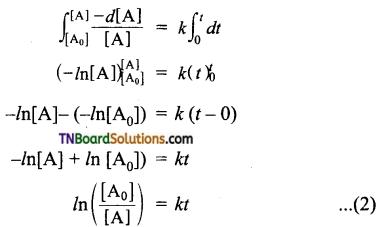
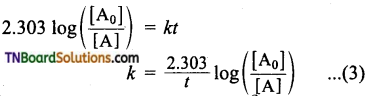
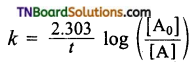
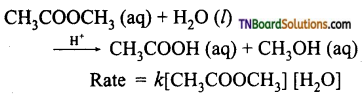
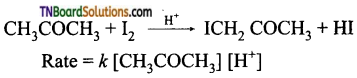
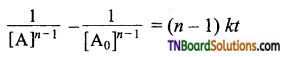
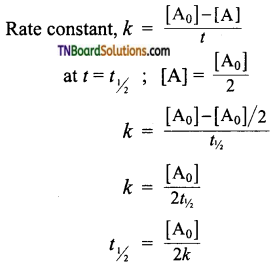

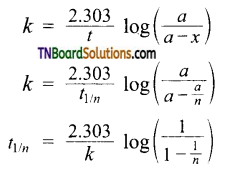
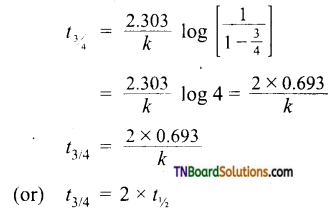

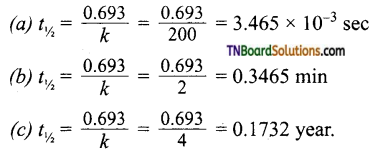

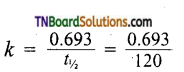
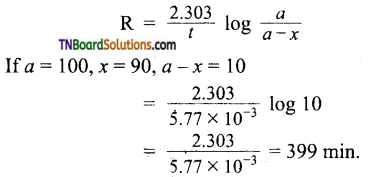
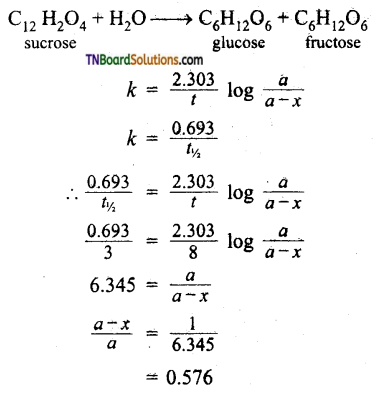

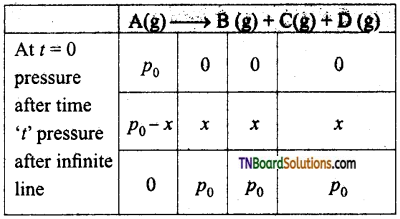


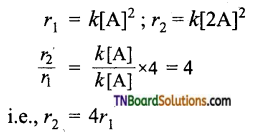
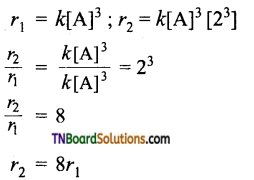
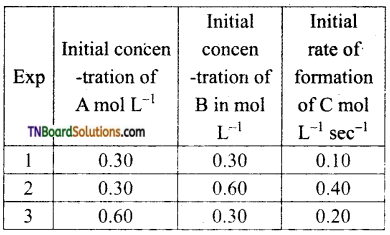
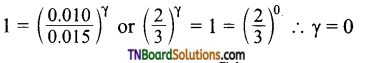
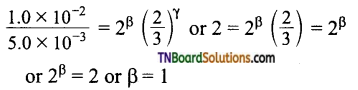
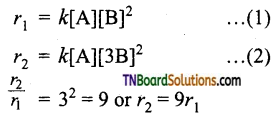
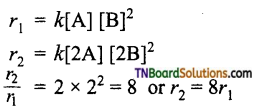
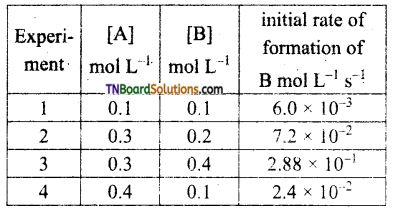

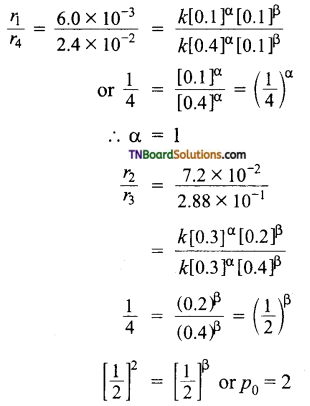
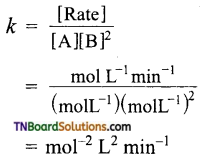
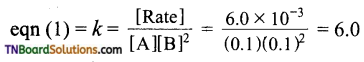
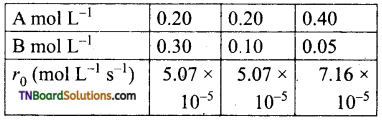
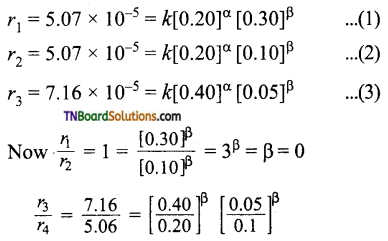

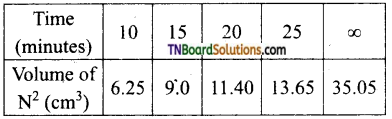
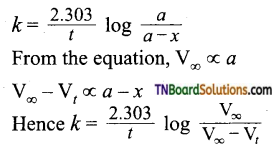
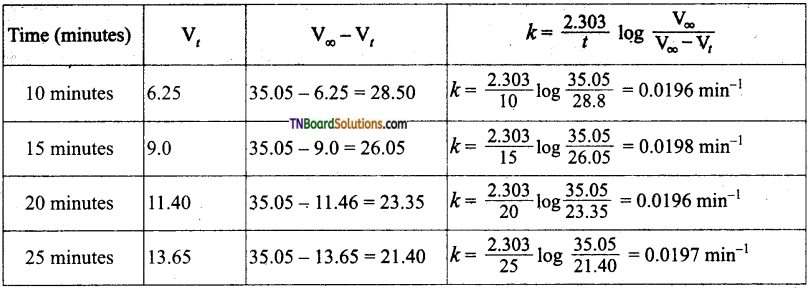
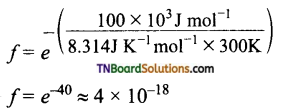
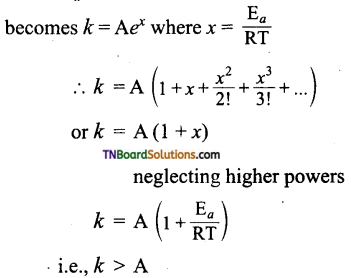

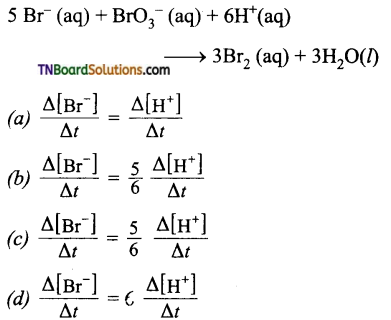
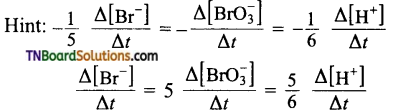

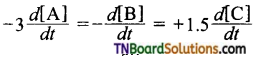
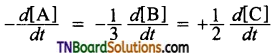
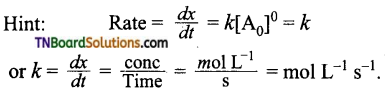

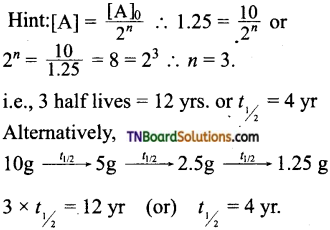
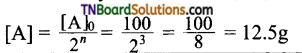
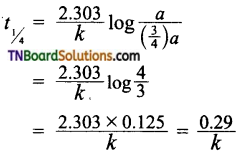
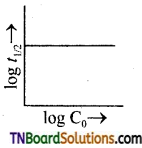

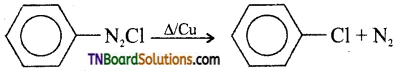
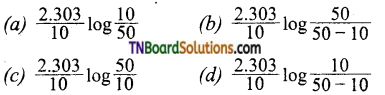



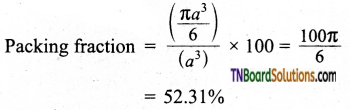
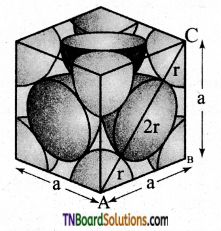
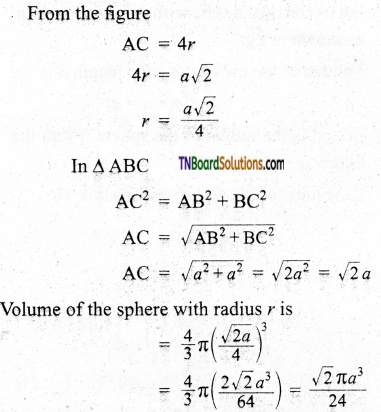
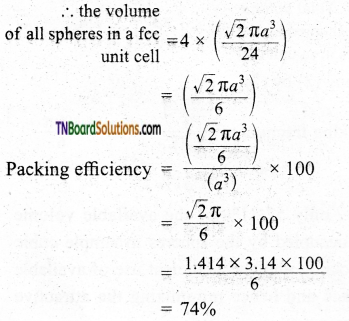













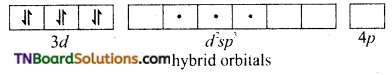
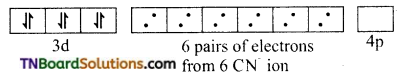
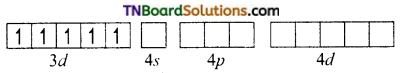
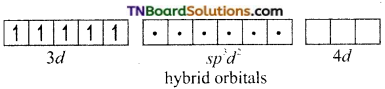
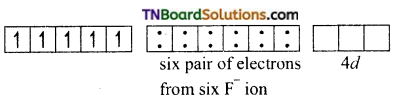
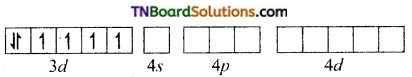
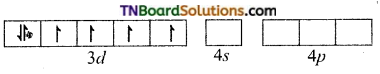
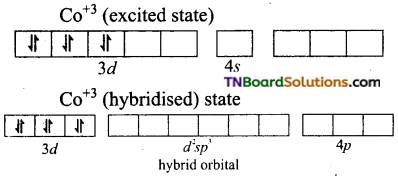
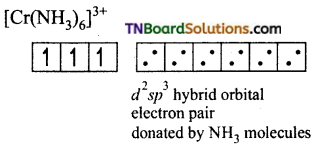

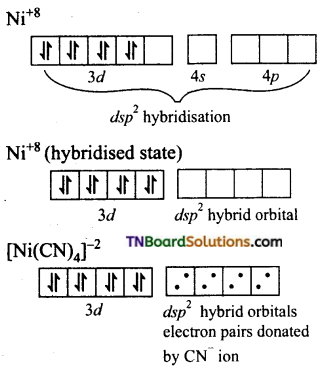

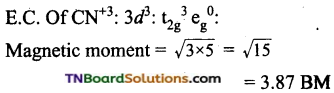

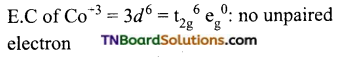

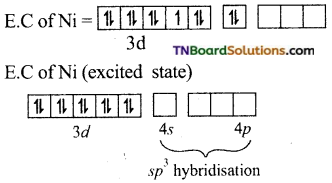
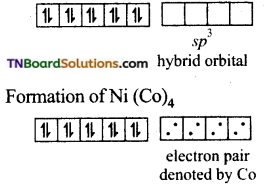
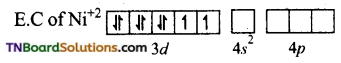
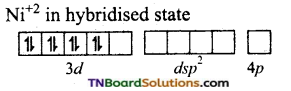
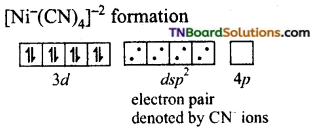
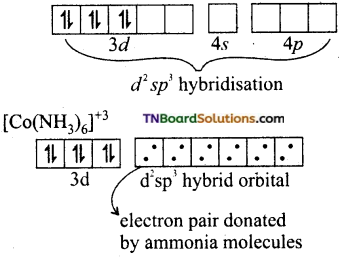
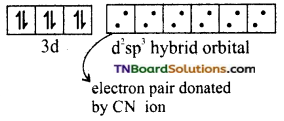
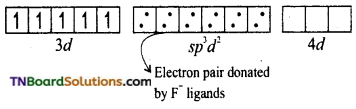
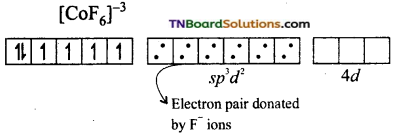
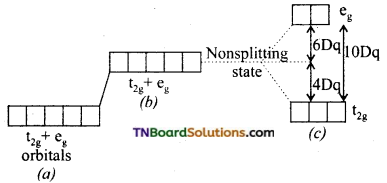
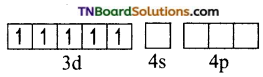
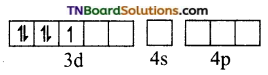
 and it has one unpaired electron.
and it has one unpaired electron.