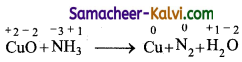
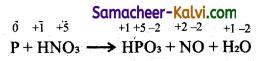
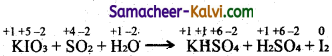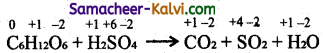TN State Board 11th Commerce Important Questions Chapter 28 Balance of Trade and Balance of Payments
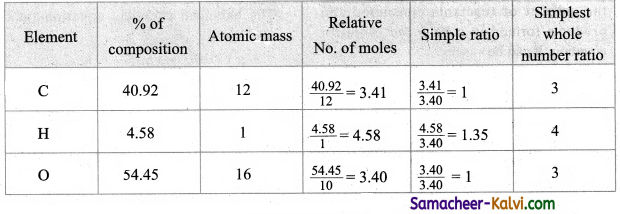
Question 1.
What do you mean by Balance of payments?
Answer:
Balance of payment refers to a systematic record of all economic transactions between the residents of one country and the residents of foreign countries during a particular period of time. For example, one year.
Question 2.
What do you mean by Balance of trade?
Answer:
Balance of trade denotes the difference between the value of import and the value of export during a year. If the export of a country exceeds its imports, it shows favourable balance of trade. If-the import exceeds the exports, it shows unfavorable balance of trade.
![]()
Question 3.
Define Balance of payments.
Answer:
According to International Monetary Fund,
“ The balance of payments for given period is a systematic records of all economic transactions taken place during the period between residents of the reporting countries.”
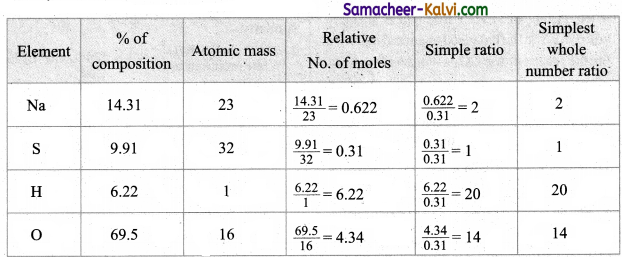
Question 4.
What is the composition of private capital?
Answer:
Private capital consists of foreign invest- mems, long term loan and foreign currency deposits.
Question 5.
Mention the components of banking capital.
Answer:
Banking capital includes movement into external financial asset and liabilities commercial and co-operative banks authorized to dealing in foreign exchange.
![]()
Question 6.
Mention the components of official capital.
Answer:
It includes RBI’s holdings of foreign currency and special drawing rights (SDR) held by the Government.
Question 7.
Why is Balance of payment prepared?
Answer:
- Balance of payment is the principal tool for analyzing the monetary position of international trade of a country just like Receipts and Payments account of enterprise revealing the net effect of cash movements happening in an enterprise during a particular period.
- Balance of payments help in framing monetary, fiscal and trade policies of country. Government keenly observes balance of payment position of its important trade partners in making policy decisions. It reveals whether a country produces enough economic output to pay for its growth. It is reported either for every quarter or for a year.
Question 8.
What does Balance of payment disclose?
Answer:
- A balance of payment surplus indicates that country’s exports are more than its imports and its government and residents are savers.
- A balance of payments deficit indicates to the fact that country’s import is more than the export. At the same time the country borrow finance from other countries to pay for its imports.
![]()
Question 9.
What are the credit items shown in currents accounts?
Answer:
- Goods export (visible).
- Invisible exports.
- Transport service sold abroad.
- Banking service sold abroad.
- Insurance service sold abroad.
- Income received on loan and investment made in foreign countries
- Expenses incurred by foreign tourists in India.
Question 10.
State the components of capital account.
Answer:
Capital account consists of three components.
- Private Capital,
- Banking Capital and
- Official Capital.
![]()
Question 11.
Write down the structure of capital account.
Answer:
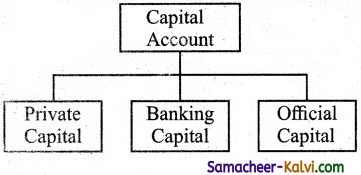
Capital account consists of three components.
(i) Private Capital.
(ii) Banking Capital.
(iii) Official Capital.
(i) Private Capital:
Private capital consists of foreign investments, long term loan and foreign currency deposits.
(ii) Banking Capital:
Banking capital includes movement into external financial asset and liabilities commercial and co-operative banks authorized to dealing in foreign exchange.
(iii) Official Capital:
It includes RBI’s holdings of foreign currency and special drawing rights (SDR) held by the Government.
Question 12.
Distinguish balance of payment and balance of trade.
Answer:
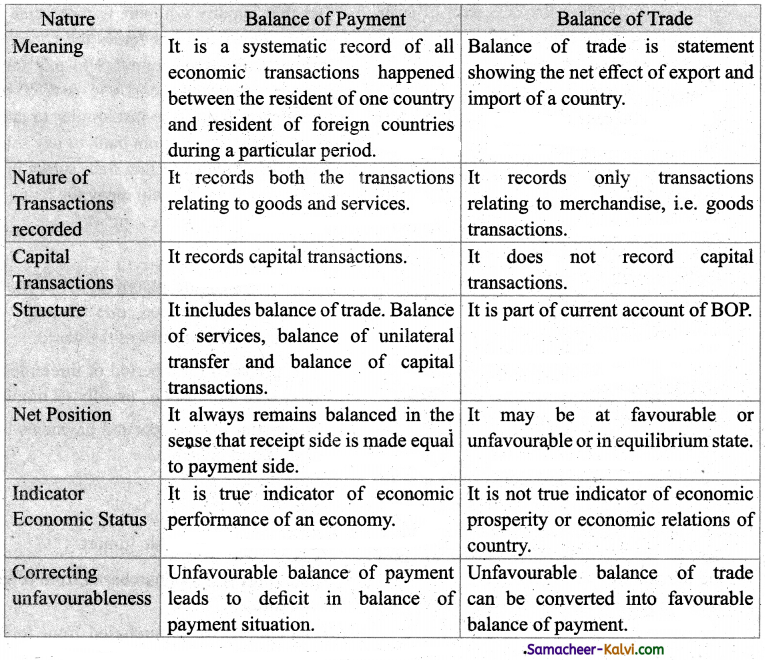
![]()
Question 13.
Highlight the features of balance of trade.
Answer:
|
Nature |
Balance of Trade |
| Meaning | Balance of trade is statement showing the net effect of export and import of a country. |
| Nature of Transactions recorded | It records only transactions relating to merchandise, i.e. goods transactions. |
| Capital Transactions | It does not record capital transactions. |
| Structure | It is part of current account of BOP. |
| Net Position | It may be at favourable or unfavourable or in equilibrium state. |
| Indicator Economic Status | It is not true indicator of economic prosperity or economic relations of country. |
| Correcting unfavourableness | Unfavourable balance of trade can be converted into favourable balance of payment. |
Question 14.
Impact of Balance of Payments and Trade.
Answer:
BOP shows a favourable or surplus position when the total receipts from foreign countries exceed the total payments to foreign countries are less than the payments to foreign countries. BOP is said to be unfavourable or indeficit.
BOP position shows the economic health of nation just like the thermometer indicates the temperature of human body. Favourable BOP indicates economic prosperity while unfavourable balance of payments shows economic weakness of a country.
![]()
Question 15.
Necessary for Global Village concept.
Answer:
(i) The current account.
(ii) The financial account.
(iii) The capital account.
Together included in the overall balance of payments.
It includes total inflows and outflows for a given nation.
The current account states that the balance of trade means the purchase and sale of goods and services.
Question 16.
Balance of Payment is key to economic development.
Answer:
Balance of payments help in framing monetary, fiscal and trade policies of country government keenly observes balance of payment position of its important trade – partners in its making policy decisions. It reveals whether a country produces enough economic output to pay for its growth.
Question 17.
Importance of BOP and BOT.
Answer:
BOP:
Balance of payment refers to a systematic record of all economic transactions
between the residents of one country and the residents of foreign countries during a particular period of time, eg: One year it contains a classified record of all receipts and payments arising from goods exported.
BOT:
Balance of trade denotes the different between the value of import and the value of export during a year. If the export of a country exceeds its imports, It shows favourable balance of trade. If the import exceeds the exports, it shows unfavourable balance of trade.
![]()
Choose the Correct Answer:
Question 1.
The Statement which discloses a record of transactions between the residents of one country and residents of foreign country:
(a) balance of payment
(b) balance of trade
(c) statement of receipts apd payments
(d) accounting statement
Answer:
(a) balance of payment
Question 2.
The Balance of Payments councils consists of:
(a) current account
(b) capital account
(c) receipts and payments account
(d) both current account and capital account
Answer:
(d) both current account and capital account
Question 3.
Foreign capital long- term loan and foreign currency reserve are recorded under:
(a) official capital
(b) private capital
(c) banking capital
(d) both private and official Capital
Answer:
(b) private capital
![]()
Question 4.
The term official capital includes:
(a) RBI holdings of foreign currencies
(b) Special Drawing Rights held by the Government
(c) Both A and B
(d) Foreign Investment
Answer:
(c) Both A and B
Question 5.
Balance of payments surplus indicates:
(a) Exports are more than the Imports
(b) Imports are more than Exports
(c) Exports and Imports are at Equilibrium
(d) Exports and Imports are above Equilibrium
Answer:
(a) Exports are more than the Imports
![]()
Samacheer Kalvi 11th Commerce Notes Chapter 28 Balance of Trade and Balance of Payments
→ Balance of trade and balance of payment are important aspects in international trade.
→ Balance of payment (BOP): Balance of payment refers to a systematic record of all economic transaction between the residents of one country and the residents of foreign countries during a particular period of time for example one year.
→ It contains a classified record of all receipts and payments arising from goods exported services rendered and capital received by residents in a country and payment made by them on accounts of goods imported, services rendered and capital transferred to non residents or foreigners out of the country.
→ According to international monetary fund. The balance of payment for given period is a systematic record of all economic transaction taken place during the period between residents of the reporting countries.
→ Structure of balance of trade, balance of services, balance of unilateral transfer and balance of capital transaction. Balance of payment it is true indicator of economic performance of an economic.
→ The balance of payments consists of two components. Namely current account and capital account.