Students can download 10th Science Chapter 8 Periodic Classification of Elements Questions and Answers, Notes, Samacheer Kalvi 10th Science Guide Pdf helps you to revise the complete Tamilnadu State Board New Syllabus, helps students complete homework assignments and to score high marks in board exams.
Tamilnadu Samacheer Kalvi 10th Science Solutions Chapter 8 Periodic Classification of Elements
Samacheer Kalvi 10th Science Periodic Classification of Elements Text Book Back Questions and Answers
I. Choose the best answer:
Question 1.
The number of periods and groups in the periodic table are:
(a) 6, 16
(b) 7, 17
(c) 8, 18
(d) 7, 18
Answer:
(d) 7, 18
Question 2.
The basis of modem periodic law is ____.
(a) atomic number
(b) atomic mass
(c) isotopic mass
(d) number of neutrons.
Answer:
(a) atomic number
Question 3.
……….. group contains the member of the halogen family.
(a) 17th
(b) 15th
(c) 18th
(d) 16th
Answer:
(a) 17th
![]()
Question 4.
_______ is a relative periodic property.
(a) atomic radii
(b) ionic radii
(c) electron affinity
(d) electronegativity.
Answer:
(b) ionic radii
Question 5.
Chemical formula of rust is:
(a) Fe0.xH2O
(b) Fe04.xH2O
(c) Fe2O3. xH2O
(d) FeO
Answer:
(c) Fe2O3. xH2O
Question 6.
In the aluminothermic process the role of Al is:
(a) oxidizing agent
(b) reducing agent
(c) hydrogenating agent
(d) sulphurising agent
Answer:
(b) reducing agent
Question 7.
The process of coating the surface of the metal with a thin layer of zinc is called ____.
(a) painting
(b) thinning
(c) galvanization
(d) electroplating.
Answer:
(c) galvanization
Question 8.
Which of the following inert gas has electrons in the outermost shell?
(a) He
(b) Ne
(c) Ar
(d) Kr
Answer:
(a) He
Question 9.
Neon shows zero electron affinity due to ____.
(a) stable arrangement of neutrons
(b) stable configuration of electrons
(c) reduced size
(d) increased density.
Answer:
(b) stable configuration of electrons
Question 10.
……….. is an important metal to form amalgam.
(a) Ag
(b) Hg
(c) Mg
(d) Al
Answer:
(b) Hg
II. Fill in the blanks:
1. If the electronegativity difference between two bonded atoms in a molecule is greater than 1.7, the nature of bonding is ………..
2. …………. is the longest period in the periodical table.
3. ………… forms the basis of modern periodic table.
4. If the distance between two Cl atoms in Cl2 molecule is 1.98 Å, then the radius of Cl atom is ………..
5. Among the given species A– A+, and A, the smallest one in size is ……….
6. The scientist who propounded the modern periodic law is …………
7. Across the period, ionic radii ………… (increases,decreases).
8. ……….. and ………… are called inner transition elements.
9. The chief ore of Aluminium is ………….
10. The chemical name of rust is ………….
Answer:
1. ionic
2. 6th (sixth) period
3. Atomic number
4. 0.99 Å
5. A+
6. Dimitri Mendeleev
7. decreases
8. Lanthanides, Actinides
9. bauxite
10. hydrated ferric oxide
![]()
III. Match the following:
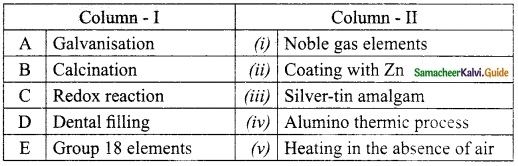
Answer:
A. (ii)
B. (v)
C. (iv)
D. (iii)
E. (i)
IV. True or False: (If false give the correct statement)
1. Moseley’s periodic table is based on atomic mass.
2. Ionic radius increases across the period from left to right.
3. All ores are minerals; but all minerals cannot be called as ores.
4. Aluminium wires are used as electric cables due to their silvery white colour.
5. An alloy is a heterogenous mixture of metals.
Answer:
1. False – Moseley’s periodic table is based on atomic number.
2. True
3. True
4. False – Aluminium wires are used as electric cables because it is a good conductor of heat and electricity.
5. False – An alloy is an homogeneous mixture of metals.
V. Assertion and Reason:
Answer the following questions using the data given below:
Question 1.
Assertion: The nature of bond in HF molecule is ionic.
Reason: The electronegativity difference between H and F is 1.9.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(a) Assertion and Reason are correct, Reason explains the Assertion.
Question 2.
Assertion: Magnesium is used to protect steel from rusting.
Reason: Magnesium is more reactive than iron.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(a) Assertion and Reason are correct, Reason explains the Assertion.
Question 3.
Assertion: An uncleaned copper vessel is covered with greenish layer. Reason: copper is not attacked by alkali.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
VI. Short answer questions:
Question 1.
A is a reddish brown metal, which combines with O2 at < 1370 K gives B, a black coloured compound. At a temperature > 1370 K, A gives C which is red in colour. Find A, B and C with reaction.
Answer:
(A) is a reddish brown metal – Copper
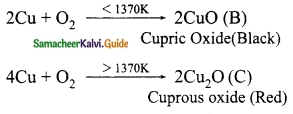
A – Copper; B – Cupric oxide; C – Cuprous oxide.
Question 2.
A is a silvery white metal. A combines with O2 to form B at 800°C, the alloy of A is used in making the aircraft. Find A and B.
Answer:
A – Silvery white metal – Aluminium
![]()
The alloys of aluminium, Duralumin and Magnalium are used in making the aircraft.
A – Aluminium; B – Aluminium oxide.
Question 3.
What is rust? Give the equation for the formation of rust.
Answer:
When iron is exposed to moist air, it forms a layer of brown hydrated ferric oxide on its surface. This compound is known as rust and the phenomenon of formation of rust is known as rusting.
4Fe + 3O2 + xH2O → 2Fe2O3. xH2O (Rust).
Question 4.
State two conditions necessary for rusting of iron.
Answer:
(i) The presence of water and oxygen is essential for the rusting of iron.
(ii) Impurities in the iron, the presence of water vapour, acids, salts and CO2 speeds up rusting.
![]()
VII. Long answer questions:
Question 1.
(a) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
Answer:
Caustic alkali is added to bauxite, to dissolve bauxite ore and obtain a solution of sodium aluminate.
(b) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for the addition.
Answer:
CaF2 (Fluorspar) is added along with cryolite and alumina. It is added to reduce the high melting point of the electrolyte.
Question 2.
The electronic configuration of metal A is 2, 8, 18, 1.
The metal A when exposed to air and moisture forms B a green layered compound. A with con. H2SO4 forms C and D along with water. D is a gaseous compound. Find A, B, C and D.
Answer:
Metal (A) with electronic configuration- 2, 8, 18, 1 is copper.
2Cu + O2 + CO2 + H2O → CuCO3. Cu(OH)2 (B)
Copper carbonate (Green layer)
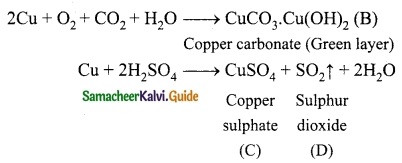
(A) – Copper (Cu)
(B) – Copper Carbonate (CuCO3. Cu(OH)2)
(C) – Copper Sulphate (CuSO4)
(D) – Sulphur dioxide (SO2)
Question 3.
Explain the smelting process.
Answer:
The roasted ore of copper is mixed with powdered coke and sand and is heated in a blast furnace to obtain matte (Cu2S + FeS) and slag. The slag is removed as waste.
2 FeS + 3O2 → 2 FeO + 2 SO2
2 Cu2S + 3O2 → 2 Cu2O + 2SO2
Cu2O + FeS → Cu2S + FeO
FeO + SiO2 → FeSiO2 (slag)
VIII. HOT questions:
Question 1.
Metal A belongs to period 3 and group 13. A in red hot condition reacts with steam to form B. A with strong alkali forms C. Find A, B and C with reactions.
Answer:
Metal A belongs to Period 3 and Group 13. So metal ‘A’ is aluminium.
(A) in red hot condition reacts with steam to form ‘B’.
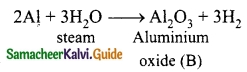
‘A’ with strong alkali forms ‘C’
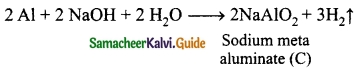
(A) – Aluminium
(B) – Aluminium oxide
(C) – Sodium meta aluminate
Question 2.
Name the acid that renders aluminium passive. Why?
Answer:
Dilute or concentrated nitric acid (HNO3) renders aluminium passive. Because nitric acid does not attack aluminium but it renders aluminium passive due to the formation of an oxide film on its surface.
Question 3.
(a) Identify the bond between H and F in HF molecule.
Answer:
Ionic, because the electronegativity difference is more than 1.7.
(b) What property forms the basis of identification?
Answer:
Electronegativity.
(c) How does the property vary in periods and in groups?
Answer:
In a period, from left to right the electronegativity increases because of the increase in the nuclear charge.
In a Group, from top to bottom, the electronegativity decreases because of the increase in size of the elements.
![]()
Samacheer Kalvi 10th Science Periodic Classification of Elements Additional Important Questions and Answers
I. Choose the correct answer:
Question 1.
The shortest period in the periodic table contains elements.
(a) 18
(b) 8
(c) 2
(d) 32
Answer:
(c) 2
Question 2.
Group number of carbon family is _____.
(a) 13
(b) 15
(c) 17
(d) 14.
Answer:
(d) 14.
Question 3.
The ore forming elements, chalcogens are present in ……… group of the modern periodic table.
(a) 18th
(b) 1st
(c) 2nd
(d) 16th
Answer:
(d) 16th
Question 4.
Valency of all the alkali metals is _____.
(a) 1
(b) 2
(c) 3
(d) 4.
Answer:
(a) 1
Question 5.
The largest atom in the 2nd period of the periodic table is:
(a) Li
(b) Be
(c) F
(d) Ne
Answer:
(a) Li
Question 6.
The covalent radii of Hydrogen, if the distance between the Hydrogen nuclei of the molecule is 0.74 Å is:
(a) 1.58 Å
(b) \(\frac{0.74}{4}\) Å
(c) 0.37 Å
(d) 0.74 Å
Answer:
(c) 0.37 Å
Question 7.
Pick out the correct ionic radii in increasing order for the following species – Na, Cl, Na+, Cl– _____.
(a) Na > Cl > Na+ > Cl–
(b) Cl > Na > Na+ > Cl–
(c) Cl– > Na > Na+ > Cl
(d) Cl < Na+ < Cl– < Na.
Answer:
(d) Cl < Na+ < Cl– < Na.
Hint:
Na = 186 pm,
Cl = 91 pm,
Na+ = 116 pm,
Cl– = 167 pm.
Question 8.
In the third period, the first ionization potential is of the order:
(a) Na > Al > Mg > Si > P
(b) Mg > Na > Si > P > Al
(c) Na < Al < Mg < Si < P
(d) Na < Al < Mg < Si < P
Answer:
(c) Na < Al < Mg < Si < P
![]()
Question 9.
Which one of the following is the least electronegative element?
(a) Bromine
(b) Chlorine
(c) Iodine
(d) Hydrogen
Answer:
(d) Hydrogen
Question 10.
Which is a widely used a scale to determine the electronegativity?
(a) Pauling scale
(b) Moseley scale
(c) Mendeleev scale
(d) none of these.
Answer:
(a) Pauling scale
Question 11.
Which one of the following orders of ionic radii is correct?
(a) H– > H+ > H
(b) Na+ > F– > O2-
(c) F > O2- > Na+
(d) None of these
Answer:
(d) None of these
Question 12.
The percentage of carbon in Pig iron is:
(a) < 0.25%
(b) 0.25 – 2%
(c) 2 – 4.5%
(d) > 5%
Answer:
(c) 2 – 4.5%
Question 13.
The chemical formula of clay is _____.
(a) Al2O3
(b) Al2O3.2H2O
(c) Al2O3. 2SiO2.2H2O
(d) Al2O3. 2SiO2.H2O.
Answer:
(c) Al2O3. 2SiO2.2H2O
Question 14.
The temperature in the combustion zone is maintained at:
(a) 1500°C
(b) 400°C
(c) 1000°C
(d) 1380°C
Answer:
(a) 1500°C
Question 15.
Oil used in Froth floatation method is _____.
(a) pine oil
(b) natural oil
(c) crude oil
(d) Synthetic oil.
Answer:
(a) pine oil
Question 16.
The first most abundant metal present in the Earth crust is:
(a) Iron
(b) Aluminium
(c) Zinc
(d) Copper
Answer:
(b) Aluminium
Question 17.
……….. metal is used for making calorimeters.
(a) Copper
(b) Tin
(c) Mercury
(d) Iron
Answer:
(a) Copper
Question 18.
More reactive metal is _____.
(a) Zn
(b) Fe
(c) Ag
(d) Na.
Answer:
(d) Na.
Question 19.
The chief ore of Iron is:
(a) Magnesite
(b) Galena
(c) Cinnabar
(d) Haematite
Answer:
(d) Haematite
Question 20.
The metal which melts at room temperature is:
(a) Zinc
(b) Lead
(c) Gallium
(d) Tin
Answer:
(c) Gallium
Question 21.
Conversion of bauxite into alumina is _____.
(a) Hall’s process
(b) Alumino thermic process
(c) Baeyer’s process
(d) Bessemerisation process.
Answer:
(c) Baeyer’s process
Question 22.
………. metal can be cut with knife.
(a) Potassium
(b) Gallium
(c) Mercury
(d) Gold
Answer:
(a) Potassium
![]()
Question 23.
………. is not a good conductor of heat and electricity.
(a) Silver
(b) Tungsten
(c) Copper
(d) Aluminium
Answer:
(b) Tungsten
Question 24.
Electrolyte used in Hall’s process ______.
(a) Pure alumina + molten cryolite + fluorspar
(b) Pure alumina + molten bauxite + fluorspar
(c) Pure bauxite + molten cryolite + fluorspar
(d) Pure bauxite + molten Haematite + fluorspar.
Answer:
(a) Pure alumina + molten cryolite + fluorspar
Question 25.
The foaming agent used for froth floatation process is:
(a) Coconut oil
(b) Pine oil
(c) Sodium chloride
(d) Groundnut oil
Answer:
(b) Pine oil
Question 26.
Three elements A, B and C are having the electronic configuration Is2 2s1, Is2 2s2 and Is2 2s2 2p1 respectively. Which element will have the lowest ionization energy?
(a) A
(b) B
(c) C
(d) B and C
Answer:
(a) A
Question 27.
Metal used in household utensils is ______.
(a) Al
(b) Co
(c) Fe
(d) Na.
Answer:
(a) Al
Question 28.
Which one of the following pair is a metalloid?
(a) Na and K
(b) F and Cl
(c) Cu and Hg
(d) Si and Ge
Answer:
(d) Si and Ge
Question 29.
The highly metallic element will have the configuration of:
(a) 2, 8, 7
(b) 2, 8, 8, 5
(c) 2, 8, 8, 1
(d) 2, 8, 2
Answer:
(c) 2, 8, 8, 1
Question 30.
The metal used in electroplating is ______.
(a) Cu
(b) Al
(c) Fe
(d) Co.
Answer:
(a) Cu
Question 31.
The flux which is used when the gangue present in the ore is acidic:
(a) Silica
(b) Calcium oxide
(c) Calcium silicate
(d) Cuprous sulphide
Answer:
(b) Calcium oxide
Question 32.
Matte is a mixture of:
(a) Cu2O + Cu2S
(b) Cu2O + FeS
(c) Cu2S + FeS
(d) Cu2O + PbS
Answer:
(c) Cu2S + FeS
Question 33.
Fe reacts with dilute nitric acid in cold condition to give ______.
(a) Ferrous nitride
(b) Ferrous nitrate
(c) Ferric nitride
(d) Ferric nitrate.
Answer:
(b) Ferrous nitrate
Question 34.
In the brass alloy, which is solvent?
(a) Zn
(b) Co
(c) Ag
(d) Cu.
Answer:
(d) Cu.
![]()
II. Fill in the blanks:
1. The major component of the matte is ………….
2. The modern periodic table is based on ………..
3. The valency of alkali metals is …………
4. The unreactive elements are present in group ………..
5. In the 2nd period, the smallest atom is ……….
6. The size of a cation is ………… than the neutral atom.
7. ……….. is the unit of ionization energy.
8. The ionization energy ……… down the group in the periodic table.
9. The electron affinities of noble gases are …………
10. ………. is the process of extracting the ore from the Earth’s crust.
11. Galena is the ore of …………..
12. The silvery white metal which is a good conductor of heat and electricity is …………
13. The slag formed during Bessemerisation process is ………….
14. Blister copper contains ………. percentage of copper.
15. Haematite ore is concentrated by ……….. washing.
Answer:
1. Cu2S
2. atomic number
3. one
4. 18
5. Neon
6. smaller
7. KJ/mol
8. decreases
9. zero
10. Mining
11. lead
12. aluminium
13. Iron silicate or FeSiO3
14. 98%
15. hydraulic
III. Match the following:
Question 1.
Match the column I with column II.
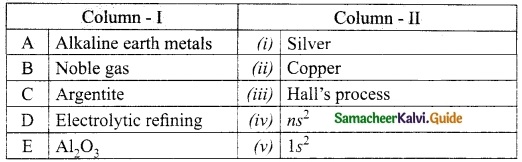
Answer:
A. (iv)
B. (v)
C. (i)
D. (ii)
E. (iii)
Question 2.
Match the column I with column II.
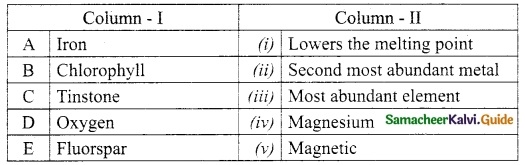
Answer:
A. (ii)
B. (iv)
C. (v)
D. (iii)
E. (i)
Question 3.
Match the column I with column II.
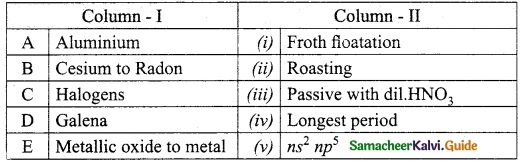
Answer:
A. (iii)
B. (iv)
C. (v)
D. (i)
E. (ii)
Question 4.
Match the column I with column II.
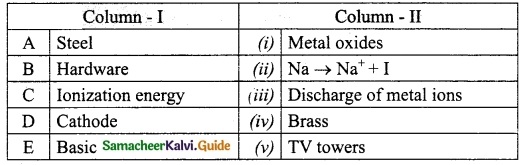
Answer:
A. (v)
B. (iv)
C. (ii)
D. (iii)
E. (i)
IV. True or false. (If false give the correct statement)
1. Alkali metals are generally extracted by the electrolysis of their ores in fused state.
2. Every mineral is an ore but every ore is not a mineral.
3. Slag is a product formed during smelting by combination of flux and impurities.
4. Reactive metals occur in native state.
5. Malachite is a sulphide ore of copper.
6. Lanthanides are present in the 6th group of the periodic table.
7. Atomic radius increases as we go across the period due to increase in size.
8. As the positive charge increases, the size of the cation decreases.
9. If the difference in electronegativity is greater than 1.7, the bond is considered to be covalent.
10. Siderite is the carbonate ore of calcium.
Answer:
1.True
2. True
3. True
4. False – Reactive metals always occur in the combined state.
5. False – Malachite is the carbonate ore of copper.
6. False – Lanthanides are present in the 6th period of the periodic table.
7. False – Atomic radius increases as we go across the period due to decrease in size.
8. True
9. False – If the difference in electronegativity is greater than 1.7, the bond is considered to be ionic.
10. False – Siderite is the carbonate ore of Iron.
![]()
V. Assertion and Reason:
Answer the following questions using the data given below:
Question 1.
Assertion: Zinc blende is concentrated by Froth floatation process.
Reason: Zinc blende is a sulphide ore.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(a) Assertion and Reason are correct, Reason explains the Assertion.
Question 2.
Assertion: In thermite welding, aluminium powder and Fe2O3 are used.
Reason: Aluminium powder is a strong reducing agent.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(a) Assertion and Reason are correct, Reason explains the Assertion.
Question 3.
Assertion: To design the body of an aircraft, aluminium alloys are used.
Reason: Aluminium becomes passive when it is treated with dil or con.HNO3
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Question 4.
Assertion: Tinstone and the impurity wolframite are seperated by magnetic separation.
Reason: Tinstone is magnetic and wolframite is non-magnetic in nature.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(a) Assertion and Reason are correct, Reason explains the Assertion.
Question 5.
Assertion: Bauxite is purified by leaching.
Reason: Bauxite undergoes thermal decomposition.
(a) Assertion and Reason are correct, Reason explains the Assertion.
(b) Assertion is correct, Reason is wrong.
(c) Assertion is wrong, Reason is correct.
(d) Assertion and Reason are correct, Reason doesn’t explains Assertion.
Answer:
(b) Assertion is correct, Reason is wrong.
VI. Short answer questions:
Question 1.
State the modern periodic law.
Answer:
The physical and chemical properties of the elements are the periodic function of their atomic number.
Question 2.
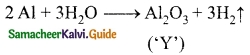
‘X’ is a silvery white metal. X reacts with O2 to form Y. The same compound is obtained from the metal on reaction with steam with the liberation of hydrogen gas. Identify X and Y.
Answer:
(i) Silvery white metal ‘X’ is Aluminium.
(ii) It reacts with O2 to form ‘Y’
![]()
(iii) Y can also be obtained on reaction with steam with the liberation of H2.
Question 3.
Write any four characteristics of periods.
Answer:
- In a period, the electrons are filled in the same valence shell of all elements.
- As the electronic configuration changes along the period, the chemical properties of the elements also change.
- The atomic size of the elements in a period decreases from left to right
- In a period, die metallic character of the element decreases, while their non-metallic character increases.
Question 4.
Write the Principle of Hydraulic washing.
Answer:
The difference in the densities or specific gravities of the ore and the gangue is the main principle behind this method.
![]()
Question 5.
What are coinage metals?
Answer:
Copper, silver and gold are called coinage metals, as they are used in making coins and jewellery.
Question 6.
How will you separate tinstone from wolframite?
Answer:
Magnetic separation method. Tinstone is magnetic in nature.
Method: The crushed ore is placed over a conveyer belt which rotates around two metal wheels, one of which is magnetic. The magnetic particles are attracted to the magnetic wheel and fall separately apart from the non¬magnetic particles.
Question 7.
What are ores?
Answer:
The mineral from which a metal can be readily and economically extracted on a large scale is said to be ore.
eg. Bauxite Al2O3.2H2O is the ore of Aluminium
Question 8.
Define electronegativity.
Answer:
It is the tendency of an element in a covalent bond to attract the shared pair of electrons towards itself. It is a relative property.
Question 9.
In what period and group will an element with z = 118 will be present?
Answer:
Elements with z = 118 will be present in Period number ‘7’ and Group number 18.
Question 10.
Why flux is added during metallurgy?
Answer:
Flux is the substance added to the ore to reduce the fusion temperature and to remove impurities.
e.g. CaO, SiO2
Question 11.
State the trends in the electronegativity in a Group and period.
Answer:
In a Group: Electronegativity decreases in a group because of the increased number of energy levels.
In a Period: The electronegativity increases because the increase in the nuclear charge.
Question 12.
Write a note about smelting.
Answer:
Smelting is a process of reducing the roasted metallic oxide to metal in a molten condition. In this process, impurities are removed by the addition of flux as slag.
Question 13.
Write the formula of the ores of Aluminium.
Answer:
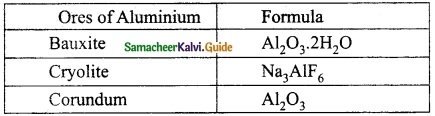
Question 14.
Explain the action of Aluminium with air.
Answer:
On heating at 800°C, aluminium bums in the air very brightly forming its oxide and nitride.
4Al + 3O2 → 2Al2O3 (Aluminium oxide)
2Al + N2 → 2AlN (Aluminium nitride).
![]()
Question 15.
What happen when Aluminium reacts with steam?
Answer:
When steam is passed over red hot aluminium, H2 gas is evolved.
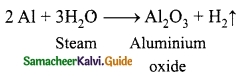
Question 16.
Write the reaction of Aluminium with Sodium hydroxide?
Answer:
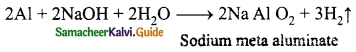
Question 17.
Explain the electrolytic refining of copper.
Answer:
Cathode: A thin plate of pure copper metal.
Anode: A block of impure copper metal.
Electrolyte: Copper sulphate solution + dilute H2SO4
When an electric current is passed through the electrolytic solution, pure copper gets deposited at the cathode and the impurities are settled at the bottom of the anode as anode mud.
Question 18.
Mention the uses of Aluminium.
Answer:
Aluminium is used in
- household utensils
- electrical cable industry
- making aeroplanes and other industrial machine parts.
Question 19.
What are the methods employed to make an alloy?
Answer:
- By fusing the metals together. Eg: Brass is made by melting zinc and copper.
- By compressing finely divided metals. Eg: wood mexai.
Question 20.
Write the components of wood metal.
Answer:
Wood metal is an alloy of Lead, Tin, Bismuth and Cadmium.
Question 21.
What are the uses of copper?
Answer:
- Copper is used in manufacturing electric cables and other electric appliances.
- Copper is used for making utensils, containers, calorimeters and coins.
- Copper is used in electroplating.
- Copper is alloyed with gold and silver for making coins and jewels.
Question 22.
Give example for non-ferrous copper and aluminium alloys. Non-ferrous copper alloys: Brass (Cu, Zn), Bronze (Cu, Sn)
Answer:
Non-ferrous aluminium alloys: Duralumin (Al, Mg, Cu, Mn), Magnalium (Al, Mg)
Question 23.
How is rust formed?
Answer:
When iron is exposed to moist air, it forms a layer of brown hydrated Ferric oxide on its surface. This compound is known as rust.
4Fe + 3O2 + xH2O → 2Fe2O3. xH2O (Rust).
Question 24.
Why are the alloys prepared?
Answer:
- To modify appearance and colour.
- To modify chemical activity.
- To lower the melting point.
- To increase hardness and tensile strength.
- To increase resistance to electricity.
Question 25.
Define corrosion.
Answer:
Corrosion is the gradual destruction of metals by chemical or electrochemical reaction with the environment.
![]()
Question 26.
What are alloys? How are they prepared?
Answer:
- An alloy is a homogeneous mixture of a metal with other metals or with non-metals that are fused together. e.g. Brass is an alloy of zinc (solute) in copper (solvent)
- Alloys are prepared by fusing the metals together.
- Alloys are prepared by compressing finely divided metals one over the other.
Question 27.
Which is known as Wet corrosion or Electrochemical corrosion?
Answer:
The corrosive action in the presence of moisture is called wet corrosion. It occurs as a result of electrochemical reaction of metal with water or aqueous solution of salt or acids or bases.
Question 28.
Write a note on Cathodic protection.
Answer:
It is the method of controlling corrosion of a metal surface protected is coated with the metal which is easily corrodible. The easily corrodible metal is called Sacrificial metal to act as anode ensuring cathodic protection.
Question 29.
What are the methods used to prevent corrosion?
Answer:
Corrosion of metals is prevented
- by coating with paints
- by coating with oil and grease
- by alloying with other metals
- by the process of galvanization
- by electroplating
- by sacrificial protection
Question 30.
A reddish brown metal ‘A’ reacts with dil.HCl in the presence of O2 and forms the compound ‘B’. ‘B’ can also be prepared by heating the metal A with Cl2. Identify A and B.
Answer:
Reddish brown metal ‘A’ is copper.
(A) reacts with dil.HCl in the presence of O2 and forms CuCl2 which is ‘B’.
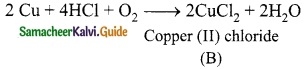
(B) can also prepared by the action of Cl2.
![]()
Question 31.
Write the uses of copper.
Answer:
- It is extensively used in manufacturing electric cables and other electric appliances.
- It is used for making utensils, containers, calorimeters and coins.
- It is used in electroplating.
- It is alloyed with gold and silver for making coins and jewels.
Question 32.
Write the name and formula of the ores of iron.
Answer:

Question 33.
Define periodicity.
Answer:
The electronic configurations of elements help us to explain the periodic recurrence of physical and chemical properties. Anything which repeats itself after a regular interval is called periodic and this behaviour is called periodicity.
Question 34.
What happens in the combustion zone during the extraction of iron.
Answer:
The temperature in the combustion zone is 150°C. In this region coke bums 02 to form CO2, when the charge comes in contact with a hot blast of air.
![]()
Question 35.
Explain the reactions taking place in the reduction zone.
Answer:
In the upper region of reduction zone, the temperature is at 400°C. In this region CO reduces ferric oxide to form spongy iron.

Question 36.
Define Metallic radius.
Answer:
It is defined as half the distance between the nuclei of adjacent metal atoms.
Question 37.
Complete the following reactions.
- 4Fe + 10HNO3 → 4Fe(NO3)2 + ………. + 3H2O
- 2Fe + 6H2SO4 → Fe2(SO4)3 + ………. + 6H2O
Answer:
- NH4NO3
- 3SO2
![]()
Question 38.
What happens when steam is passed over red hot iron?
Answer:
When steam is passed over red hot iron magnetic oxide is formed.
3Fe + 4H2O (steam) → Fe3O4 + 4H2↑
Question 39.
Define Electron affinity.
Answer:
Electron affinity is the amount of energy released when a gaseous atom gains an electron to form its anion. It is also measured in kJ / mol.
Question 40.
Complete the table.
Answer:
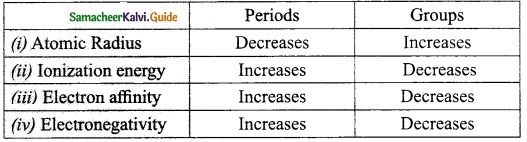
Question 41.
Define Metallurgy.
Answer:
Metallurgy is a science of extracting metals from their ores and modifying the metals into alloys for various uses, based on their physical and chemical properties and their structural arrangement of atoms.
Question 42.
Write a short note on leaching or chemical process.
Answer:
This method is employed when the ore is in a very pure form. The ore is treated with a suitable reagent such that the ore is soluble in it but the impurities are not. The impurities are removed by filtration. The solution of the ore, ie., the filtrate is treated with a suitable reagent which precipitates the ore.
E.g. Bauxite Al2O3.2H2O, the ore of aluminium.
Question 43.
Relate all the four columns of the table with their unique properties.
Answer:
Question 44.
Guess Who I am?
(i) I am preserved in Kerosene.
Answer:
Sodium
(ii) My ore is leached with NaOH.
Answer:
Aluminium
(iii) I sacrifice myself to protect my friend Iron.
Answer:
Magnesium
(iv) I am being used in propellers
Answer:
Nickel steel
Question 45.
Explain the method of making alloys.
Answer:
- By fusing the metals together. E.g. Brass is made by melting zinc and copper.
- By compressing finely divided metals. E.g. Wood metal: an alloy of lead, tin, bismuth and cadmium powder is a fusible alloy.
Question 46.
Write the differences between a mineral and a ore.
Answer:
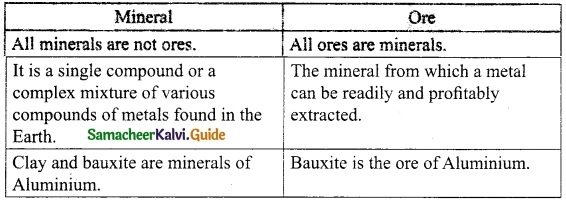
![]()
VII. Long answer questions:
Question 1.
Write the reactions taking place during Bessemerisation of copper.
Answer:
2 FeS + 3O2 → 2FeO + 2SO2↑
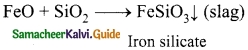
2Cu2S + 3O2 → 2 Cu2O + 2 SO2↑
2Cu2O + Cu2S → 6 Cu + SO2↑
Question 2.
How do electronegativity values help to find out the nature of bonding between atoms?
Answer:
- If the difference in electronegativity between two elements is 1.7, the bond has 50% ionic character and 50 % covalent character.
- If the difference is less than 1.7, the bond is considered to be covalent.
- If the difference is greater than 1.7, the bond is considered to be ionic.
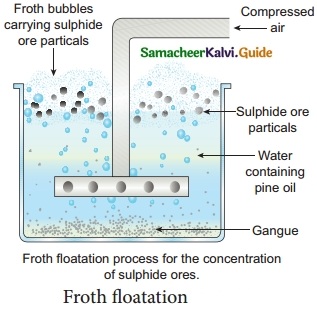
Question 3.
Explain Froth floatation with diagram.
Answer:
Principle: This process depends on the preferential wettability of the ore with oil (pine oil) and the gangue particles by water. Lighter ores, such as sulphide ores, are concentrated by this method. Eg: Zinc blende (ZnS).
Question 4.
Explain the Baeyer’s process of conversion of Bauxite into alumina.
Answer:
(i) Bauxite ore is finely ground and heated under pressure with a solution of concentrated caustic soda solution at 150°C to obtain sodium meta aluminate.
(ii) On diluting sodium meta aluminate with water, a precipitate of aluminium hydroxide is formed.
(iii) The precipitate is filtered, washed, dried and ignited at 1000°C to get alumina.
![]()
Question 5.
Explain the Hall’s Process of electrolytic reduction of alumina with diagram.
Answer:
Hall’s Process:
Aluminium is produced by the electrolytic reduction of fused alumina (Al2O3) in the electrolytic cell.
Cathode : Iron tank linked with graphite
Anode : A bunch of graphite rods suspended in molten electrolyte.
Electrolyte : Pure alumina + molten cryolite + fluorspar (fluorspar lowers the fusion temperature of electrolyte)
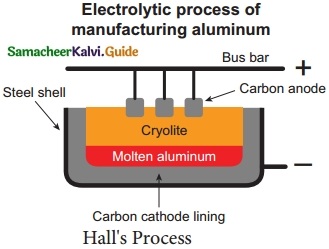
Temperature: 900 – 950 °C
Voltage used: 5 – 6 V
Overall reaction: 2 Al2O3 → 4 Al + 3O2↑
Question 6.
Write the reaction involved in the middle region of blast furnace during the extraction of iron.
Answer:
The Middle Region (Fusion Zone): The temperature prevails at 1000°C. In this region, CO2 is reduced to CO.
![]()
Limestone decomposes to calcium oxide and CO2.
![]()
These two reactions are endothermic due to absorption of heat. Calcium oxide combines with silica to form calcium silicate slag.
CaO + SiO2 → CaSiO3
Question 7.
What are the three different types of iron? Write their uses.
Answer:
(i) Pig iron (Iron with 2-4.5% of carbon): It is used in making pipes, stoves, radiators, railings, manhole covers and drain pipes.
(ii) Steel (Iron with < 0.25% of carbon): It is used in the construction of buildings, machinery, transmission cables and T. V towers and in making alloys.
(iii) Wrought iron (Iron with 0.25-2% of wraught carbon): It is used in making springs, anchors and electromagnets.
![]()
Question 8.
What is corrosion? Write the chemistry behind the formation of rust.
Answer:
(i) The slow and steady destruction of a metal by chemical or electro chemical reaction with the environment.
(ii) When the surface of iron is exposed to moisture and other gases present in the atmosphere, the following chemical reaction takes place.
Fe → Fe2+ + 2e–
O2 + 2H2O + 4e– → 4OH–
O2 + 4H+ + 4e– → 2H2O
The Fe2+ ions are oxidised to Fe3+ ions.
The Fe3+ ions combine OH– ions to form Fe(OH)3. This becomes rust which is hydrated ferric oxide with the formula Fe2O3.xH2O. It is a reddish brown substance.
Question 9.
Explain the methods of preventing corrosion.
Answer:
(i) Alloying : The metals can be alloyed to prevent the process of corrosion. Eg: Stainless Steel
(ii) Surface Coating : It involves application of a protective coating over the metal. It is of the following types:
(a) Galvanization: It is the process of coating zinc on iron sheets by using electric current.
(b) Electroplating: It is a method of coating one metal over another metal by passing electric current.
(c) Anodizing: It is an electrochemical process that converts the metal surface into a decorative, durable and corrosion resistant. Aluminium is widely used for anodizing process.
(d) Cathodic Protection: It is the method of controlling corrosion of a metal surface protected is coated with the metal which is easily corrodible. The easily corrodible metal is called sacrificial metal to act as anode ensuring cathodic protection.
Question 10.
Discuss the main featured of Periods in the modern periodic table (or) long form of periodic table.
Answer:
The horizontal rows are called periods.There are seven periods in the periodic table.
- First period (Atomic number 1 and 2): This is the shortest period. It contains only two elements (Hydrogen and Helium).
- Second period (Atomic number 3 to 10): This is a short period. It contains eight elements (Lithium to Neon).
- Third period (Atomic number 11 to 18): This is also a short period. It contains eight elements (Sodium to Argon).
- Fourth period (Atomic number 19 to 36): This is a long period. It contains eighteen elements (Potassium to Krypton). This includes 8 normal elements and 10 transition elements.
- Fifth period (Atomic number 37 to 54): This is also a long period. It contains 18 elements (Rubidium to Xenon). This includes 8 normal elements and 10 transition elements.
- Sixth period (Atomic number 55 to 86): This is the longest period. It contains 32 elements (Caesium to Radon). This includes 8 normal elements, 10 transition elements and 14 inner transition elements (Lanthanides).
- Seventh period (Atomic number 87 to 118): Like the sixth period, this period also accommodates 32 elements. Recently 4 elements have been included by IUPAC.
Question 11.
Discuss the main feature of Groups in the long form of periodic table.
Answer:
(i) The vertical columns in the periodic table starting from top to bottom are called groups. There are 18 groups in the periodic table.
(ii) Based on the common characteristics of elements in each group, they can be grouped as various families.
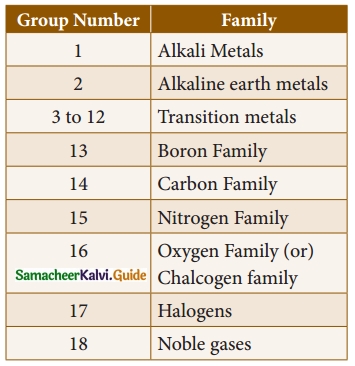
(iii) The Lanthanides and Actinides, which form part of Group 3 are called inner transition elements.
(iv) Except ‘group O’, all the elements present in each group have the same number of electrons in their valence shell and thus have the same valency. Eg: all the elements of group 1 have one electron in their valence shells (Is1). So, the valency of all the alkali metals is ‘ 1’.
(v) As the elements present in a group have identical valence shell electronic configurations, they possess similar chemical properties.
(vi) The physical properties of the elements in a group such as melting point, boiling point and density vary gradually.
(vii) The atoms of the ‘group 0’ elements have stable electronic configuration in their valence shells and hence they are unreactive.
VIII. Hot Questions:
Question 1.
Why noble gases have zero electron affinity value?
Answer:
Noble gases show no tendency to accept electrons because the outers and p orbitals of noble gases are completely filled. No more electrons can be added to them and hence their electron affinities are zero.
![]()
Question 2.
Arrange the following ions in order of their increasing ionic radii.
Answer:
Li+, Mg2+, K+ Al3+
Al3+ < Li+ < Mg2+ < K+
Question 3.
Cationic radius is smaller than its corresponding neutral atom. Why?
Answer:
When a neutral atom lose one or more electrons it forms a cation.
Na → Na+ + e–
The radius of this cation (rNa+)is decreased than its parent atom (rNa).
When an atom is charged to cation, the number of nuclear charges becomes greater than the number of orbital electrons. Florence the remaining electrons are more strongly attracted by the nucleus. Hence the cationic radius is smaller than its corresponding neutral atom.