Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Chemistry Guide Pdf Chapter 11 Fundamentals of Organic Chemistry Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 11 Fundamentals of Organic Chemistry
11th Chemistry Guide Fundamentals of Organic Chemistry Text Book Back Questions and Answers
Textbook Evaluation:
I. Choose the best answer:
Question 1.
Select the molecule which has only one π bond.
a) CH3 – CH = CH – CH3
b) CH3 – CH = CH – CHO
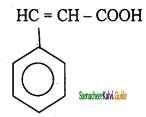
c) CH3 – CH = CH – COOH
d) All of these
Answer:
a) CH3 – CH = CH – CH3
Question 2.
In the hydrocarbon ![]() the state of hybridization of carbon 1, 2, 3, 4 and 7 are in the following sequence.
the state of hybridization of carbon 1, 2, 3, 4 and 7 are in the following sequence.
a) sp, sp, sp3, sp2, sp3
b) sp2, sp, sp3, sp2, sp3
c) sp, sp, sp2, sp, sp3
d) none of these
Answer:
a) sp, sp, sp3, sp2, sp3
Question 3.
The general formula for alkadiene is
a) CnH2n
b) CnH2n – 1
c) CnH2n – 2
d) CnHn – 2
Answer:
c) CnH2n – 2
Question 4.
Structure of the compound whose IUPAC name is 5, 6 – dimethylhept – 2 – ene is
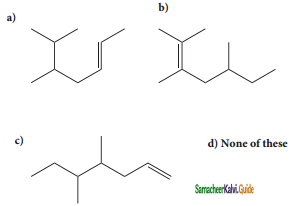
Answer:
a) 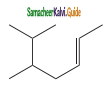
Question 5.
The IUPAC name of the compound is
a) 2, 3 – Dimethylheptane
b) 3 – methyl – 4 – ethyloctane
c) 5 – ethyl – 6- methyloctane
d) 4 – Ethyl – 3 methyloctane
Answer:
d) 4 – Ethyl – 3 methyloctane
![]()
Question 6.
Which one of the following names does not fit a real name?
a) 3 – Methyl – 3- hexanone
b) 4 – Methyl – 3 – hexanone
c) 3 – Methyl – 3 hexanol
d) 2 – Methyl cyclo hexanone
Answer:
a) 3 – Methyl – 3- hexanone
Question 7.
The IUPAC name of the compound CH3 – CH = CH – C ≡ CH is
a) Pent – 4- yn – 2 – ene
b) Pent – 3- en – 1- yne
c) Pent – 2 – en – 4 – yne
d) Pent – 1 yn – 3 – ene
Answer:
b) Pent – 3- en – 1- yne
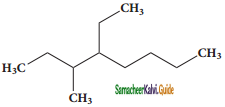
Question 8.
IUPAC name of 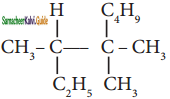 is
is
a) 3, 4, 4 – Trimethylheptane
b) 2 – Ethyl – 3, 3, – dimethyl heptane
c) 3, 4, 4 – Trimethyloctane
d) 2 – Butyl – 2 – methyl – 3 ethyl – butane
Answer:
c) 3, 4, 4 – Trimethyloctane
Question 9.
The IUPAC name of
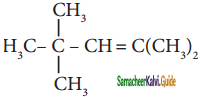 is
is
a) 2,4,4 – Trimethylpent – 2 – ene
b) 2,4,4 – Trimethylpent – 3 – ene
c) 2,2,4 – Trimethylpent – 3 – ene
d) 2,2,4 – Trirnethylpent – 2 – ene
Answer:
c) 2,2,4 – Trimethylpent – 3 – ene
Question 10.
The IUPAC name of the compound 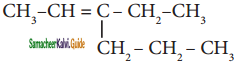 is
is
a) 3 – Ethyl – 2 – hexene
b) 3 – Propyl – 3 – hexene
c) 4 – Ethyl – 4 – hexene
d) 3 – Propyl – 2 – hexene
Answer:
a) 3 – Ethyl – 2 – hexene
![]()
Question 11.
The IUPAC name of the compound
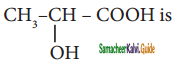 is
is
a) 2 – Hydroxypropionic acid
b) 2 – Hydroxy Propanoic acid
c) Propan – 2 – ol – 1 – oic acid
d) 1 – Carboxyethanol
Answer:
b) 2 – Hydroxy Propanoic acid
Question 12.
The IUPAC name of 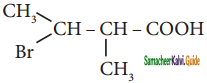 is
is
a) 2 – Bromo – 3- methyl butanoic acid
b) 2 – methyl – 3 bromo butanoic acid
c) 3 – Bromo – 2 – methylbutanoic acid
d) 3 – Bromo – 2, 3 – dimethyl propanoic acid
Answer:
c) 3 – Bromo – 2 – methylbutanoic acid
Question 13.
The structure of isobutyl group in an organic compound is
a) CH3 – CH2 – CH2 – CH2
b) 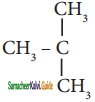
c) 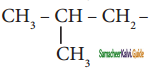
d) ![]()
Answer:
c) 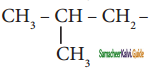
Question 14.
The number of stereoisomers of 1, 2 – dihydroxy cyclopentane
a) 1
b) 2
c) 3
d) 4
Answer:
c) 3
Question 15.
Which of the following is optically active?
a) 3 – Chloropentane
b) 2- Chloro propane
c) Meso – tartaric acid
d) Glucose
Answer:
d) Glucose
![]()
Question 16.
The isomer of ethanol is
a) acetaldehyde
b) dimethyl ether
c) acetone
d) methyl carbinol
Answer:
b) dimethyl ether
Question 17.
How many cyclic and acyclic isomers are possible for the molecular formula C3H6O?
a) 4
b) 5
c) 9
d) 10
Answer:
c) 9
Question 18.
Which one of the following shows functional isomerism?
a) ethylene
b) Propane
c) ethanol
d) CH2Cl2
Answer:
c) ethanol
Question 19.
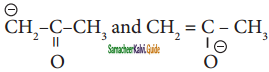 are
are
a) resonating structure
b) tautomers
c) Optical isomers
d) Conformers
Answer:
b) tautomers
Question 20.
Nitrogen detection in an organic compound is carried out by Lassaigne’s test. The blue colour formed is due to the formation of
a) Fe3[Fe(CN)6]2
b) Fe4 [ Fe(CN)6]3
c) Fe4[Fe(CN)6]2
d) Fe3[Fe(CN)6]3
Answer:
b) Fe4 [ Fe(CN)6]3
![]()
Question 21.
Lassaigne’s test for the detection of nitrogen fails in
a) H2N – CO – NH.NH2.HCl
b) NH2 – NH2.HCl
c) C6H5 – NH – NH2.HCl
d) C6H5CONH2
Answer:
c) C6H5 – NH – NH2.HCl
Question 22.
Connect pair of compounds which give blue colouration / precipitate and white precipitate respectively, when their Lassaigne’s test is separately done.
a) NH2 NH2 HCl and ClCH2 – CHO
b) NH2 CS NH2 and CH3 – CH2Cl
c) NH2 CH2 COOH and NH2 CONH2
d) C6H5NH2 and ClCH2 – CHO
Answer:
d) C6H5NH2 and ClCH2 – CHO
Question 23.
Sodium nitropruside reacts with sulphide ion to give a purple colour due to the formation of
a) [Fe (CN)5 NO]3-
b) [Fe (NO)5 CN]+
c) [Fe (CN)5 NOS]4-
d) [Fe (CN)5 NOS]3-
Answer:
c) [Fe (CN)5 NOS]4-
Question 24.
An organic Compound weighing 0.15 g gave on carius estimation, 0.12 g of silver bromide. The percentage of bromine in the Compound will be close to
a) 46 %
b) 34 %
c) 3.4 %
d) 4.6 %
Answer:
b) 34 %
Question 25.
A sample of 0.5 g of an organic compound was treated according to Kjeldahl’s method. The ammonia evolved was absorbed in 50mL of 0.5 M H2SO4 The remaining acid after neutralization by ammonia consumed 80 mL of 0.5 M NaOH. The percentage of nitrogen in the organic compound is.
a) 14 %
b) 28 %
c) 42 %
d) 56 %
Answer:
b) 28 %
![]()
Question 26.
In an organic compound, phosphorus is estimated as
a) Mg2P2O7
b) Mg3(PO4)2
c) H3PO4
d) P2O 5
Answer:
a) Mg2P2O7
Question 27.
Ortho and para – nitro phenol can be separated by
a) azeotropic distillation
b) destructive distillation
c) steam distillation
d) cannot be separated
Answer:
c) steam distillation
Question 28.
The purity of an organic – compound is determined by
a) Chromatography
b) Crystallization
c) melting or boiling point
d) both (a) and (c)
Answer:
d) both (a) and (c)
Question 29.
A liquid which decomposes at its boiling point can be purified by
a) distillation at atmospheric pressure
b) distillation under reduced pressure
c) fractional distillation
d) steam distillation
Answer:
b) distillation under reduced pressure
Question 30.
Assertion:
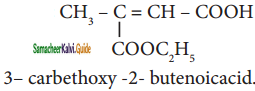
Reason:
The principal functional group gets lowest number followed by double bond (or) triple bond.
a) both the assertion and reason are true and the reason is the correct explanation of assertion.
b) both assertion and reason are true and the reason is not the correct explanation of assertion.
c) assertion is true but reason is false.
d) both the assertion and reason are false
Answer:
a) both the assertion and reason are true and the reason is the correct explanation of assertion.
![]()
II. Write brief answers to the following questions:
Question 31.
Give the general characteristics of organic compounds.
Answer:
All organic compounds have the following characteristic properties.
- They are covalent compounds of carbon and generally insoluble in water and readily soluble in organic solvent such as benzene, toluene, ether, chloroform etc…
- Many of the organic compounds are inflammable (except CCl4). They possess low boiling and melting points due to their covalent nature.
- Organic compounds are characterized by functional groups. A functional group is an atom or a specific combination of bonded atoms that react in a characteristic way, irrespective of the organic molecule in which it is present. In almost all the cases, the reaction of an organic compound takes place at the functional group. They exhibit isomerism which is a unique phenomenon.
Question 32.
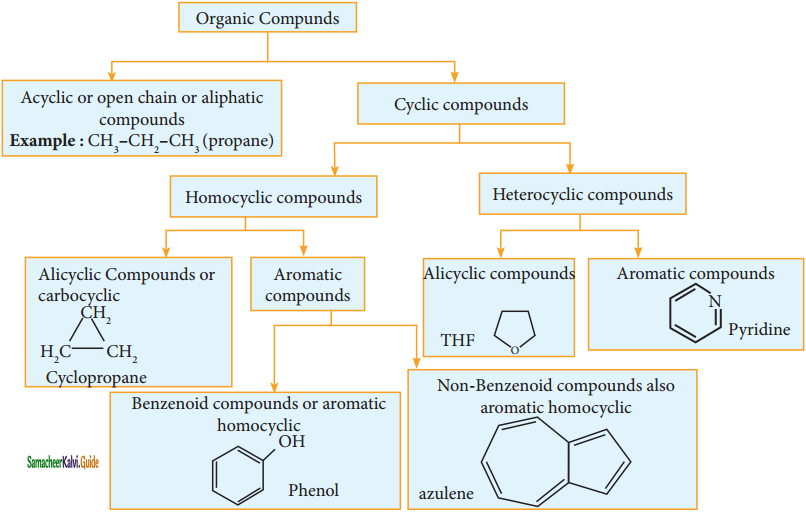
Describe the classification of organic compounds based on their structure.
Answer:
Question 33.
Write a note on homologous series.
Answer:
Homologous series:
A series of organic compounds each containing a characteric functional group and the successive members differ from each other in molecular formula by a CH2 group is called homologous series.
Example:
Alkanes:
Methane (CH4), Ethane (C2H6), Propane (C3H8) etc..
Alcohols:
Methanol (CH3OH), Ethanol (C2H5OH) Propanol (C3H7OH) etc…
![]()
Question 34.
What is meant by a functional group?
Identify the functional group in the following compounds.
a) acetaldehyde
b) oxalic acid
e) dimethyl ether
d) methylamine
Answer:
Functional group:
An atom or group of atoms within a molecule that shows characteristics set of physical and chemical properties.
a) acetaldehyde → – CHO
b) oxalic acid → – COOH
c) di methyl ether → – O –
d) methyiamine → – NH2
Question 35.
Give the general formula for the following classes of organic compounds
a) Aliphatic monohydric alcohol
b) Aliphatic ketones
c) Aliphatic amines
Answer:
a) Aliphatic monohydric alcohol → CnH2n + 2O
b) Aliphatic ketones → CnH2nO
c) Aliphatic amines → CnH3n + 2N
Question 36.
Write the molecular formula of the first six members of homologous series of nitre alkanes.
Answer:
- CH3NO2
- C2H5NO2
- C3H7NO2
- C4H9NO2
- C5H11NO2
- C6H13NO2
![]()
Question 37.
Write the molecular formula and possible structural formula of the first four members of homologous series of carboxylic acids.
Answer:
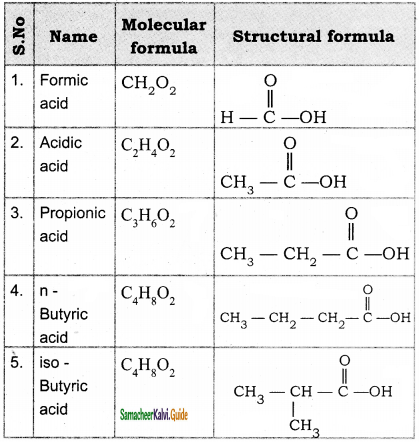
Question 38.
Give the IUPAC names of the following compounds.
i) (CH3)2CH – CH2 – CH(CH3) – CH(CH3)2
ii) 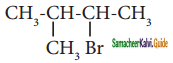
iii) CH3 – O – CH3
iv) 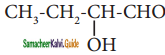
v) CH2 = CH – CH – CH2
vi) 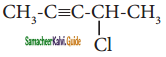
vii) 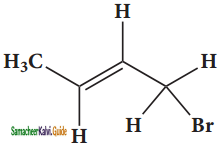
viii) 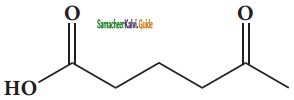
ix) 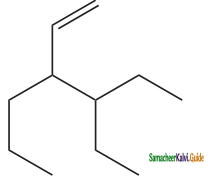
x) 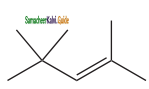
xi) 
xii) 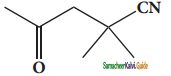
xiii) 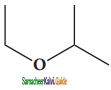
xiv) 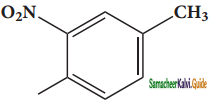
xv) 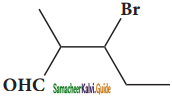
xvi) 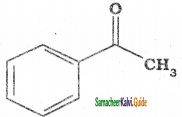
Answer:
(i) 2, 3, 5 – trimethyihexane
(ii) 2 – bromo – 3 – methylbutane
(iii) methoxymethane
(iv) 2 – hydroxybutanal
(y) buta – 1 ,3 – diene
(vi) 4 – chloropent – 2 – yne
(vii) 1 – bromobut – 2 – ene
(viii) 5 – oxohexanoic acid
(ix) 3 – ethyl – 4 – ethenylheptane
(x) 2, 4, 4 – trimethylpent – 2 – ene
(xi) 2 – methyl -1 – phenyipropan – 1 -amine
(xii) 2, 2 – dimethyl – 4oxopentanenitrile
(xiii) 2 – ethoxypropane
(xiv) 1 – fluoro – 4 – methyl – 2 -nitrobenzene
(xv) 3 – bromo – 2 – methylpentanal
(xvi) Acetophenone
Question 39.
Give the structure for the following compound
(i) 3 – ethyl – 2 methyl – 1 – pentene
(ii) 1, 3, 5 – Trimethyl cyclohex – 1 – ene
(iii) tetry butyl iodide
(iv) 3 – Chlorobutanal
(V) 3 – Chlorobutanol
(vi) 2 – Chloro – 2 – methyl propane
(vii) 2, 2 – dimethyl – 1 – chloropropane
(viii) 3 – methylbut -1- ene
(ix) Butan – 2, 2 – diol
(x) Octane – 1, 3 – diene
(xi) 1, 3 – Dimethylcyclohexane
(xii) 3 – Chlorobut – 1 – ene
(xiii) 2 – methylbutan – 3 – ol
(xiv) acetaldehyde
Answer:
(i) 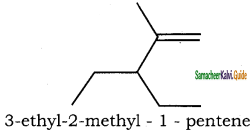
(ii) 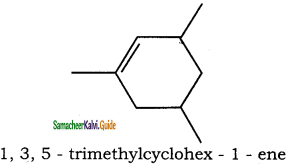
(iii) 
(iv) 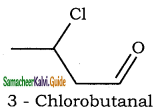
(v) 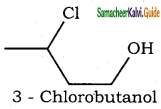
(vi) 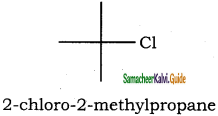
(vii) 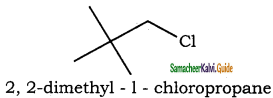
(viii) 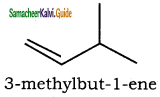
(ix) 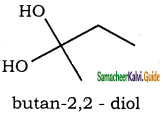
(x) 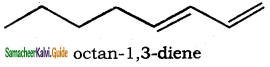
(xi) 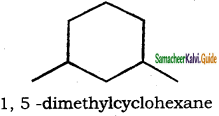
(xii) 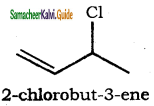
(xiii) 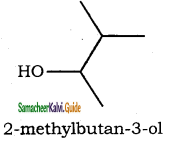
(xiv) 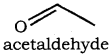
Question 40.
Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.
Answer:
This method involves the conversion of covalently bonded N, S or halogen present in the organic compounds to corresponding water soluble ions in the form of sodium salts For this purpose a freshly cut piece of Na of the size of a paper, dried it by pressing between the folds of a filter paper is taken in a fusion tube in an iron tand clamping it just near the upper end and it is gently heated.
When it melts to a shiñing globule, put a pinch of the organic compound on it. Heat the tube with the tip of the flame till all reaction ceases and it becomes red hot. Now plunges it in about 50 mL of distilled water taken in a china dish and break the bottom of the tube by striding against the dish. Boil the contents of the dish for about 10 mts and filter. This filtrate is known as lassaignes extract or sodium fusion extract and it used for qualitative analysis of nitrogen, sulfur and halogens present in organic compounds.
Test for Nitrogen:
If nitrogen is present it gets converted to sodium cyanide which on reaction with freshly prepared ferrous sulphate and ferric ion followed by cone. HCl gives a Prussian blue color or green color or precipitate. It confirms the presence of nitrogen. HCl is added to dissolve the greenish precipitate of ferrous hydroxide produced by the excess of NaOH on FeSO4 which would otherwise mark the Prussian blue precipitate. The following reaction takes part in the formation of Prussian blue.

![]()
Question 41.
Give the principle involved in the estimation of halogen in an organic compound by Carius method.
Answer:
Estimation of halogens (Carius method):
A known mass of the organic compound is heated with fuming HNO3 along with AgNO3. C, H & S gets oxidized to CO2, H2O, SO2 and halogen combines with AgNO3 to form a precipitate of silver halide.
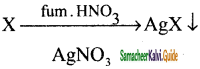
The ppt of AgX is filtered, washed, dried and weighed. From the mass of AgX and the mass of the organic compound taken, percentage of halogens are calculated.
A known mass of the substance is taken along with fuming HNO3 and AgNO3 is taken in a clean carius tube. The open end of the Carius tube is sealed and placed in a iron tube for 5 hours in the range at 530 – 540 K Then the tube is allowed to cool and a small hole is made in the tube to allow gases produced to escape. The tube is broken and the ppt is filtered, washed, dried and weighed. From the mass of AgX obtained, calculations are made.
Calculation:
Weight of the organic compound: w g
Weight of AgCl precipitate = a g
143. 5 g of AgCl contains 35.5 g of Cl
∴ a g of AgCl contains \(\frac{35.5}{143.5}\) × a
w g Organic compound gives a g AgCl
Percentage of Cl in w g organic compound = \(\left(\frac{35.5}{143.5} \times \frac{\mathrm{a}}{\mathrm{w}} \times 100\right) \%\)
Let Weight of silver Bromide be ‘b’ g
188 g of AgBr contains 80 g of Br
∴ b g of AgBr contains \(\frac{80}{180} \times \frac{b}{w}\) of Br
w g Organic compound gives b g AgBr
Percentage of Br in w g organic compound = \(\left(\frac{80}{180} \times \frac{b}{w} \times 100\right) \%\)
Let Weight of silver Iodide be ‘c’ g
235 g of AgI contains 127 g of I
∴ C g of AgI contains \(\left(\frac{127}{235} \times \frac{c}{w}\right)\) of I
w g Organic compound gives c g AgI
percentage of I in w g organic compound = \(\left(\frac{80}{180} \times \frac{b}{w} \times 100\right) \%\)
Question 42.
Give a brief description of the principles of
(i) Fractional distillation
(ii) Column Chromatography
Answer:
(i) Fractional distillation:
This is one method to purify and separate liquids present in the mixture having their boiling point close to each other. In the fractional distillation, a fractionating column is fitted with distillation flask and a condenser. A thermometer is fitted in the fractionating column near the mouth of the condenser. This will enable to record the temperature of vapour passing over the condenser.
The process of separation of the components in a liquid mixture at their respective boiling points in the form of vapours and the subsequent condensation of those vapours is called fractional distillation. The process of fractional distillation is repeated, if necessary. This method finds a remarkable application in distillation of petroleum, coal-tar and crude oil.
(ii) Column Chromatography:
This is the simplest chromatographic method carried out in long glass column having a stop cock near the lower end. This method involves separation of a mixture over a column of adsorbent (Stationery phase) packed in a column. In the column a plug of cotton or glass wool is placed at the lower end of the column to support the adsorbent powder. The tube is uniformly packed with suitable adsorbent constitute the stationary phase. (Activated aluminum oxides (alumina), Magnesium oxide, starch are also used as adsorbents).
The mixture to be separated is placed on the top of the adsorbent column. Eluent which is a liquid or a mixture of liquids is allowed to flow down the column slowly. Different components depending upon the degree to which the components are adsorbed and complete separation takes place. The most readily adsorbed substances are retained near the top and others come down to various distances in the column.
![]()
Question 43.
Explain paper chromatography.
Answer:
Paper chromatography (PC) is an example of partition chromatography. The same procedure is followed as in thin layer chromatography except that a strip of ‘ paper acts as an adsorbent. This method involves continues differential portioning of components of a mixture between stationary and mobile phase. In paper chromatography, a special quality paper known as chromatography paper is used. This paper act as a stationary phase.
A strip of chromatographic paper spotted at the base with the solution of the mixture is suspended in a suitable solvent which act as the mobile phase. The solvent rises up and flows over the spot. The paper selectively retains different components according to their different partition in the two phases where a chromatogram is developed. The spots of the separated colored compounds are visible at different heights from the position of initial spots . on the chromatogram. The spots of the separated colorless compounds may be observed either under ultraviolent light or by the use of an appropriate spray reagent.
Question 44.
Explain various types of constitutional isomerism (structural isomerism) in organic compounds.
Answer:
Structural isomerism:
This type of isomers have same molecular formula but differ in their bonding sequence.
(a) Chain or nuclear or skeletal isomerism:
These isomers differ in the way in which the carbon atoms are bonded to each other in a carbon chain or in other words isomers have similar molecular formula but differ in the nature of the carbon skeleton (ie. Straight or branched).
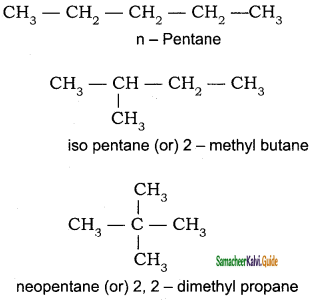
(b) Position isomerism:
If different compounds belonging to same homologous series with the same molecular formula and carbon skeleton, but differ in the position of substituent or functional group or an unsaturated linkage are said to exhibit position isomerism.
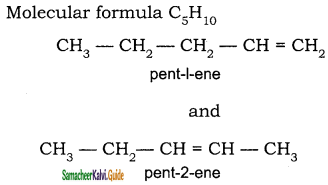
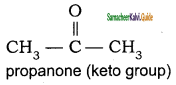
(c) Functional isomerism:
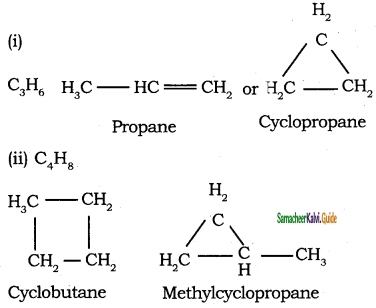
Different compounds having same molecular formula but different functional groups are said to exhibit functional isomerism.
Molecular formula C3H6O
CH3 – CH2 – CHO
propanal (aldehyde group)
Question 45.
Describe optical isomerism with suitable example.
Answer:
Compounds having same physical and chemical property but differ only in the rotation of plane of the polarized light are known as optical isomers and the phenomenon is known as optical isomerism.
Example:
Some organic compounds such as glucose have the ability to rotate the plane of the plane polarized light and there called are said to be optically active compounds and this property of a compound is called optical activity. The optical isomer, which rotates the plane of the plane polarised light to the right or in clockwise direction is said to be dextrorotary (dexter means right) denoted by the sign (+), whereas the compound which rotates to the left or anticlockwise is said to be leavorotatory (leavues means left) denoted by sign (-). Dextrorotatory compounds are represented as ‘d’ or by sign (+) and leavorotatory compounds are represented as ‘l’ or by sign (-).
![]()
Question 46.
Briefly explain geametrical isomerism in alkene by considering 2-butene as an example.
Answer:
Geometrical isomers are the stereoisomers which have different arrangement of groups or atoms around a rigid frame work of double bonds. This type of isomerism occurs due to restricted rotation of double bonds, or about single bonds in cyclic compounds.
In alkenes, the carbon-carbon double bond is sp2 hybridized. The carbon-carbon double bond consists of a σ bond and a π bond. The π bond is formed by the head on overlap of sp2 hybrid orbitals. The n bond is formed by the side-wise overlap of ‘p’ orbitals. The presence of the π bond lock the molecule in one position. Hence, rotation around C = C bond is not possible. This restriction of rotation about C – C double bond is responsible for geometrical isomerism in alkenes.
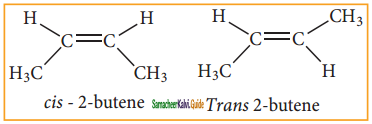
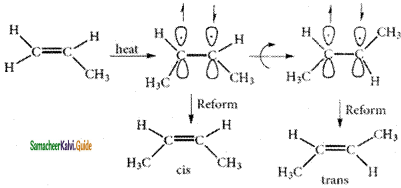
These two compounds are termed as geometrical isomers and are distinguished from each other by the terms c is and trans. The c is isomer is one in which two similar groups are on the same side of the double bond. The trans isomers is that in which the two similar groups are on the opposite side of the double bond, hence this type of isomerism is often called cis- trans isomerism.
The cis-isomer can be converted to trans isomer or vice versa is only if either isomer is heated to a high temperature or absorbs light. The heat supplies the energy (about 62kcal/ mole) to break the n bond so that rotation about a bond becomes possible. Upon cooling, the reformation of the n bond can take place in two ways giving a mixture both cis and trans forms of trans-2-butene and cis-2-butene.
Question 47.
0.30 g of a substance gives 0.88 g of carbon dioxide and 0.54 g of water calculate the percentage of carbon and hydrogen in it.
Solution:
Weight of organic compound = 0.30 g
Weight of carbon dioxide = 0.88 g
Weight of water = 0.54 g
Percentage of carbon:
44 g of carbondioxide contains, carbon = 12 g
0.88 g of carbon dioxide contains, carbon = \(\frac{12 \times 0.88}{44}\) g
0.30 g substance contains,
carbon = \(\frac{12 \times 0.88}{44}\) g
100 g substance Contains \(\frac{12 \times 0.88}{44}\) × \(\frac{100}{0.30}\) = 80 g of carbon
Percentage of carbon = 80 %
Percentage of hydrogen:
18 g of water contains, hydrogen = 2 g
0.54 g of water contains, hydrogen = \(\frac{2 \times 0.54}{18}\) g
0.30 g of substance contains hydrogen = \(\frac{2 \times 0.54}{18 \times 0.30}\) g
100 g of substance contains = \(\frac{2 \times 0.54}{18 \times 0.30}\) × 100 g = 20 g of hydrogen
Percentage of hydrogen = 20 %
![]()
Question 48.
The ammonia evolved form 0.20 g of an organic compound by Kjeldahl method neutralized 15 ml of N / 20 sulphuric acid solution. Calculate the percentage of Nitrogen.
Answer:
weight of organic compound = 0.20 g
Normality of acid = \(\frac{\mathrm{N}}{20}\)
Volume of standard acid neitralized by ammonia = 15 ml
1000 ml of N ammonia contains = 14 g of nitrogen
15 ml of ammonia of normality \(\frac{\mathrm{N}}{20}\) contains nitrogen = \(\frac{14 \times 15 \times 1}{1000 \times 20}\)
0.20 g of compound contains nitrogen = \(\frac{14 \times 15}{1000 \times 20}\)
100 g of compound contains nitrogen = \(\frac{14 \times 15 \times 100}{1000 \times 20 \times 0.20}\) = 5.25 g
Percentage of nitrogen = 5.25 %
Question 49.
0.32 g of an organic compound, after heating with fuming nitric acid and barium nitrate crystals is a sealed tube gave 0. 466 g of barium sulphate. Determine the percentage of sulphur in the compound.
Solution:
Mass of the substance taken = 0.32 g
Mass of BaSO4 formed = 0.466 g
Molecular mass of BaSO4 = 137 + 32 + 64 = 233
Then, mass of S in 0.466 g of BaSO4 = \(\frac{0.466 \times 32}{233}\)
Percentage of S in compound= \(\frac{0.466 \times 32 \times 100}{233 \times 0.32}\) = 20 %
Question 50.
0.24 g of an organic compound gave 0.287 g of silver chloride in the carius method. Calculate the percentage of chlorine in the compound.
Solution:
Mass of organic compound = 0.24 g
Mass of silver chloride = 0.287 g
143. 5 g AgCl contains = 35.5 g chlorine
0.287 g of AgCl contains = \(\frac{35.5}{143.5}\) × 0.287
Percentage of chlorine = \(\frac{35.5}{143.5} \times \frac{0.287}{0.24}\) × 100 = 29.58 %
Question 51.
In the estimation of nitrogen present in an organic compound by Dumas method 0.35 g yielded 20.7 mL of nitrogen at 15° C and 760 mm pressure. Calculate the percentage of nitrogen in the compound.
Solution:
Volume of N2 at NTP = \(\frac{V \times P}{t+273} \times \frac{273}{760}\)
= V0 ml
Substituting the various values in the above equation,
V0 = \(\frac{20.7 \times 760}{288} \times \frac{273}{760}\) = 19.62 ml
weight of 19.62 ml of Nitrogen = \(\frac{28}{22400}\) × 19.62 g
∴ Percentage of Nitrogen = \(\frac{28}{22400}\) × 19.62 × \(\frac{100}{0.35}\)
= 4.9 %
![]()
11th Chemistry Guide Fundamentals of Organic Chemistry Additional Questions and Answers
I. Choose the correct answer:
Question 1.
Organic compounds can be formed by
a) Plants only
b) Animals only
c) Plants and Animals
d) Plants, animals and can be synthesized in laboratory
Answer:
d) Plants, animals and can be synthesized in laboratory
Question 2.
Generally, organic compounds are
a) Amorphous
b) Complexes
c) Covalent
d) Electrovalent
Answer:
c) Covalent
Question 3.
The vital force theory was proposed by
a) Wohler
b) Berthlot
c) Berzelius
d) Kolbe
Answer:
c) Berzelius
Question 4.
The first carbon compound prepared from its elements is
a) Urea
b) Acetic acid
c) Methane
d) benzene
Answer:
b) Acetic acid
Question 5.
The first organic compound was synthesized in laboratory by
a) Wohler
b) Kolbe
c) Berzelius
d) Neil Barthlot
Answer:
a) Wohler
![]()
Question 6.
The first organic compound synthesized in the laboratory from an inorganic compound is
a) NH4NCO
b) NH2 – CO – NH2
c) CH3COOH
d) CH4
Answer:
b) NH2 – CO – NH2
Question 7.
Marsh gas mainly contains
a) C2H2
b) C2H4
c) CH4
d) C2H6
Answer:
c) CH4
Question 8.
Hybridization at 2nd carbon in CH2 = CH – CH3 is
a) sp
b) sp2
c) sp3
d) sp3d
Answer:
b) sp2
Question 9.
Number of possible position isomers for Dichlorobenzene is
a) 2
b) 3
c) 4
d) 5
Answer:
b) 3
Question 10.
n – Butane and isobutane are a pair of
a) chain isomers
b) position isomers
c) metamers
d) functional isomers
Answer:
a) chain isomers
![]()
Question 11.
Alkanols and Alkoxyalkanes are
a) Functional isomers
b) Keto – enol tautomers
c) Geometrical isomers
d) Not isomers at all
Answer:
a) Functional isomers
Question 12.
n – propyl alcohol and isopropyl alcohol are examples of
a) Position isomerism
b) Chain isomerism
c) Tautomerism
d) Geometrical isomerism
Answer:
a) Position isomerism
Question 13.
The number of structural alcoholic isomers for C4H10O is
a) 2
b) 3
c) 4
d) 5
Answer:
c) 4
Question 14.
Cycloalkanes are isomeric with
a) Alkadienes
b) Alkynes
c) Aromatic compounds
d) Olefins
Answer:
d) Olefins
Question 15.
Number of possible monochloro benzenes is
a) 1
b) 3
c) 5
d) 6
Answer:
a) 1
![]()
Question 16.
Diethyl ether and n – propyl methyl ether are
a) Metamers
b) Chain isomers
c) Geometrical isomers
d) Position isomers
Answer:
a) Metamers
Question 17.
The total number of structural isomers for the compound of the formula C4H10O is
a) 7
b) 6
c) 4
d) 3
Answer:
a) 7
Question 18.
The number of primary alcoholic isomers with the formula C4H10O is
a) 1
b) 2
c) 3
d) 4
Answer:
b) 2
Question 19.
The compound which is not isomeric with diethyl ether is
a) n – propyl methyl ether
b) Butan – 1 – ol
c) 2 – Methylpropan – 2 – ol
d) Butanone
Answer:
d) Butanone
Question 20.
The number of isomeric amines possible for the formula C3H9N
a) 4
b) 3
c) 5
d) 6
Answer:
a) 4
![]()
Question 21.
Which hybrid orbitals are involved in the CH3 – CH = CH – CH3 compound
a) sp and sp3
b) sp2 and sp3
c) sp and sp2
d) only sp3
Answer:
b) sp2 and sp3
Question 22.
Which of the following bonds is strongest?
a) 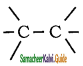
b) > C = C <
c) 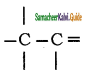
d) – C – C –
Answer:
a) 
Question 23.
According to Huckel’s rule a compound, is said to be aromatic if’ it contains
a) 4n bonds
b) 4n atoms
c) (4n + 2) atoms
d) (4n + 2) π electrons
Answer:
d) (4n + 2) π electrons
Question 24.
Which of the following is an aromatic compound
a) Phenol
b) Naphthalene
c) Pyridine
d) All
Answer:
d) All
Question 25.
Which is a saturated compound?
a) alkanes
b) alkenes
c) alkynes
d) cyclo alkenes
Answer:
a) alkanes
![]()
Question 26.
Which is an alicyclic compound?
a) benzene
b) cyclohexane
c) pyridine
d) pyrrole
Answer:
b) cyclohexane
Question 27.
Which of the following is not a cyclic compound?
a) Anthracene
b) Pyrrole
c) Phenol
d) Isobutylene
Answer:
d) Isobutylene
Question 28.
Functional group present in amides is
a) – COOH
b) – NH2
c) – CONH2
d) – COO –
Answer:
c) – CONH2
Question 29.
IUPAC name of ester is
a) Alkoxy alkane
b) Alkyl alkanoate
c) Alkanoyl halide
d) Alkanoic anhydride
Answer:
b) Alkyl alkanoate
Question 30.
IUPAC name of methyl cyanide is
a) Cyano methane
b) Ethanenitrile
c) Methane nitrile
d) Methyl – n – butyl amine
Answer:
b) Ethanenitrile
![]()
Question 31.
The correct IUPAC name of 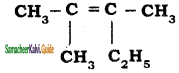 is
is
a) 1, 2 – diethyl butene
b) 2 – ethyl – 3- methyl pentene
c) 3, 4 – dimethyl hex – 3 – ene
d) 2, 3 – dimethyl pent – 2 – ene
Answer:
d) 2, 3 – dimethyl pent – 2 – ene
Question 32.
IUPAC name of CH2OH – CH2OH is
a) 1, 2 – dihydroxy ethane
b) ethylene glycol
c) ethane – 1, 2 – diol
d) ethane – 1, 2 – dial
Answer:
c) ethane – 1, 2 – diol
Question 33.
IUPAC name of CH ≡ C – CH = CH2 is
a) but – 3 – ene – 1 – yne
b) but – 1 – ene – 3 – yne
c) but – 1 – yne – 3 – ene
d) but – 3 – yne – 1 – ene
Answer:
b) but – 1 – ene – 3 – yne
Question 34.
The IUPAC name of 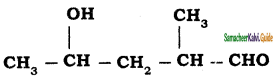 is
is
a) 4 – hydroxy – 2 – methyl pentanal
b) 2 – hydroxy – 4 – methyl pentanal
c) 4 – hydroxy – 2 – methyl pentanol
d) 2 – hydroxy – 4 – methyl pentanol
Answer:
a) 4 – hydroxy – 2 – methyl pentanal
Question 35.
3 – methyl penta -1, 3- diene is
a) CH2 = CH (CH2)2 CH3
b) CH2 = CHCH (CH3) CH2CH3
c) CH3CH = C(CH3)CH = CH2
d) CH3 = C = CH (CH3)2
Answer:
c) CH3CH = C(CH3)CH = CH2
![]()
Question 36.
The structural formula of methyl amino methane is
a) (CH3)2 CH NH2
b) (CH3)3 N
c) (CH3)2 NH
d) CH3NH2
Answer:
c) (CH3)2 NH
Question 37.
Which of the following is the functional isomer of methyl acetate?
a) Ethyl acetate
b) Propanoic acid
c) Ethyl formate
d) Propanone
Answer:
b) Propanoic acid
Question 38.
The compound which is not isomeric with diethyl ether is
a) n – propyl methyl ether
b) 1 – Butanol
c) 2 – Methyl – 2 – propanol
d) Butanone
Answer:
d) Butanone
Question 39.
Which of the following pairs of compounds are tautomers?
a) Propanol & propanone
b) Ethanol & vinyl alcohol
c) Ethanol & allyl alcohol
d) Vinyl alcohol & ethanal
Answer:
d) Vinyl alcohol & ethanal
Question 40.
Which of the following compounds does not have any tertiary hydrogen atoms?
a) (CH3)3 CCH2 CH3
b) (CH3)2 CHCH2 CH3
c) (CH3)2 CHCH (CH3)2
d) (CH3)3 CH
Answer:
a) (CH3)3 CCH2 CH3
![]()
Question 41.
The IUPAC name of 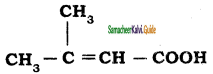 is
is
a) 2 – Methyl – 2 – butenoic acid
b) 3 – Methyl – 3 – butenoic acid
c) 3 – Methyl – 2 – butenoic acid
d) 2 – Methyl – 3 – butenoic acid
Answer:
c) 3 – Methyl – 2 – butenoic acid
Question 42.
The IUPAC name of Cinnamaldehyde is
a) 3 – Phenyl prop – 2 – enal
b) 1 – Phenyl – prop – 1 – enal
c) 1 – Phenyl – prop – 2 – enal
d) 3 – Phenyl – prop – 1 – enal
Answer:
a) 3 – Phenyl prop – 2 – enal
Question 43.
The IUPAC name of the Compound CH3 – CH(OH) – COOH is
a) Lactic acid
b) 2 – Hydroxy propanoic acid
c) 3 – Hydroxy propanoic acid
d) Carboxy propanol
Answer:
b) 2 – Hydroxy propanoic acid
Question 44.
The IUPAC name of the compound 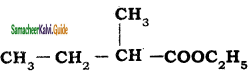 is
is
a) 2 – Ethyl – ethyl acetate
b) Ethyl – 3 – methy1butanoate
c) Ethyl – 2 – methyl butanoate
d) 2- methyl butanoic acid
Answer:
c) Ethyl – 2 – methyl butanoate
Question 45.
The IUPAC name of the given compound 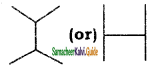 is
is
a) 2,2 — Dimethyl butane
b) lsohexane
c) 2, 3 – Dimethyl butane
d) Di isohexane
Answer:
c) 2, 3 – Dimethyl butane
![]()
Question 46.
The IUPAC name of the given compound 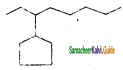 is
is
a) Octyl cyclopentane
b) 3 – cyclopentyl octane
c) Cyclopentane octane
d) 6 – cyclopentyl octane
Answer:
b) 3 – cyclopentyl octane
Question 47.
The IUPAC name of 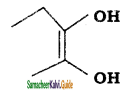 is
is
a) but – 2- ene – 2,3 – diol
b) pent – 2- ene – 2,3 – diol
c) 2 – methyl but – 2 – ene – 2,3 – diol
d) hex – 2- ene – 2,3 – diol
Answer:
b) pent – 2- ene – 2,3 – diol
Question 48.
The structure of 3-bromoprop-1-ene is
a) 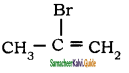
b) CH3 – CH = CH – Br
c) 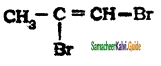
d) Br – CH2 – CH ≡ CH2
Answer:
d) Br – CH2 – CH ≡ CH2
Question 49.
Neo-heptyl alcohol is correctly represented as
a) 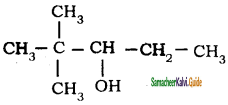
b) 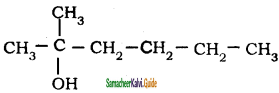
c) 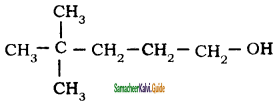
d) 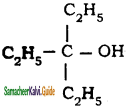
Answer:
c) 
Question 50.
Number of dibromo derivatives possible for propane are
a) 2
b) 3
c) 1
d) 4
Answer:
d) 4
![]()
Question 51.
The number of aromatic isomers possible for C7H8O is
a) 2
b) 3
c) 4
d) 5
Answer:
d) 5
Question 52.
Isomers of propanoic acid are
a) HCOOC2H5 and CH3COOCH3
b) H – COOC2H5 and C3H7COOH
c) CH3COOCH3 and C3H7OH
d) C3H7OH and CH3COCH3
Answer:
a) HCOOC2H5 and CH3COOCH3
Question 53.
IUPAC name of CH3 – CH (OCH3) – CH2 – NH2
a) 2-methoxy propanamine
b) 1-amino – 2-methoxy propane
c) 1-amino – 2-methyl – 2-methoxy ethane
d) 1 – methoxy- 2-amino propane
Answer:
a) 2-methoxy propanamine
Question 54.
IUPAC name of 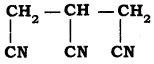 is
is
a) 3 – cyanopentane – 1, 5 – dinitrile
b) Propane – 1, 2, 3-tri nitrile
c) 1, 2, 3-tri cyano propane
d) Propane 1, 2, 3-tricarbonitrile
Answer:
d) Propane 1, 2, 3-tricarbonitrile
Question 55.
The IUPAC name of the compound 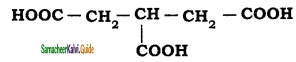 is
is
a) 3 – Carboxylic pentane – 1,5 -dioic acid
b) Propane – 1, 2, 3 – trioic acid
c) 1, 2, 3- tricarboxylic propane
d) Propane – 1,2, 3 – tricarboxylic acid
Answer:
b) Propane – 1, 2, 3 – trioic acid
![]()
Question 56.
The IUPAC name of the following compound
CH3 – C(CH3)2 – CH2 – CH = CH2 is
a) 2, 2 – Dimethyl – 4 – pentene
b) 4, 4 – Dimethyl – 1 – pentene
c) 1, 1, 1 – trimethyl – 3 – butene
d) 4, 4, 4 – trimethyl – 1 – butene
Answer:
b) 4, 4 – Dimethyl – 1 – pentene
Question 57.
The IUPAC name of 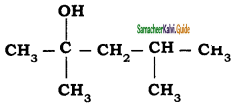 is
is
a) 2, 4 – Dimethyl pentan – 2 – ol
b) 2, 4 – Dimethyl pentan – 4 – ol
c) 2,2 – Dimethyl butan – 2- ol
d) Butan – 2 – ol
Answer:
a) 2, 4 – Dimethyl pentan – 2 – ol
Question 58.
The IUPAC name the compound 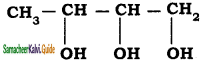 is
is
a) Butane – 2, 3, 4 – triol
b) Butane – 1,2, 3 – triol
c) Pentane – 1, 2, 3 – triol
d) 2, 3 dihydroxy butanol
Answer:
b) Butane – 1,2, 3 – triol
Question 59.
The IUPAC name the compound 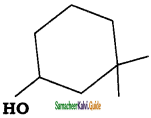 is
is
a) 3, 3 – Dimethyl – 1 – cyclohexanol
b) 1, 1- Dimethyl – 3 – hydroxy cyclohexane
c) 3, 3 – Dimethyl – 1 – hydroxy cyclohexane
d) 1, 1 – Dimethyl – 3 – cyclohexanol
Answer:
a) 3, 3 – Dimethyl – 1 – cyclohexanol
Question 60.
The IUPAC name of the compound 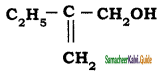 is
is
a) 2 – Ethylprop – 2 – en – 1 – ol
b) 2 – Hydroxymethyl butan – 1- ol
c) 2 – Methylene butan – 1 – ol
d) 2 – Ethyl -3 hydroxyprop – 1 – ene
Answer:
a) 2 – Ethylprop – 2 – en – 1 – ol
![]()
Question 61.
The number of possible alkynes with molecular formula C5H8 is
a) 2
b) 3
c) 4
d) 5
Answer:
b) 3
Question 62.
What is the IUPAC name of the following 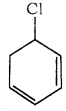 is
is
a) 3 – chloro cyclo hexa – 1, 5 – diene
b) 5 – chloro cyclo hexa – 1, 3 – diene
c) 1 – chloro cyclo hexa – 2, 5 – diene
d) 2 – chloro cyclo hexa – 1, 4 – diene
Answer:
b) 5 – chloro cyclo hexa – 1, 3 – diene
Question 63.
What is the IUPAC name of the following 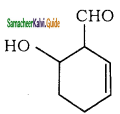 is
is
a) 6 – hydroxy cyclohex – 2 – ene – 1 – al
b) 4 – hydroxy cyclohex – 1 – ene – 3 – al
C) 2 – hydroxy cyclohex – 5 – ene – 1 – al
d) 1 – formyl cyclohex – 5 – ene – 2 – ol
Answer:
a) 6 – hydroxy cyclohex – 2 – ene – 1 – al
Question 64.
What is the IUPAC name of the following?
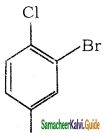
a) 1 – chloro – 2 – bromo – 4 – nitrobenzene
b) 1 – bromo – 2- chloro – 4 – mtrobenzene
c) 3 – bromo – 4 – chloro – nitrobenzene
d) 2 – bromo – 1 – chloro – 4- nitrobenzene
Answer:
d) 2 – bromo – 1 – chloro – 4- nitrobenzene
Question 65.
What is the IUPAC name of the following?
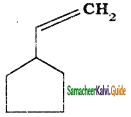
a) Ethenyl cyclo pentane
b) cyclopentyl ethene
c) cyclopentyl ethylene
d) vinyl cyclopentane
Answer:
b) cyclopentyl ethene
![]()
Question 66.
The hybridization of carbon atoms in C – C single bond is HC ≡ C – CH = CH2 is
a) sp3 – sp3
b) sp2 – sp3
c) sp – sp2
d) sp3 – sp
Answer:
c) sp – sp2
Question 67.
The correct IUPAC name of the compound 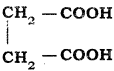 is
is
a) 1, 4 – Butane dioicacid
b) Ethane – 1, 2 – dicarboxylic acid
c) Succinic acid
d) 1, 2 – Ethane dioic acid
Answer:
a) 1, 4 – Butane dioic acid
Question 68.
Correct statements about CH3 – CH2 – CN is
a) common name of the compound is ethylcyanide.
b) IUPAC name of the compound propane – 1 – nitrile.
c) secondary suffix of the compound is nitrile.
d) IUPAC name of the compound is ethane nitrile.
Answer:
a) common name of the compound is ethylcyanide.
Question 69.
Tautomerism is shown by
a) R – C ≡ N
b) R – NO2
c) R – OH
d) R – COOH
Answer:
b) R – NO2
Question 70.
Stereo isomers have different
a) Molecular mass
b) Molecular formula
c) Structural formula
d) Configuration
Answer:
d) Configuration
![]()
Question 71.
Geometrical isomerism may be exhibited by compounds having atleast.
a) One double bond
b) One triple bond
c) One asymmetric carbon
d) One polar bond
Answer:
a) One double bond
Question 72.
The prefixes syn – and anti – are used to denote
a) structural isomers
b) conformational isomers
c) geometrical isomers
d) optical isomers
Answer:
c) geometrical isomers
Question 73.
d – tartaric acid and l – tartaric acid are
a) geometrical isomers
b) conformers
c) enantiomers
d) diastereomers
Answer:
c) enantiomers
Question 74.
The method of separation of enantiomers from racemic mixture is known as
a) inversion
b) recemisation
c) resolution
d) asymmetric synthesis
Answer:
c) resolution
Question 75.
Racemic mixture is optically inactive due to
a) internal compensation
b) external compensation
c) inversion
d) plane of symmetry
Answer:
c) inversion
![]()
Question 76.
Meso isomers are possible when the organic compound contains
a) one asymmetric carbon
b) two or more dissimilar asymmetric carbons
c) similar asymmetric carbons
d) unsaturation
Answer:
c) similar asymmetric carbons
Question 77.
Optical inactivity of meso isomer is due to
a) element of symmetry and element of asymmetry
b) internal compensation
c) due to lack of asymmetric carbon
d) External compensation
Answer:
b) internal compensation
Question 78.
A racemic mixture is a mixture of
a) meso and its isomers
b) d and l isomers of same compound in equimolar proportions
c) d and l isomers of same compound in different proportions
d) mixture of d and meso isomers
Answer:
b) d and 1 isomers of same compound in equimolar proportions
Question 79.
Which of the following is optically active?
a) HOOC – CH2 – COOH
b) CH3 – CO – COOH
c) CH3 – CH(OH) – COOH
d) CH3 – CH2 – COOH
Answer:
c) CH3 – CH(OH) – COOH
Question 80.
Which of the following is optically active?
a) n – propanal
b) 2 – chlorobutane
c) n – butanal
d) 3 – pentanol
Answer:
b) 2 – chlorobutane
![]()
Question 81.
The number of optical enantiomers of tartaric acid
a) 3
b) 2
c) 4
d) 1
Answer:
b) 2
Question 82.
Geometrical isomers differ in
a) position of substituents
b) Position of double bond
c) C – C bond length
d) Spatial arrangement of groups
Answer:
d) Spatial arrangement of groups
Question 83.
Which of the following exhibit cis – trans isomerism
a) propene
b) 1 – butene
c) 2 – butene
d) benzene
Answer:
c) 2 – butene
Question 84.
Which of the following show geometrical isomerism?
a) CH3CH = CHCH3
b) (CH3)2C = CH2
c) C2H5CH = CH2
d) CH3CH = CH2
Answer:
a) CH3CH = CHCH3
Question 85.
Which of the following does not show geometrical isomerism?
a) 1, 2 – dichloro – 1 – pentene
b) 1, 3 – dichloro – 2 – pentene
c) 1, 1 – dichloro – 1 – pentene
d) 1, 4 – dichloro – 2 – pentene
Answer:
c) 1, 1 – dichloro – 1 – pentene
![]()
Question 86.
Geometrical isomerism is not shown by
a) 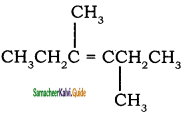
b) 
c) CH2 = C(CI) CH3
d) CH3 – CH = CH – CH = CH2
Answer:
c) CH2 = C(CI) CH3
Question 87.
Which of the following is optically active?
a) Glycerine
b) Acetaldehyde
c) Glyceraldehyde
d) Acetone
Answer:
c) Glyceraldehyde
Question 88.
The minimum number of C atoms for a hydrocarbon to exhibit optical isomerism
a) 4
b) 5
c) 6
d) 7
Answer:
d) 7
Question 89.
Which of the following can form cis – trans isomers?
a) C2H5Br
b) (CH)2(COOH)2
c) CH3CHO
d) (CH2)2COOH
Answer:
b) (CH)2(COOH)2
Question 90.
No.of geometrical isomers possible for the compound CH3 – CH = CH – CH = CH – C2H5
a) 2
b) 3
c) 4
d) 5
Answer:
c) 4
![]()
Question 91.
The number of geometrical isomers of CH3 – CH = CH – CH = CH – CH = CHCl is
a) 2
b) 4
c) 6
d) 8
Answer:
d) 8
Question 92.
Minimum number of C atoms for an alkene hydrocarbon, that shows geometrical & optical isomerism both
a) 5
b) 6
c) 7
d) 8
Answer:
c) 7
Question 93.
Among the following compounds which exhibits optical isomerism?
a) propanol
b) 2 – propanol
c) 1 – butanol
d) 2 – butanol
Answer:
d) 2 – butanol
Question 94.
Which of the mesoisomer?
a) CH2OHCHOHCHO
b) CH2OHCHOHCHOHCHO
c) HOOCCHOHCHOHCOOH
d) HOH2CCHOHCHOHCOOH
Answer:
c) HOOCCHOHCHOHCOOH
Question 95.
d – tartaric acid and l – tartaric acid can be separated by
a) Salt formation
b) Fractional distillation
c) Fractional crystallization
d) Chromatography
Answer:
a) Salt formation
![]()
Question 96.
Paper chromatography is
a) Adsorption chromatography
b) partition chromatography
c) Ion exchange chromatography
d) all of these
Answer:
b) partition chromatography
Question 97.
Simple distillation can be used to separate liquids which differ in their boiling points at least by
a) 5°C
b) 10°C
c) 40 – 50°C
d) 100°C
Answer:
c) 40 – 50°C
Question 98.
In adsorption chromatography mobile phase will be
a) Only solid
b) Only liquid
c) Only gas
d) Liquid as well as gas
Answer:
d) Liquid as well as gas
Question 99.
Which of the following can be used as adsorbent in adsorption chromatography?
a) Silica gel
b) Alumina
c) Cellulose powder
d) All of these
Answer:
d) All of these
Question 100.
Two substances when separated out on the basis of their extent of adsorption by one material, the phenomenon is called
a) Chromatography
b) Crystallization
c) Sublimation
d) Steam distillation
Answer:
a) Chromatography
![]()
Question 101.
Chromatographic technique is used for the separation of
a) Camphor
b) Alcohol & Water
c) Acetone and Methanol
d) Plant pigments
Answer:
d) Plant pigments
Question 102.
In column chromatography stationary phase is
a) only solid
b) only liquid
c) only gas
d) All of these
Answer:
a) only solid
Question 103.
Which of the following method is used for the purification of solids?
a) Distillation under reduced pressure
b) Distillation
c) Strain distillation
d) Sublimation
Answer:
d) Sublimation
Question 104.
Vacuum distillation is used to purify liquids which
a) are highly volatile
b) are explosive in nature
c) soluble in water
d) decomposes below their B.P’s
Answer:
c) soluble in water
Question 105.
Impure Napthalene is purified by
a) Fractional crystallization
b) Fractional distillation
C) solvent extraction
d) sublimation
Answer:
d) sublimation
![]()
Question 106.
A very common adsorbent used in coloun^n chromatography is
a) Powdered charcoal
b) Alumina
c) Chalk
d) Sodium carbonate
Answer:
b) Alumina
Question 107.
Simple distillation of liquids involves simultaneously
a) Vapourisation and condensation
b) Condensation and vapourisation
c) Vapourisation and sublimation
d) Sublimation and condensation
Answer:
a) Vapourisation and condensation
Question 108.
The latest technique for the purification of organic compounds is
a) Fractional distillation
b) Chromatography
c) Vacuum distillation
d) Crystallization
Answer:
b) Chromatography
Question 109.
Fixed melting point of an organic compound informs
a) Purity of an organic compound
b) Conductivity of compound
c) Chemical nature of compound
d) Whether the compound is liquid or gas
Answer:
a) Purity of an organic compound
Question 110.
Lassaigne’s test is used in qualitative analysis to detect
a) Nitrogen
b) Sulphur
c) Chlorine
d) All of these
Answer:
d) All of these
![]()
Question 111.
In Lassaigne’s method organic compound is fused with
a) Sodium metal
b) Zinc dust
c) Sodium carbonate and Zinc dust
d) Calcium metal
Answer:
a) Sodium metal
Question 112.
Presence of nitrogen in organic compound in Lassaigne’s extract as
a) Nitrogen gas
b) NH3
c) NO
d) CN–
Answer:
d) CN–
Question 113.
Medium of Sodium extract is
a) Neutral
b) Basic
c) Acidic
d) Depends on organic compound
Answer:
b) Basic
Question 114.
H2O vapours on passing through anhydrous CuSO4 turns it to
a) Green
b) Blue
c) Violet
d) White
Answer:
b) Blue
Question 115.
When a nitrogenous organic compound is fused with sodium, the nitrogen present in the compound is converted into
a) Sodium Nitrate
b) Sodium nitrite
c) Sodamide
d) Sodium cyanide
Answer:
d) Sodium cyanide
![]()
Question 116.
In the Lassainge’s test the Sulphur present in the organic compound first changes into
a) Na2SO3
b) CS2
c) Na2SO4
d) Na2S
Answer:
d) Na2S
Question 117.
Which of the following elements in an organic compound cannot be detected by Lassaigne’s test?
a) N
b) S
c) Cl
d) H
Answer:
d) H
Question 118.
A compound which does not give a positive result in the Lassaigne’s test for nitrogen is
a) Urea
b) Hydroxyl amine
c) Glycine
d) Phenylhydrazine
Answer:
b) Hydroxyl amine
Question 119.
Lassaigne’s test gives a violet colouration with sodium nitroprusside, it indicates presence of
a) N
b) S
c) O
d) Cl
Answer:
b) S
Question 120.
The presence of halogen in an organic compound is detected by
a) Iodoform test
b) Silver nitrate test
c) Beilstein’s test
d) Million’s test
Answer:
c) Beilstein’s test
![]()
Question 121.
The Beilstein’s test in a rapid test used for . organic compound^ to^ detect
a) Phosphorous
b) Sulphur
c) Halogens
d) Nitrogen
Answer:
c) Halogens
Question 122.
Liebig’s method is used for the estimation of
a) Nitrogen
b) Sulphur
c) Carbon and hydrogen
d) Halogens
Answer:
c) Carbon and hydrogen
Question 123.
In Kjeldahl’s method of estimation of nitrogen, copper sulphate act as
a) Oxidizing agent
b) reducing agent
c) Catalytic agent
d) Hydrolysing agent
Answer:
c) Catalytic agent
Question 124.
Percentage of carbon in an organic compound is determined by
a) Duma’s method
b) Kjeldahl’s method
c) Carius method
d) Liebig’s method
Answer:
d) Liebig’s method
Question 125.
Halogen can be estimated by
a) Duma’s method
b) Carius method
c) Leibig’s method
d) All of these
Answer:
b) Carius method
![]()
Question 126.
In Garius method halogens are estimated
a) X2
b) BaX2
c) PbX2
d) AgX
Answer:
d) AgX
Question 127.
In Duma’s method nitrogen in organic compound is estimated in the form of
a) N2
b) NO
c) NH3
d) N2O5
Answer:
a) N2
Question 128.
In Kjeldahl’s method to estimate nitrogen, compound is heated with conc.H2SO4 in presence of
a) CaSO4
b) (NH4)2SO4
c) CuSO4
d) P2O5
Answer:
c) CuSO4
Question 129.
In organic compounds, Sulphur is estimated as
a) BaSO4
b) BaCl2
c) Ba3(PO4)2
d) H2SO4
Answer:
a) BaSO4
Question 130.
In the Liebig’s method, if ‘w’ is the mass of compound taken and ‘x’ is the amount of C0„ formed then
a) %C = \(\frac{12 \times x}{16 \times w}\)
b) %C = \(\frac{12}{44} \times \frac{\mathrm{w}}{\mathrm{x}} \times 100\)
c) %C = \(\frac{12}{44} \times \frac{x}{w} \times 100\)
d) %C = \(\frac{12}{44} \times \frac{x}{w}\)
Answer:
c) %C = \(\frac{12}{44} \times \frac{x}{w} \times 100\)
![]()
Question 131.
In Dumas method for estimating nitrogen in organic compound, the gas finally collected is
a) N2
b) NO
c) NH3
d) H2
Answer:
a) N2
Question 132.
In Dumas method, the gas which is collected in Nitrometer is
a) N2
b) NO
c) NH3
d) H2
Answer:
a) N2
Question 133.
In Kjeldahl’s method, the nitrogen presence is estimated as
a) N2
b) NH3
c) NO2
d) N2O3
Answer:
b) NH3
Question 134.
In Kjeldahl’s method, nitrogen present in the organic compound is first converted into
a) NH3
b) (NH4)2SO4
c) N2
d) NO
Answer:
b) (NH4)2SO4
Question 135.
In Liebig’s method for the estimation of C and H, the combustion tube is passed over
a) CuO pellets
b) Copper turnings
c) Iron fillings
d) Zinc – copper couple
Answer:
a) CuO pellets
![]()
Question 136.
Which gas is introduced into the combustion tube in Liebig’s method?
a) Pure and dry CO2
b) Pure and dry N2
c) Pure and dry O2
d) Pure and dry He
Answer:
c) Pure and dry O2
Question 137.
Chromatographic techniques of purification can be used for
a) Coloured compounds
b) Liquids
c) Solids
d) All of these
Answer:
d) All of these
Question 138.
Two substances when separated on the basis of partition co – efficient between two liquid phase, then the technique is known as
a) column chromatography
b) Paper chromatography
c) GLC
d) TLC
Answer:
b) Paper chromatography
Question 139.
Ortho and para nitro phenols can be separated by
a) crystallization
b) distillation
c) sublimation
d) solvent extraction
Answer:
b) distillation
Question 140.
In steam distillation, the sum of the vapour pressure of the volatile compound and that of water is
a) Equal to atmospheric pressure
b) Less than atmospheric pressure
c) More than atmospheric pressure
d) Exactly half of the atmospheric pressure
Answer:
a) Equal to atmospheric pressure
![]()
Question 141.
Organic compound is fused with metallic sodium for testing nitrogen, sulphur, and halogens because
a) To make the solution alkaline
b) To convert into elemental state of nitrogen, sulphur, and halogens
c) To convert covalent compound into ionic compound
d) To decrease fusion temperature
Answer:
c) To convert covalent compound into ionic compound
Question 142.
Sodium extract gives blood red colour when treated with FeCl3, Formation of blood-red colour confirms the presence of
a) Only nitrogen
b) Only sulphur
c) Only halogens
d) Both Nitrogen and Sulphur
Answer:
d) Both Nitrogen and Sulphur
Question 143.
The compound not formed in the positive test for nitrogen with the Lussaigne’s solution of an organic compound is
a) Fe4[Fe(CN)6]3
b) Na3[Fe(CN)6]
c) Fe(CN)3
d) Na3[Fe(CN)5NOS]
a) b, c, d
b) a, b
c) a, b, c
d) a only
Answer:
a) b, c, d
Question 144.
The Lassaigne’s solution when heated with ferrous sulphate and acidified with sulphuric acid gave intense blue colour indicating the presence of nitrogen. The blue colour is due to the formation of
a) Na4[Fe(CN)6]
b) Fe3[Fe(CN)6]2
c) Fe2[Fe(CN)6]
d) Fe4[Fe(CN)6]3
Answer:
d) Fe4[Fe(CN)6]3
Question 145.
Which of the following compounds will answer Lassaigne’s test for nitrogen?
a) NH2NH2
b) NH2OH
c) NaCN
d) NaNO3
Answer:
c) NaCN
![]()
Question 146.
In Dumas method 0.5 g of an organic compound containing nitrogen gave 112 ml of nitrogen at S.T.P. The percentage of nitrogen in the given compound is
a) 28
b) 38
c) 18
d) 48
Answer:
a) 28
Question 147.
0.73 g of organic compound on oxidation gave 1.32 g of carbon dioxide. The percentage of carbon in the given compound will be
a) 49.32
b) 59.32
c) 29.32
d) 98.64
Answer:
a) 49.32
Question 148.
In an estimation of S by Carius method 0.217 g of the compound gave 0.5825 g of BaSO4. Percentage of S is
a) 36.78 %
b) 35.50 %
c) 36.48 %
d) 35.69 %
Answer:
a) 36.78 %
![]()
II. Very short question and answers (2 Marks):
Question 1.
What are Acyclic compounds? Give suitable example.
Answer:
These are the compounds in which carbon atoms are linked to form open chain (straight or branched). These compounds may be saturated (all single bonds) or unsaturated (multiple bonds).
Example:
These acyclic compounds are known as open chain or aliphatic compounds.
Question 2.
What arc Alicyclic Compounds? Give suitable example.
Answer:
These are saturated or unsaturated carbo-cyclic compounds which resemble the corresponding acylic compounds in their properties.
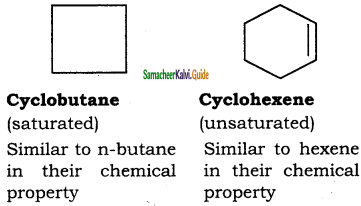
Question 3.
What are Aromatic heterocyclic Compounds? Give example.
Answer:
These are the heterocyclic compounds which possess aromaticity and resemble, the corresponding aromatic compounds in most of their properties. These are also called non-benzenoid aromatic compounds.
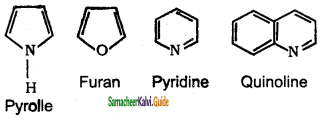
Question 4.
What is functional group? Give example.
Answer:
An atom or group of atoms within a molecule that shows a characteristics set of physical and chemical properties.
Example:
(i) – NH2 – amines
(ii) = NH – Imines
(iii) 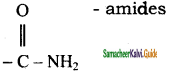
Question 5.
Explain the following terms in IUPAC system of nomenclature. of organic compounds.
(i) Root word
(ii) prefix
(iii) suffix
Answer:
(i) Root word:
Root word denotes the number of carbon atoms in the longest continuous chain in molecules.
(ii) prefix:
Prefix denotes the group(s) attached to the main chain which is placed before the root.
(iii) suffix:
Suffix denotes the funtional group and is paced after the root word.
![]()
Question 6.
What is Metamerism? With suitable examples.
Answer:
Metamerism:
This type of isomerism is a special kind of structural isomerism arises due to the unequal distribution of carbon atoms on either side of the functional group or different alkyl groups attached to the either side of the same functional group and having same molecular formula. This isomerism is shown by compounds having functional group such as ethers, ketones, esters and secondary amines between two alkyl groups.
C4H10O
CH3 – O – C3H7 – Methyl propyl ether 1 – methoxypropane
C2H5 – O – C2H5 – diethyl ether ethoxyethane
 – methyl iso – propyl ether 2 – methoxypropane.
– methyl iso – propyl ether 2 – methoxypropane.
Question 7.
What is meant by stereochemistry?
Answer:
The isomers which have same bond connectivity but different arrangement of groups or atoms in space are known as stereoisomers. This branch of chemistry dealing with the study of three-dimensional nature (spactial arrangement) of molecules is known as stereo chemistry. The metabolic activities in living organisms, natural synthesis and drug synthesis involve various stereoisomers.
Question 8.
What is enantiomerism?
Answer:
An optically active substance may exist in two or more isomeric forms which have same physical & chemical properties but differ in terms of direction of rotation of plane polarized light, such optical isomers which rotate the plane of polarized light with equal angle but in opposite direction are known as enantiomers and the phenom-enon is known as enantiomerism.
Example: d and l lactic acid.
Question 9.
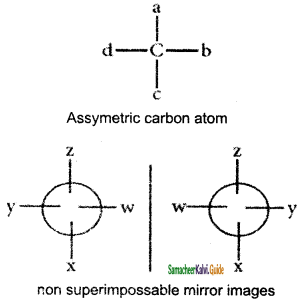
What are the conditions for an organic compound is said to be optically active?
Answer:
(1) The molecule must contains at least one chiral or Asymmetric carbon atom.
(2) The object molecule should not be super impossable with its own mirror image.
Question 10.
How will you detect phosphorus present in the given organic compound?
Answer:
Test for phosphorous:
A solid compound is strongly heated with a mixture of Na2CO3 & KNO3. Phosphorous present in the compound is oxidized to sodium phosphate. The residue is extracted with water and boiled with Conc. HNO3.. A solution of ammonium molybdate is added to the above solution. A canary yellow coloration or precipitate shows the presence of phosphorous.
![]()
Question 11.
Explain the sublimation process for the purification of organic compounds.
Answer:
Few substances like benzoic acid, naphthalene and camphor when heated pass directly from solid to vapor without melting (ie liquid). On cooling the vapours will give back solids. Such phenomenon is called sublimation. It is a useful technique to separate volatile and non-volatile solid. It has limited application because only a few substance will sublime.
Example:
naphthalene, benzoic acid.
Question 12.
What is the need for purifying an organic compound?
Answer:
In order to study the structure, physical properties, chemical properties and biological properties of organic compounds they must be in the pure state.
III. Short question and answers (3 Marks):
Question 1.
What is Homologous Series? Give suitable example.
Answer:
Homologous series:
It is a series of compounds in which the adjacent members differ by a – CH2 unit. Individual members of such series are called homologues, and the phenomenon is called as homology. All the members of such a series of alkane have general formula CnH2n + 2. Few members of this family are
CH4 – Methane
C2H6 – Ethane
C3H8 – Propane
C4H10 – Butane
C5H12 – Pentane
Question 2.
What are Alicycic heterocyclic Compounds? Give example.
Answer:
These heterocyclic compounds resemble the corresponding aliphatic compounds in most of their properties.
Example:
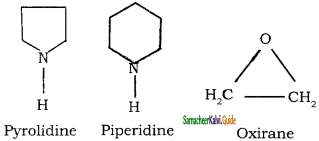
Question 3.
Write the IUPAC name of the following compounds.
a) 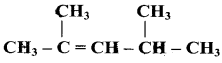
b) 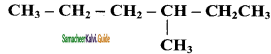
c) 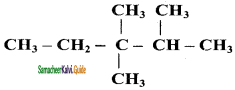
d) 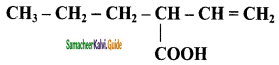
Answer:
a) 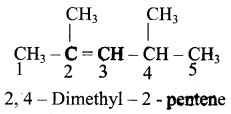
b) 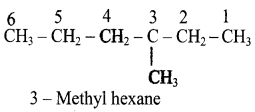
c) 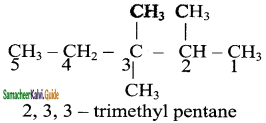
d) 
![]()
Question 4.
Write the IUPAC name of the following compounds.
Answer:
a) 
b) 
c) 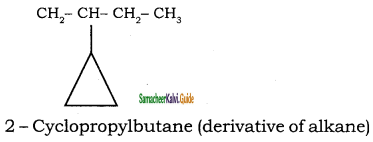
d) 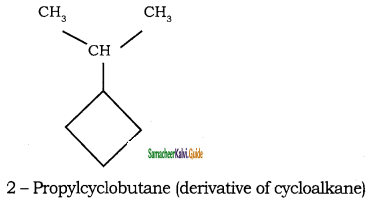
Question 5.
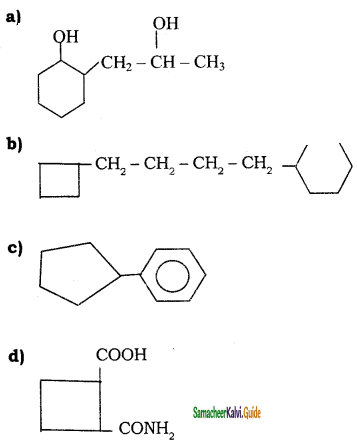
Write the IUPAC name of the following compounds.
a) 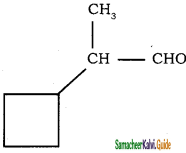
b) 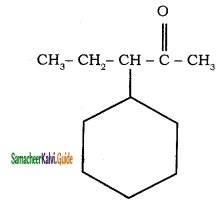
c) 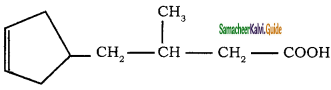
d) 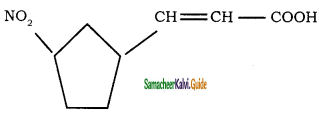
Answer:
a) 
b) 
c) 
d) 
Question 6.
Write the IUPAC name of the following compounds.
Answer:
a) 
b) 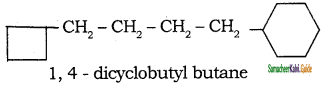
c) 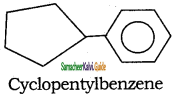
d) 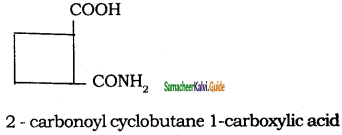
![]()
Question 7.
Write the IUPAC name of the following compounds.
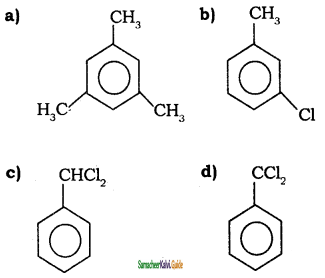
Answer:
a) 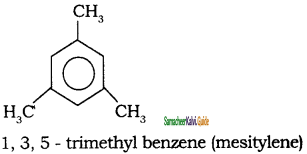
b) 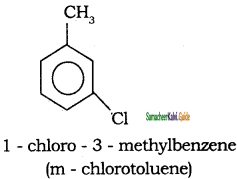
c) 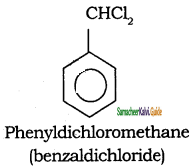
d) 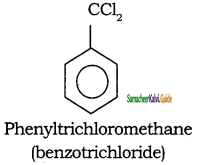
Question 8.
Classify the following compounds based on the structure.
i) CH ≡ C – CH2 – C ≡ CH
ii) CH3 – CH2 – CH2 – CH2 – CH3
iii) 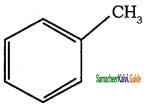
iv) 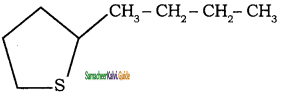
Answer:
i) CH ≡ C – CH2 – C ≡ CH is unsaturated open chain compound
ii) CH3 – CH2 – CH2 – CH2 – CH3 is saturated open chain compound
iii)  is aromatic benzenoid compound
is aromatic benzenoid compound
iv)  is alicyclic compound
is alicyclic compound
Question 9.
Give two examples for each of the following type of organic compounds.
(i) non-benzenoid aromatic
(ii) aromatic heterocyclic
(iii) alicyclic
(iv) aliphatic open chain
Answer:
(i) non-benzenoid aromatic

(ii) aromatic heterocyclic
(iii) alicyclic
(iv) aliphatic open chain
Question 10.
Explain the copper oxide test for the detection of carbon and hydrogen present in the given organic compound.
Answer:
Copper oxide test:
The organic substance is mixed intimately with about three times its weight of dry copper oxide by grinding. The mixture is then placed in a hard glass test tube fitted with a bent delivery tube. The other end of which is dipping into lime water in an another test tube. The mixture is heated strongly and the following reaction take place.
C + 2CuO → CO2 + 2CuO
2H + CuO → H2O + Cu
Thus if carbon is present, it is oxidized to CO2 which turns lime water millcy. If hydrogen is also present, it will be oxidized to water which condenses in small droplets on the cooler wall of the test tube and inside the bulb. Water collected in the bulb is separated on anhydrous CuSO4 which turn blue. This confirms the presence of C and H in the compound. If however, H is not present water droplet is not obtained in the bulb.
![]()
Question 11.
In an estimation of sulphur by carius method. 0.2175 g of the substance gave 0.5825 g of BaSO4 calculate the percentage composition of S in the compound.
Solution:
Weight of organic compound = 0.2175 g
Weight of BaSO4 = 0.5825 g
233 g of BaSO4 contains = \(\left(\frac{32}{233} \times \frac{0.5825}{0.2175}\right)\)
0.5825 g of BaSO4 contains
Percentage of S = \(\left(\frac{32}{233} \times \frac{0.5825}{0.2175} \times 100\right)\)
= 36.78 %
Question 12.
0.16 g of an organic compound was heated in a carius tube and H2SO4 acid formed
was precipitated with BaCl2. The mass of BaSO4 was 0.35 g. Find the percentage of
sulphur [30.04]
Answer:
Weight of organic substance (w) = 0.16 g
Weight of Barium sulphate (x) = 0.35 g
Percentage of sulphur = \(\left(\frac{32}{233} \times \frac{x}{w} \times 100\right)\)
= \(\left(\frac{32}{233} \times \frac{0.35}{0.16} \times 100\right)\)
= 30.04 %
Question 13.
0.284 g of an organic substance gave 0.287 g AgCl in a Carius method for the estimation of halogen. Find the percentage of Cl in the compound.
Solution:
Weight of the organic substance = 0.284 g
Weight of AgCl is = 0.287 g
143.5 g of AgCl contains = 35.5 g of chlorine
0.287 g of AgCl contains = \(\left(\frac{35.5}{143.5} \times \frac{0.287}{0.284}\right)\)
% of chlorine is = \(\left(\frac{35.5}{143.5} \times \frac{0.287}{0.284} \times 100\right)\)
= 24.56 %
Question 14.
0.185 g of an organic compound when treated with Conc. HNO3 and silver nitrate gave 0.320 g of silver bromide. Calculate the % of bromine in the compound.
(Ag = 108, Br = 80)
Answer:
Weight of organic substance (w) = 0.185 g
Weight of silver bromide (x) = 0.320 g
Percentage of bromine = \(\left(\frac{80}{188} \times \frac{x}{w} \times 100\right)\)
= \(\left(\frac{80}{188} \times \frac{0.32}{0.185} \times 100\right)\)
= 73.6%
![]()
Question 15.
0.40 g of an lodo – substituted organic compound gave 0.235 g of AgI by carlus method. Calculate the percentage of iodine in the compound. (Ag = 108 I = 127).
Solution:
Weight of organic substance (w) = 0.40 g
Weight of silver iodide (x) = 0.235 g
Percentage of iodine = \(\left(\frac{127}{235} \times \frac{x}{w} \times 100\right)\)
= \(\left(\frac{127}{235} \times \frac{0.235}{0.40} \times 100\right)\)
= 31.75%
Question 16.
0.24 g of organic compound containing phosphorous gave 0.66 g of Mg2P2O7 by the usual analysis. Calculate the percentage of phosphorous in the compound.
Solution:
Weight of an organic compound = 0.24 g
Weight of Mg2P2O7 = 0.66 g
222 g of Mg2P2O7 contains = 62 g of P
0.66 g contains = \(\frac{62}{222} \times \frac{0.66}{0.24}\)
Percentage of p = \(\frac{80}{180} \times \frac{0.66}{0.24}\) = 76.80%
Question 17.
0.33 g of an organic compound containing phosphorous gave 0.397 g of Mg2P2O7 by the analysis. Calculate the percentage of P in the compound.
Solution:
Weight of organic substance (w) = 0.33 g
Weight of Mg2P2O7 (x) = 0.397 g
Percentage of phosphorous = \(\frac{62}{222} \times \frac{x}{w} \times 100\)
= \(\frac{62}{222} \times \frac{0.397}{0.33} \times 100\) = 33. 59 %
Question 18.
Explain simple distillation process with suitable example.
Answer:
The process of distillation involves the impure liquid when boiled gives out vapour and the vapour so formed is collected and condensed to give back the pure liquid in the receiver. This method is called simple distillation. Liquids with large difference in boiling point(about 40k) and do not decompose under ordinary pressure can be purified by simple distillation.
Example:
The mixture of C6H5NO2 (b.p 484K) &
C6H6 (354 K) and mixture of diethyl ether (b.p 308K) and ethyl alcohol (b.p 35 K).
![]()
Question 19.
How will you purify an organic compound by differential extraction process?
Answer:
The process of removing a substance from its aqueous solution by shaking with a suitable organic solvent is termed extraction. When an organic substance present as solution in water can be recovered from the solution by means of a separating funnel. The aqueous solution is taken in a separating funnel with little quantity of ether or chloroform (CHCl3).
The organic solvent immiscible with water will form a separate layer and the contents are shaken gently. The solute being more soluble in the organic solvent is transfered to it. The solvent layer is then separated by opening the tap of the separating funnel, and the substance is recovered.
Question 20.
Explain the steam distillation process for purifying organic compound.
Answer:
This method is applicable for solids and liquids. If the compound to be steam distilled the compound should not decompose at the steam temperature, should have a fairly high vapour pressure at 373 K, it should be insoluble in water; the impurities present should be non-volatile.
The impure liquid along with little water is taken in a round-bottom flask which is connected to a boiler on one side and water condenser on the other side, the flask is kept in a slanting position so that no droplets of the mixture will enter into the condenser on the brisk boiling and bubbling of steam. The mixture in the flask is heated and then a current of steam passed in to it. The vapours of the compound mix up with steam and escape into the condenser.
The condensate obtained is a mixture of water and organic compound which can be separated. This method is used to recover essential oils from plants and flowers, also in the manufacture of aniline and turpentine oil.
![]()
Question 21.
Write a note on azeotropic distillation with suitable example.
Answer:
These are the mixture of liquids that cannot be separated by fractional distillation. The mixtures that can be purified only by azeotropic distillation are called as azeotropes. These azeotropes are constant boiling mixtures, which distil as a single compound at a fixed temperature. Ethanol and water are the most common examples of azeotropic mixture in the ratio of 95.87 : 4.13.
In this method apart from azeotropic mixture a third component like C6H6, CCl4, ether, glycerol, glycol which act as a dehydrating agent depress the partial pressure of one component so that the boiling point of that component is raised sufficiently and thus other component will distill over.
Dehydrating agents like C6H6, CCl4 have low boiling points and reduce the partial vapour pressure of alcohol more than that of water whereas glycerol & glycol, etc. have high boiling point and reduce the partial vapour pressure of water more than that of alcohol.
Question 22.
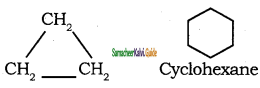
What is Ring chain isomerism? Give an example.
Answer:
In this type of isomerism, compounds having same molecular formula but differ terms of bonding of carbon atom to form open-chain and cyclic structures.
Question 23.
Briefly explain geometrical isomerism in oximes with suitable example.
Ans:
Restricted rotation around C = N (oximes) gives rise to geometrical isomerism in oximes. Here ‘syn’ and ‘anti’ are used instead of cis and trans respectively. In the syn isomer the H atom of a doubly bonded carbon and -OH group of doubly bonded nitrogen lie on the same side of the double bond, while in the anti isomer, they lie on the opposite side of the double bond.
For eg:
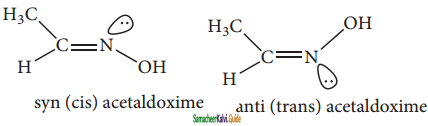
![]()
IV. Long Question and answers (5 Marks):
Question 1.
Write the structure of the following compounds.
a) Cinnamic acid
b) Lactic acid
c) Phthalic acid
d) Tartaric acid
d) Benzoic acid
Answer:
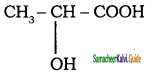
a) Cinnamic acid
b) Lactic acid
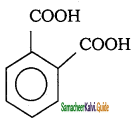
c) Phthalic acid
d) Tartaric acid
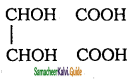
d) Benzoic acid
C6H5COOH
Question 2.
Explain the methods for the representation of structure of organic compounds with suitable example.
Answer:
The structure of an organic compound can he represented using any one of the below mentioned methods.
- Lewis structure or dot structure,
- Dash structure or line bond structure,
- Condensed structure
- Bond line structure
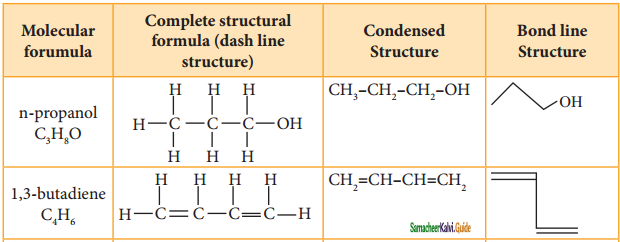
We know how to draw the Lewis structure for a molecule. The line bond structure is obtained by representing the two electron covalent bond by a dash or line (-) in a Lewis structure. A single line or dash represents single a covalent bond, double line represents double bond (1 σ bond, 2 π bond) and a triple line represents triple bond (1 σ bond, 2 π bond). Lone pair of electrons on hetero atoms may or may not be shown. This represents the complete structural formula.
This structural formula can be further abbreviated by omitting some or all of the dashes representing covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. The resulting expression of the compound is called a condensed structural formula.
For further simplification, organic chemists use another way of representing the structures in which only lines are used. In this type of representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are shown in a zigzag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. These representations can be easily understood by the following illustration.
![]()
Question 3.
Explain the molecular model method for the representation of structure of organic compounds.
Answer:
Molecular models:
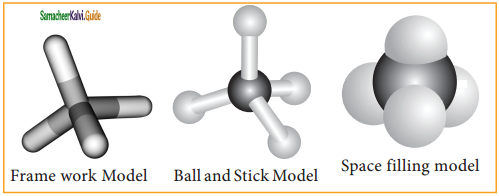
Molecular models are physical devices that are used for a better visualisation and perception of three dimensional shapes of organic molecules. These are made of wood, plastic or metal and are commercially available.
(i) Frame work model
(ii) Ball and stick model &
(iii) space filling model.
In the frame work model only the bonds connecting the atoms themselves are shown. This model emphasizes the pattern of bonds of a molecule while ignoring the size of the atom.
In the ball and stick model, both the atoms and the bonds are shown. Ball represent atoms and the stick a bond. Compounds containing C = C can be best represented by using springs in place of sticks and this model is termed as ball and spring model.
The space filling model emphasizes the relative size of each atom based on its Vander Waals radius.
Question 4.
Explain briefly
(i) Fisher projection
(ii) sawhorse projection
(iii) Newman projection formula with neat example.
Answer:
(i) Fisher projection formula:
This is a method of representing three dimensional structures in two dimension. In this method, the chiral atom(s) lies in the plane of paper. The horizontal substituents are pointing towards the observer and the vertical substituents are away from the observer. Fisher projection formula for tartaric acid is given below.
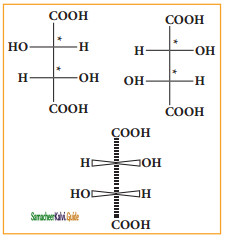
Sawhorse projection formula:
Here the bond between two carbon atoms is drawn diagonally and slightly elongated. The lower left hand carbon is considered lying towards the front and the upper right hand carbon towards the back. The Fischer projection inadequately portrays the spatial relationship between ligands attached to adjacent atoms. The sawhorse projection attempts to clarify the relative location of the groups.
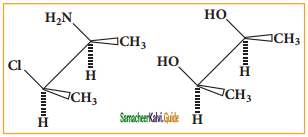
Newman projection formula:
In this method the molecules are viewed from the front along the carbon-carbon bond axis. The two carbon atom forming a bond is represented by two circles. One behind the other so that only the front carbon is seen. The front carbon atom is shown by a point whereas the carbon lying further from the eye is represented by the origin of the circle. Therefore, the C-H bonds of the front carbon are depicted from the circle while C-H bonds of the back carbon are drawn from the circumference of the circle with an angle of 120° to each other.
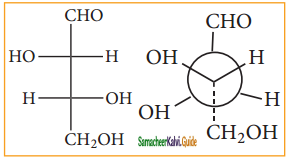
![]()
Question 5.
What is Tautomerism? Explain different types of Tautomerism with suitable example.
Answer:
Tautomerism:
It is a special type of functional isomerism in which a single compound exists in two readily inter con-vertible structures that differ markedly in the relative position of atleast one atomic nucleus, generally hydrogen. The two dif¬ferent structures are known as tautomers. There are several types of tautomerism and the two important types are dyad and triad systems.
Dyad system:
In this system hydrogen atom oscillates between two directly linked polyvalent atoms.
Example:
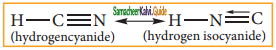
In this example hydrogen atom oscillates between carbon & nitrogen atom.
Triad system:
In this system hydrogen atom oscillates between three polyvalent atoms. It involves 1, 3 migration of hydrogen atom from one polyvalent atom to other within the molecule. The most important type of triad system is keto-enol tautomerism and the two groups of tautomers are ketoform and enol-form. The polyvalent atoms involved are one oxygen and two carbon atoms. Enolisation is a process in which keto-form is converted to enol form. Both tautomeric forms are not equally stable. The less stable form is known as labile form.
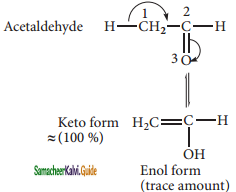
Question 6.
How is sulphur detected by Lassaigne test?
Answer:
a) To a portion of the lassaigne’s extract, add freshly prepared sodium nitro prusside solution. A deep violet colouration is obtained. This test is also used to detect S2- in inorganic salt analysis.
Na2S + Na2 [Fe(CN5) NO] → Na4 [Fe (CN5) NOS]
sodium nitro prusside
b) Acidify another portion of lassaigne’s extract with acetic acid and add lead acetate solution. A black precipitate is obtained.
(CH3COO)2Pb + Na2S → PbS↓ + 2CH3COONa
(black ppt)
c) Oxidation test:
The organic substances are fused with a mixture of KNO3 and Na2CO3. The sulphur, if present is oxidized to sulphate.
Na2CO3 + S + 3O + Na2SO4 + CO2
The fused mass is extracted with water, acidified with HCl and then BaCl2 solution is added to it. A white precipitate indicates the pressure of sulphur.
BaCl2 + Na2SO4 -> BaSO4 + 2NaCl
![]()
Question 7.
How is halogen detected by using Lassaigne test?
Answer:
To another portion of the Lassaigne’s filtrate add dil HNO3 warm gently and add AgNO3
Solution:
a) Appearance of curdy white precipitate soluble in ammonia solution indicates the presence of chlorine.
b) Appearance of pale yellow precipitate sparingly soluble in ammonia solution indicates the presence of bromine.
c) Appearance of a yellow precipitate insoluble in ammonia solution indicates the presence of iodine.
Na + ![]() NaX ( where X = Cl, Br, I)
NaX ( where X = Cl, Br, I)
from organic compound
NaX + AgNO3 → AgX + NaNO3
If N or S is present in the compound along with the halogen, we might obtain NaCN and Na2S in the solution, which interfere with the detection of the halogen in the AgNO3 test Therefore we boil the lassaignes extract with HNO3 which decomposes NaCN and Na2S as
NaCN + HNO3 ![]() NaNO3 + HCN T
NaNO3 + HCN T
Na2S + 2HNO3 ![]() 2NaNO3 + H2S
2NaNO3 + H2S
NaCN + AgNO3 ![]() AgCN + NaNO3
AgCN + NaNO3
white ppt confusing with AgCl
Na2S + AgNO3 → Ag2S ↓ + NaNO3
black ppt
Question 8.
How will you estimate carbon and hydrogen present in the given organic compound?
Answer:
Both carbon and hydrogen are estimated by the same method. A known weight of the organic substance is burnt in excess of oxygen and the carbon and hydrogen present in it are oxidized to carbon dioxide and water, respectively.
CXHY + O2 + XCO2 + \(\frac{\mathrm{y}}{2}\)H2O
The weight of carbon dioxide and water thus formed are determined and the amount of carbon and hydrogen in the original substance is calculated. The apparatus employed for the purpose consists of three units (1) oxygen supply (2) combustion tube (3) absorption apparatus.
Oxygen supply:
To remove the moisture from oxygen it is allowed to bubble through sulphuric acid contained in a drech gel bottle and then passed through a U-tube charged with soda lime to remove CO2. The oxygen gas free from moisture and carbon dioxide enters the combustion tube.
Combustion tube:
A hard glass tube open at both end is used for the combustion of the organic substance. It is filled with
(i) a role of oxidized copper gauze to prevent the backward diffusion of the product of combustion
(ii) a porcelain boat containing a known weight of the organic substance
(iii) coarse copper oxide packed in about 2/3 of the entire length of the tube, and kept in position by loose asbestos plugs on either side and
(iv) a roll of oxidized copper gauze placed towards the end of the combustion tube to prevent any vapours of the organic substance having the tube unoxidized. The combustion tube is enclosed in a furnace, and heated by a gas burner.
Absorption Apparatus:
The products of combustion containing moisture and carbon dioxide are then passed through the absorption apparatus which consists of
(i) a weighed U-tube packed with pumice soaked in Conc. H2SO4 to absorb water
(ii) a set of bulbs containing strong solution of KOH to absorb CO2 and finally
(iii) a guard tube filled with anhydrous CaCl2 to prevent the entry of moisture from atmosphere.
Procedure:
To start with, before loading it with the boat, the combustion tube is detached from the absorption unit. The tube is heated strongly to dry its content and CO2 present in it is removed by passing a current of pure, dry oxygen through it. It is then cooled slightly and connected to the absorption apparatus. The other end of the combustion tube is open for a while and the boat containing weighed organic substance is introduced.
The tube is again heated strongly till the substance in the boat is burnt away. This takes about 2 hours. Finally, a strong current of oxygen is passed through the combustion tube to sweep away any traces of carbon dioxide or moisture which may have been left in it. The U-tube and the potash bulbs are then detached and the increase in weight of each of them is determined.
Calculation:
Weight of the organic substance taken = w g
Increase in weight of H2O = x g
Increase in weight of CO2 = y g
18 g of H2O contain 2 g of hydrogen
∴ x g of H2O contain = \(\left(\frac{2}{18} \times \frac{x}{w}\right)\)
% of hydrogen = \(\left(\frac{2}{18} \times \frac{x}{w} \times 100\right)\) %
44 g of CO2 contains = 12 g of carbon
∴ y g of CO2 contain = \(\left(\frac{12}{44} \times \frac{y}{w}\right)\) g of carbon
Percentage of carbon = \(\left(\frac{12}{44} \times \frac{y}{w} \times 100\right)\) %
![]()
Question 9.
Explain the Carius method for the estimation of sulphur in an organic compound.
Answer:
Estimation of sulphur Carius method:
A known mass of the organic substance is heated strongly with fuming HNO3, C & H get oxidized to CO2 & H2O while sulphur is oxidized to sulphuric acid as per the following reaction.
C ![]() CO2
CO2
2H ![]() H2O
H2O
S → SO2 ![]() H2SO4
H2SO4
The resulting solution is treated with excess of BaCl2 solution H2SO4 present in the solution in quantitatively converted into BaSO4, from the mass of BaSO4, the mass of sulphur and hence the percentage of sulphur in the compound can be calculated.
Procedure:
A known mass of the organic compound is taken in clean carius tube and added a few mL of fuming HNO3. The tube is the sealed. It is then placed in an iron tube and heated for about 5 hours. The tube is allowed to cool to temperature and a small hole is made to allow gases produced inside to escape.
The carius tube is broken and the content collected in a beaker. Excess of BaCl2 is added to the beaker H2SO4 acid formed as a result of the reaction is converted to BaSO4. The precipitate of BaSO4 is filtered, washed, dried and weighed. From the mass of BaSO4, percentage of S is found.
Mass of the organic compound = w g
Mass of the BaSO4 formed = x g
233 g of BaSO4 contains = 32 g of sulphur
∴ x g of BaSO4 contain = \(\left(\frac{32}{233} \times \frac{x}{w}\right)\) g of S
Percentage of sulphur = (\(\left(\frac{32}{233} \times \frac{x}{w}\right)\) × 100) %
Question 10.
0.26 g of an organic compound gave 0.039 g of water and 0.245 g of carbon dioxide on combustion. Calculate the percentage of C & H.
Answer:
Weight of organic compound = 0.26 g
Weight of water = 0.039 g
Weight of CO2 = 0.245 g
Percentage of hydrogen
18 g of water contain 2 g of hydrogen
0.039 of water contain = \(\left(\frac{2}{18} \times \frac{0.039}{0.26}\right)\) of hydrogen
% of hydrogen = \(\left(\frac{0.039}{0.26} \times \frac{2}{18} \times 100\right)\) = 1.66%
Percentage of Carbon
44 g of CO2 contain 12 g of C
0.245 g of CO2 contains \(\left(\frac{12}{44} \times \frac{0.245}{0.26}\right)\) g of C
% of Carbon = \(\left(\frac{12}{44} \times \frac{0.245}{0.26} \times 100\right)\) = 25.69 %
![]()
Question 11.
0.2346 of an organic compound yielded C, H & 0 0.2754 g of H2O and 0.4488 g CO2. Calculate the % composition.
Solution:
Weight of organic substance w = 0.2346 g
Weight of water (x) = 0.2754 g
Weight of CO2 (y), = 0.4488 g
Percentage of carbon = \(\left(\frac{12}{44} \times \frac{y}{w} \times 100\right)\)
= \(\left(\frac{12}{44} \times \frac{0.4488}{0.2346} \times 100\right)\) = 52.17 %
Percentage of hydrogen = \(\left(\frac{2}{18} \times \frac{x}{w} \times 100\right)\)
= \(\left(\frac{2}{18} \times \frac{0.2754}{0.2346} \times 100\right)\) = 13. 04%
Percentage of oxygen= [100 – (52.17 + 13.04)]
= 100 – 65.21 = 34.79 %
Question 12.
Explain the estimation of phosphours by Carius method.
Answer:
Carius method:
A known mass of the organic compound (w) containing phosphorous is heated with fuming HNO in a sealed tube where C is converted into CO2 and H to H2O. phosphorous present in organic compound is oxidized to phosphoric acid which is precipitated, as ammonium phosphomolybdate by heating with Cone. HNO3 and then adding ammonium molybdate.
H3PO4 + 12(NH4)2MoO4 + 21 HNO3 ![]() (NH4)3PO4 + 21 NH4NO3 + 12HNO3
(NH4)3PO4 + 21 NH4NO3 + 12HNO3
The precipitate of ammonium phosphomolybdate thus formed is filtered washed, dried, and weighed. In an alternative method, the phosphoric acid is precipitated as magnesium-ammonium phosphate by adding magnesia mixture (a mixture containing MgCl2, NH4Cl, and ammonia) This ppt is washed, dried, and ignited to get magnesium pyrophosphate which is washed, dried a weighed. The following are the reaction that takes place.
By knowing the mass of the organic compound and the, mass of ammonium phosphomolybdate or magnesium pyrophosphate formed, the percentage of P is calculated.
Mass of organic compound = w g
Weight of ammonium phosphomolybdate = x g
Weight of magnesium pyrophosphate = y g
Mole mass of (NH4)3PO4. 12MoO3 is = 1877 g
[3 × (14 + 4) + 31 + 4(16)]+ 12(96 + 3 × 16)
Molar mass of Mg3P2O7 is 222 g
(2 × 24) + (31 × 2) + (7 × 16)
1877 g of (NH4)3PO4. 12 MoO3 contains 31 g of P
X g of (NH4)3PO4. 12 MoO3 in w g of organic
compound contains \(\frac{31}{1877} \times \frac{x}{w}\) of phosphorous
Percentage of phosphorous = \(\frac{31}{1877} \times \frac{x}{w}\) (or)
227 of Mg2P2O7 contains 62 g of P
y g of Mg2P2O2 in w g of Organic compound contains = \(\frac{62}{227} \times \frac{y}{w}\) of P
Percentage of phosphorous = \(\frac{62}{227} \times \frac{y}{w}\)%
![]()
Question 13.
Explain the estimation of nitrogen by Dumas method.
Answer:
1. Dumas method:
This method is based upon the fact that nitrogenous compound when heated with cupric oxide in an atmosphere of CO2 yields free nitrogen. Thus
Cx Hy Nz + (2x + y/2) CuO → x CO2 + y/2 H2O Z/2 N2 +(2X + y/2)Cu.
Traces of oxide of nitrogen, which may be formed in some cases, are reduced to elemental nitrogen by passing overheated copper spiral. The apparatus used in Dumas method consists of CO2 generator, combustion tube, Schiffs nitrometer.
CO2 generator:
CO2 needed in this process is prepared by heating magnetite or sodium bicarbonate contained in a hard glass tube or by the action of dil. HCl on marble in a kipps apparatus. The gas is passed through the combustion tube after being dried by bubbling through Cone. H2SO4 contained in a Drechel bottle.
Combustion Tube:
The combustion tube is heated in a furnace is charged with
a) A roll of oxidized copper gauze to prevent the back diffusion of the products of combustion and to heat the organic substance mixed with CuO by radiation
b) a weighed amount of the organic substance mixed with an excess of CuO,
c) a layer, of course, CuO packed in about 2/3 of the entire length of the tube and kept in position by loose asbestos plug on either side; this oxidizes the organic vapors passing through it, and
d) a reduced copper spiral which reduces any oxides of nitrogen formed during combustion to nitrogen.
Schiff’s nitro meter:
The nitrogen gas obtained by the decomposition of the substance in the combustion tube is mixed with considerable excess of CO2 It is estimated by passing nitrometer when CO2 is absorbed by KOH and the nitrogen gets collected in the upper part of graduated tube.
Procedure:
To start with the tap of nitro meter is left open. CO2 is passed through the combustion tube to expels the air in it. When the gas bubbles rising through, the potash solution fails to reach the top of it and is completely absorbed it shows that only CO2 is coming and that all air has been expelled from the combustion tube. The nitrometer is then filled with KOH solution by lowering the reservoir and the tap is closed. The combustion tube is now heated in the furnace and the temperature rises gradually.
The nitrogen set free from the compound collects in the nitro meter. When the combustion is complete a strong current of CO2 is sent through, the apparatus in order to sweep the last trace of nitrogen from it. The volume of the gas gets collected is noted after adjusting the reservoir so that the solution in it and the graduated tube is the same. The atmospheric pressure and the temperature are also recorded.
Calculation:
Weight of the substance taken = w g
Volume of nitrogen = V1 L
Room Temperature = T1 K
Atmospheric Pressure = P mm of Hg
Aqueous tension at room temperature = P’ mm of Hg
Pressure of diy nitrogen = (P – P’) = P’ mm of Hg.
Let P0V0 and T0 be the pressure, volume and temperature respectively of dry nitrogen at STP,
Then,
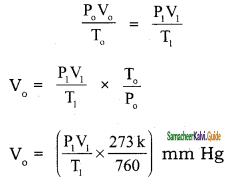
Calculation of percentage of nitrogen, 22.4 L of N2 at STP weight 28 g of N2
∴ V0 L of N2 at S.T.P weigh = \(\frac{28}{22.4}\) × V0
Wg Organic compound contain = \(\left(\frac{28}{22.4} \times \frac{\mathrm{V}_{\mathrm{o}}}{\mathrm{W}}\right)\) of nitrogen
∴ percentage of nitrogen = \(\left(\frac{28}{22.4} \times \frac{\mathrm{V}_{\mathrm{o}}}{\mathrm{W}}\right)\) × 100
![]()
Question 14.
0.1688 g when analyzed by the Dumas method yield 31.7 ml of moist nitrogen measured at 14°C and 758 mm mercury pressure. Determine the % of N in the substance (Aqueous tension at 14°C = 12 mm)
Solution:
Weight of Organic compound = 0.168 g
Volume of moist nitrogen (V1) = 31.7 mL
= 31.7 × 10-3 L
Temperature (T1) = 14°C = 14 + 273 = 287 K
Pressure of Moist nitrogen (P) = 758 mm Hg
Aqueous tension at 14°C =12 mm of Hg
∴ Pressure of dry nitrogen = (P – P1)
= 758 – 12 = 746 mm of Hg
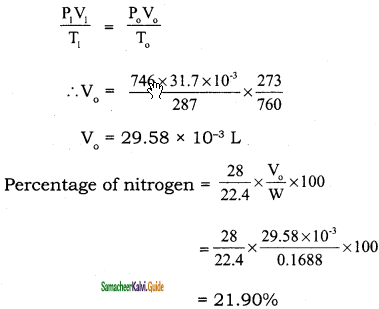
Question 15.
Explain the estimation of nitrogen by Kjeldahl’s method.
Answer:
This method is carried much more easily than the Dumas method. It is used largely in the analysis of foods and fertilizers. Kjeldahl’s method is based on the fact that when an organic compound containing nitrogen is heated with Conc. H2SO4, the nitrogen in it is quantitatively converted to ammonium sulphate. The resultant liquid is then treated with excess of alkali and then liberated ammonia gas absorbed in excess of standard acid. The amount of ammonia (and hence nitrogen) is determined by finding the amount of acid neutralized by back titration with same standard alkali.
Procedure:
A weighed quantity of the substance (0.3 to 0.5 g) is placed in a special long – necked Kjeldahl flask made of pyrex glass. About 25 mL of Cone. H2SO4 together with a little K2SO4 and CuSO 4 (catalyst) are added to it the flask is loosely stoppered by a glass bulb and heated gently in an inclined position. The heating is continued till the brown color of the liquid first produced, disappears leaving the contents clear as before. At this point all the nitrogen in the substance is converted to (NH4)2SO4.
The Kjeldahl flask is then cooled and its contents are diluted with same distilled water and then carefully transferred into a 1 lit round bottom flask. An excess NaOH solution is poured down the side of the flask and it is fitted with a Kjeldahl trap and a water condenser. The lower end of the condenser dips in a measured volume of excess the H2SO4 solution. The liquid in the round bottom flask is then heated and the liberated ammonia is distilled into sulphuric acid. The Kjeldahl trap serves to retain any alkali splashed up on vigorous boiling.
When no more ammonia passes over (test the distillate with red litmus) the receiver is removed. The excess of acid is then determined by titration with alkali, using phenolphthalein as the indicator.
Calculation:
Weight of the substance = w g
Volume of H2SO4 required for the complete neutralization of evolved NH3 = V ml.
Strength of H2S04 used to neutralize NH3 = N
Let the Volume and the strength of NH3 formed are V1 and N1 respectively.
We know that V1N1 = VN
The amount of nitrogen present in the = \(\frac{14 \times \mathrm{NV}}{1 \times 1000 \times \mathrm{w}}\)
Percentage of Nitrogen = \(\left(\frac{14 \times \mathrm{NV}}{1000 \times \mathrm{w}}\right)\) × 100 %
![]()
Question 16.
0.6 g of an organic compound was kjeldalised and NH3 evolved was absorbed into 50 mL of semi – normal solution of H2SO4. The residual acid solution was diluted with distilled water and the volume made up to 150 mL. 20 mL of this diluted solution required 35 mL of \(\frac{\mathbf{N}}{20}\) NaOH solution for complete neutralization. Calculate the % of N in the compound.
Solution:
Weight of Organic compound = 0.6 g
Volume of sulphuric acid taken = 50 mL
Strength of sulphuric acid taken = 0.5 N
20ml of diluted solution of unreacted sulphuric acid was neutralized by 35 mL of 0.05 N Sodium hydroxide
Strength of the diluted sulphuric aicd = \(\frac{35 \times 0.05}{20}\) = 0.0875 N
Volume of the sulphuric acid remaining after reaction with Organic compound = V1 mL
Strength of the diluted H2SO4 = 0.5 N
Volume of the diluted H2SO4 = 150 mL
Strength of the diluted H2SO4 = 0.0875 N
V1 = \(\frac{150 \times 0.0875}{0.5}\) = 26.25 ml
Volume of H2SO4 consumed by ammonia = 50 – 26.25 = 23.75 ml
23.75 ml of 0.5 N H2SO4 = 23.75 ml of 0.5 N NH3
The amount of Nitrogen present in the 0.6 g of organic compound = \(\frac{14 g}{1000 m L \times 1 N}\) × 23.75 × 0.5 N = 0.166 g
Percentage of Nitrogen = \(\frac{0.166}{0.6}\) × 100 = 27.66%
Question 17.
Explain the various steps involved in the purification of organic compounds by crystallization process.
Answer:
(i) Selection of solvent:
Most of the organic substances being covalent do not dissolve in polar solvents like water, hence selection of solvent (suitable) becomes necessary. Hence the powdered organic substance is taken in a test tube and the solvent is added little by little with constant stirring and heating, till the amount added is just sufficient to dissolve the solute (ie) organic compound. If the solid dissolves upon heating and throws out maximum crystals on cooling, then the solvent is suitable. This process is repeated with other solvents like benzene, ether, acetone, and alcohol till the most suitably one is sorted out.
(ii) Preparation of solution:
The quantity to be purified is taken in a conical flash fitted with a reflux condenser. The solvent selected in first stage is also taken along with solute and the quantity of the solvent just enough to dissolve the whole solid on boiling. Small amount of are animal char-coal can be added before boiling to decolorize any colored substance. The heating may be done over a wire gauze or water bath depending upon the nature of liquid (ie) whether the solvent is low boiling or high boiling.
(iii) Filtration of hot solution:
The solution so obtained is filtered through a fluted filter paper placed in a hot water funnel.
(iv) Crystallization:
The hot filtrate is then allowed to cool. Most of the impurities are removed on the filter paper, the pure solid substance separate as crystal. When copious amount of crystal has been obtained, then the crystallization is complete. If the rate of crystallization is slow, it is induced either by scratching the walls of the beaker with a glass rod or by adding a few crystals of the pure compounds to the solution.
(v) Isolation and drying of crystals:
The crystals are separated from the mother liquor by filtration. Filtration is done under reduced pressure using a Bucher funnel. When the whole of the mother liquor has been drained into the filtration flask, the crystals are washed with small quantities of the pure cold solvent and then dried.
![]()
Question 18.
How will you purify an organic compound by Thin layer chromatography?
Answer:
This method is another type of adsorption chromatography with this method it is possible to separate even minute quantities of mixtures. A sheet of a glass is coated with a thin layer of adsorbent (cellulose, silica gel or alumina). This sheet of glass is called chromoplate or thin layer chromatography plate. After drying the plate, a drop of the mixture is placed just above one edge and the plate is then placed in a closed jar containing eluent (solvent). The eluent is drawn up to the adsorbent layer by capillary action.
The components of the mixture move up along with the eluent to different distances depending upon their degree of adsorption of each component of the mixture. It is expressed in terms of its retardation factors (ie) Rf value
Rf = \(\frac{\text { Distance moved by the substance from baseline }(\mathrm{x})}{\text { Distance moved by the solvent from baseline }(\mathrm{y})}\)
The spots of colored compounds are visible on TLC plate due to their original color. The colorless compounds are viewed under UV light or in another method using iodine crystals or by using an appropriate reagent.