Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Chemistry Guide Pdf Chapter 9 Solutions Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions
11th Chemistry Guide Solutions Text Book Back Questions and Answers
Textbook Evaluation:
I. Choose the best answer:
Question 1.
The molality of a solution containing 1.8 g of glucose dissolved in 250 g of water is
a) 0.2 M
b) 0.01 M
c) 0.02 M
d) 0.04 M
Answer:
d) 0.04 M
Question 2.
Which of the following concentration terms is / are independent of temperature
a) molality
b) molarity
c) mole fraction
d) a and b
Answer:
d) a and b
Question 3.
Stomach acid, a dilute solution of HCl can be neutralized by reaction with aluminium hydroxide Al(OH)3 + 3HCl (aq) -> AlCl3 + 3H2O. How many milliliters of 0.1 M Al(OH)3 solution is needed to neutralize 21 ml of 0.1 M HCl?
a) 14 mL
b) 7 mL
c) 21 mL
d) none of these
Answer:
b) 7 mL
Question 4.
The partial pressure of nitrogen in air is 0.76 atm and its Henry’s law constant is 7.6 × 104 atm at 300 K. What is the mole fraction of nitrogen gas in the solution obtained when air is bubbled through water at 300 K ?
a) 1 × 10-4
b) 1 × 104
c) 2 × 10-5
d) 1 × 10-5
Answer:
d) 1 × 10-5
Question 5.
The Henry’s law constant for the solubility of Nitrogen gas in water at 350 K is 8 × 104 atm. The mole fraction of nitrogen in air is 0.5. The number of moles of Nitrogen from air dissolved in 10 moles of water at 350 K and 4 atm pressure is
a) 4 × 10-4
b) 4 × 104
c) 2 × 10-2
d) 2.5 × 10-4
Answer:
d) 2.5 × 10-4
![]()
Question 6.
Which one of the following is incorrect for an ideal solution?
a) ∆Hmix = 0
b) ∆Umix =0
c) ∆P = Pobserved – Pcalculated by Raoults law = 0
d) ∆Gmix = 0
Answer:
d) ∆Gmix = 0
Question 7.
Which one of the following gases has the lowest value of Henry’s law constant?
a) N2
b) He
c) CO2
d) H2
Answer:
c) CO2
Question 8.
P1 and P2 are the vapour pressures of pure liquid components, 1 and 2 respectively of an ideal binary solution If x1 represents the mole fraction of component 1, the total pressure of the solution formed by 1 and 2 will be
a) P1 + x1 (P2 – P1)
b) P2 – x1 (P2 + P1)
c) P1 – x2(P1 – P2)
d) P1 + x2(P1 – P2)
Answer:
c) P1 – x2(P1 – P2)
Question 9.
Osomotic pressure (π) of a solution is given by the relation
a) π = nRT
b) πV = nRT
c) πRT = n
d) none of these
Answer:
b) πV = nRT
Question 10.
Which one of the following binary liquid mixtures exhibits positive deviation from Raoults law?
a) acetone + chloroform
b) water + nitric acid
c) HCl + water
d) ethanol + water
Answer:
d) ethanol + water
![]()
Question 11.
The Henry’s law constants for two gases A and B are x and y respectively. The ratio of mole fractions of A to B 0.2. The ratio of mole fraction of B and A dissolved in water will be
a) \(\frac{2 x}{y}\)
b) \(\frac{y}{0.2 x}\)
c) \(\frac{0.2 x}{y}\)
d) \(\frac{5 x}{y}\)
Answer:
d) \(\frac{5 x}{y}\)
Question 12.
At 100°C the vapour pressure of a solution containing 6.5g a solute in 100g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
a) 102°C
b) 100°C
c) 101°C
d) 100.52°C
Answer:
c) 101°C
Question 13.
According to Raoults law, the relative lowering of vapour pressure for a solution is equal to
a) mole fraction of solvent
b) mole fraction of solute
c) number of moles of solute
d) number of moles of solvent
Answer:
b) mole fraction of solute
Question 14.
At same temperature, which pair of the following solutions are isotonic?
a) 0.2 M BaCl2 and 0.2 M urea
b) 0.1 M glucose and 0.2 M urea
c) 0.1 M NaCl and 0.1 M K2SO4
d) 0.1 M Ba(NO3)2 and 0.1 M Na2SO4
Answer:
d) 0.1 M Ba(NO3)2 and 0.1 M Na2SO4
Question 15.
The empirical formula of a non – electrolyte (X) is CH2O. A solution containing six grams of X exerts the same osmotic pressure as that of 0.025 M glucose solution at the same temperature. The molecular formula of X is
a) C2H4O2
b) C8H16O8
c) C4H8O4
d) CH2O
Answer:
b) C8H16O8
![]()
Question 16.
The KH for the solution of oxygen dissolved in water is 4 × 104 atm at a given temperature. If the partial pressure of oxygen in air is 0.4 atm, the mole fraction of oxygen in solution is
a) 4.6 × 103
b) 1.6 × 104
c) 1 × 10-5
d) 1 × 105
Answer:
c) 1 × 10-5
Question 17.
Normality of 1.25 M sulphuric acid is
a) 1.25 N
b) 3.75 N
c) 2.5 N
d) 2.25 N
Answer:
c) 2.5 N
Question 18.
Two liquids X and Y on mixing gives a warm solution. The solution is
a) ideal
b) non-ideal and shows positive deviation from Raoults law
c) ideal and shows negative deviation from Raoults Law
d) non-ideal and shows negative deviation from Raoults Law
Answer:
d) non-ideal and shows negative deviation from Raoults Law
Question 19.
The relative lowering of vapour pressure of a sugar solution in water is 2.5 × 10-3. The mole fraction of water in that solution is
a) 0.0035
b) 0.35
c) 0.0035/18
d) 0.9965
Answer:
d) 0.9965
Question 20.
The mass of a non – volatile solute (molar mass 80 g mol-1) which should be dissolved in 92g of toluene to reduce its vapour pressure to 90%
a) 10 g
b) 20 g
c) 9.2 g
d) 8 g
Answer:
d) 8 g
![]()
Question 21.
For a solution, the plot of osmotic pressure (π) versus the concentration (c in mol L-1) gives a straight line with slope 310 R where ‘R’ is the gas constant. The temperature at which osmotic pressure measured is
a) 310 × 0.082 K
b) 310° C
c) 37°C
d) \(\frac{310}{0.082}\) K
Answer:
c) 37°C
Question 22.
200 ml of an aqueous solution of a protein contains 1.26 g of protein. At 300 K, the osmotic pressure of this solution is found to be 2.52 × 10-3 bar. The molar mass of protein will be (R = 0.083 L bar mol-1 K-1}
a) 62.22 kg mol-1
b) 12444 g mol-1
c) 300 g mol-1
d) None of these
Answer:
a) 62.22 kg mol-1
Question 23.
The Van’t Hoff factor (i) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
a) 0
b) 1
c) 2
d) 3
Answer:
d) 3
Question 24.
Which is the molality of a 10% w/w aqueous sodium hydroxide solution?
a) 2.778
b) 2.5
c) 10
d) 0.4
Answer:
b) 2.5
Question 25.
The correct equation for the degree of an associating solute, ‘n’ molecules of which undergoes association in solution, is
a) α = \(\frac{\mathrm{n}(\mathrm{i}-1)}{\mathrm{n}-1}\)
b) α2 = \(\frac{n(1-i)}{(n-1)}\)
c) α = \(\frac{\mathrm{n}(\mathrm{i}-1)}{1-\mathrm{n}}\)
d) α = \(\frac{\mathrm{n}(1-\mathrm{i})}{\mathrm{n}(1-\mathrm{i})}\)
Answer:
c) α = \(\frac{\mathrm{n}(\mathrm{i}-1)}{1-\mathrm{n}}\)
![]()
Question 26.
Which of the following aqueous solutions has the highest boiling point?
a) 0.1 M KNO3
b) 0.1 M Na3PO4
c) 0.1 M BaCl2
d) 0.1 M K2SO4
Answer:
b) 0.1 M Na3PO4
Question 27.
The freezing point depression constant for water is 1.86° K Kg mol-1. If 5 g Na2SO4 is dissolved in 45 g water, the depression in freezing point is 3.64°C. The Vant Hoff factor for Na2SO4 is
a) 2.57
b) 2.63
c) 3.64
d) 5.50
Answer:
a) 2.57
Question 28.
Equimolal aqueous solutions of NaCl and KCl are prepared,. If the freezing point of NaCl is -2°C, the freezing point of KCl solution is expected to be
a) -2°C
b) -4°C
c) -1°C
d) 0°C
Answer:
a) -2°C
Question 29.
Phenol dimerises in benzene having van’t Hoff factor 0.54. What is the degree of association?
a) 0.46
b) 92
c) 46
d) 0.92
Answer:
d) 0.92
Question 30.
Assertion:
An ideal solution obeys Raoults Law.
Reason:
In an ideal solution, solvent – solvent as well as solute – solute interactions are similar to solute-solvent interactions.
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false
Answer:
a) both assertion and reason are true and reason is the correct explanation of assertion
![]()
II. Write brief answer to the following questions:
Question 31.
Define:
(i) Molality
(ii) Normality
Answer:
(i)Molality :
Molality (m) is defined as the number of moles of the solute dissolved in one kilogram (Kg) of the solvent. The units of molality are moles per kilogram, i.e., mole kg-1. The molality is preferred over molarity if volume of the solution is either expanding or contracting with temperature.
molality (m) = \(\frac{\text { Number of mole of solute }}{\text { mess of solvent in } \mathrm{kg}}\)
ii) Normality:
Normality (N) of a solution is defined as the number of gram equivalents of the solute present in one liter of the solution. Normality is used in acid-based redox titrations.
Normality (N) = \(\frac{\text { Number of gram equivalents of solute }}{\text { Volume of solution in litre }}\)
Question 32.
a) What is a vapour pressure of liquid?
Answer:
“The pressure exerted by the vapors above the liquid surface which is in equilibrium with the liquid at a given temperature is called vapor pressure”.
b) What is a relative lowering of vapour pressure?
Answer:
The relative lowering of vapour pressure is defined as the ratio of lowering of vapour pressure to the vapour pressure
of pure solvent (P0) RLVP = \(\frac{p^{0}-P}{P^{0}}\)
Question 33.
State and explain Henry’s law.
Answer:
“The partial pressure of the gas in vapor phase (vapour pressure of the solute) is directly proportional to the mole fraction (x) of the gaseous solute in the solution at low concentrations”. This statement is known as Henry’s law.
Henry’s law can be expressed as,
Psolute α xsolute in solution
Psolute = KH xsolute solution
Here, Psolute represents the partial pressure of the gas in vapour state which is commonly called as vapour pressure. Xsolute in solution represents the mole fraction of solute in the solution. KH is a empirical constant with the dimensions of pressure.
Question 34.
State Raoult law and obtain expression for lowering of vapour pressure when nonvolatile solute is dissolved In solvent.
Answer:
In an ideal solution, the vapour pressure of the solution is decreased when a non-volatile solute is dissolved in a solvent. The magnitude of decrease in the vapour pressure of the solution depends on the amount of solute added.
Let us consider the solution with the following features.
Mole fraction of the solvent = xA
Mole fraction of the solute = xB
Vapour pressure of the pure solvent = P°A
Vapour pressure of solution = P
As the solute is nonvolatile, the vapour pressure of the solution is only due to the solvent. Therefore, the vapour pressure of the solution (P) will be equal to the vapour pressure of the solvent (PA) over the solution.
i.e., P = PA
According to Raoult’s law, the vapour pressure of solvent over the solution is equal to the product or its vapour pressure in a pure state and its mole fraction.
PA = P°A xA or
P = P°A xA
![]()
Question 35.
What is molal depression constant? Does it depend on nature of the solute?
Answer:
If m = 1 then ∆Tf = Kf
“Then Kf is equal to the depression in freezing point for 1 molal solution”. No, it does not depends on nature of the solute.
Question 36.
What is osmosis?
Answer:
“The phenomenon of the flow of solvent through a semipermeable membrane from pure solvent to the solution is called osmosis”. Osmosis can also be defined as “the excess pressure which must be applied to a solution to prevent the passage of solvent into it through the semipermeable membrane”. Osmotic pressure is the pressure applied to the solution to prevent osmosis.
Question 37.
Define the term ‘isotonic solution’.
Answer:
Two solutions having same osmotic pressure at a given temperature are called isotonic solutions.
Question 38.
You are provided with a solid. ‘A’ and three solutions of A dissolved in water – one saturated, one unsaturated, and one supersaturated. How would you determine which solution is which?
Answer:
(A) Unsaturated solution:
It can dissolve salt an additional to it.
(B) Saturated solution:
Further solubility of salt does not takes place but solubility can takes place on heating.
(c) Supersaturated solution:
Solubility of salt do not takes place on even an further heating.
Question 39.
Explain the effect of pressure on the solubility.
Answer:
Generally, the change in pressure does not have any significant effect in the solubility of solids and liquids as they are not compressible. However, the solubility of gases generally increases with increase of pressure.
Consider a saturated solution of a gaseous solute dissolved in a liquid solvent in a closed container. In such a system, the following equilibrium exists.
Gas ⇌ Gas
(in gaseous state) (in solution)
According to Le-Chatelier principle, the increase in pressure will shift the equilibrium in the direction which will reduce the pressure. Therefore, more number of gaseous molecules dissolves in the solvent and the solubility increases.
![]()
Question 40.
A sample of 12 M Concentrated hydrochloric acid has a density 1.2 M gL-1 calculate the molality.
Solution:
Given:
Molarity = 12 M HCl
density of solution = 1.2 g L-1
In 12 M HCl solution, there are 12 moles of HCl in 1 litre of the solution.
Molality = \(\frac{\text { no of moles of solute }}{\text { mass of solvent (in } \mathrm{kg} \text { ) }}\)
Calculate mass of water(solvent)
mass of 1 litre HCl solution = density × volume
= 1.2 × gmL-1 × 1000 mL = 1200 g
mass of HCl = no. of moles of HCl × molar mass of HCl
= 12 mol × 36.5 g mol-1
= 438 g.
mass of water = mass of HCl solution – mass of HCl
mass of water = 1200 – 438 = 762 g
molality(m) = \(\frac{12}{0.762}\) = 15.75 m
Question 41.
A 0.25 M glucose solution, at 370.28 K has approximately the pressure as blood does what is the osmotic pressure of blood?
Solution:
C = 0.25 M
T = 370.28 K
(π)glucose = CRT
(π) = 0.25 mol L-1 × 0.082L atm K-1mol-1 × 370.28K
= 7.59 atm
Question 42.
Calculate the molality of a solution containing 7.5 g glycine(NH2-CH2-COOH) dissolved in 500g of water.
Solution:
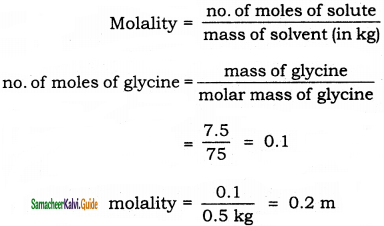
Question 43.
Which solution has the lower freezing point? 10 g of methanol (CH3OH) in 100g g of water (or) 20 g of ethanol (C2H5OH) in 200 g of water.
Solution:
∆Tf = Kf i.e
∆Tf α m
mCH3-OH = \(\frac{\left(\frac{10}{32}\right)}{0.1}\)
= 3.125 m
mC2H5-OH = \(\frac{\left(\frac{20}{46}\right)}{0.2}\)
= 2.174 m
∴ Depression in freezing point is more in methanol solution and it will have lower freezing point.
Question 44.
How many moles of solute particles are present in one liter of 10-4 M potassium sulphate?
Answer:
In 10-4 M K2SO4 solution, there are 10-4 moles of potassium sulphate.
K2SO4 molecule contains 3 ions (2K+ and 1 SO42-)
1 mole of K2SO4 molecule contains 3 × 6.023 × 1023 ions
10-4 mole of K2SO4 contains 3 × 6.023 × 1023 × 10-4 ions
= 18. 069 × 1019
![]()
Question 45.
Henry’s law constant for solubility of methane in benzene is 4.2 × 10-5 mm Hg at a particular constant temperature. At this temperature calculate the solubility of methane at
i) 750 mm Hg
ii) 840 mm Hg.
Solution:
(KH)benzene = 4.2 × 10-5 mm
Solubility of methane =?
P = 750 mm Hg P = 840 mm Hg
According to Henrys Law,
P = KH Xin solution
750 mm Hg = 4.2 × 10-5 mm Hg. Xin solution
⇒ Xin solution = \(\frac{750}{4.2 \times 10^{-5}}\)
i. e solubility = 178. 5 × 105
similarly at P = 840 mm Hg
solubility = \(\frac{840}{4.2 \times 10^{-5}}\) = 200 × 10-5
Question 46.
The observed depression in freezing point of water for a particular solution is 0.093°C calculate the concentration of the solution in molality. Given that molal depression constant for water is 1.86 K Kg mol-1.
Solution:
∆Tf = 0.093°C = 0.093 K, m = ?
Kf = 1.86 K Kg mol-1
∆Tf = Kf.m
∴ m = \(\frac{\Delta \mathrm{T}_{\mathrm{f}}}{\mathrm{K}_{\mathrm{f}}}=\frac{0.093 \mathrm{~K}}{1.86 \mathrm{~K} \mathrm{Kg} \mathrm{mol}^{-1}}\)
= 0.05 mol Kg-1 = 0.05 m
![]()
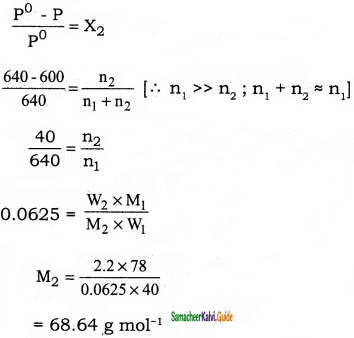
Question 47.
The vapour pressure of pure benzene (C6H6) at a given temperature is 640 mm Hg. 2.2 g of non – volatile solute is added to 40 g of benzene. The vapour pressure of the solution is 600 mm Hg. Calculate the molar mass of the solute?
Solution:
P°C6H6 = 640 mm Hg
W2 = 2.2 g (non volatile solute)
W1 = 40 g (benzene)
Psolution = 600 mm Hg
M2 =?
11th Chemistry Guide Solutions Additional Questions and Answers
I. Choose the best answer:
Question 1.
6.02 × 1020 molecules of urea ate present in 200 ml of its solution, The concentration of urea solution is (N0 = 6.02 × 1023 mol-1)
a) 0.001 M
b) 0.01M
c) 0.02 M
d) 0.10 M
Answer:
c) 0.02 M
Question 2.
Calculate the molarity and normality of a solution containing 0.5 g of NaOH dissolved in 500 ml solution
a) 0.0025 M, 0.025 N
b) 0.025 M, 0.025 N
c) 0.25 M, 0.25 N
d) 0.025M, 0.0025 N
Answer:
b) 0.025 M, 0.025 N
Question 3.
5 ml of N HCl, 20 ml of N/2 H2SO4 and 30 ml of N/3 HNO3 are mixed together and volume made to one liter. The normality of the resulting solution is
a) \(\frac{\mathrm{N}}{40}\)
b) \(\frac{\mathrm{N}}{10}\)
c) \(\frac{\mathrm{N}}{20}\)
d) \(\frac{\mathrm{N}}{5}\)
Answer:
a) \(\frac{\mathrm{N}}{40}\)
Question 4.
At 25°C, the density of 15 M H2SO4 is 1.8 g cm-3. Thus, mass percentage of H2SO4 in aqueous solution is
a) 2%
b) 81.6%
c) 18%
d) 1.8%
Answer:
b) 81.6%
Question 5.
Mole fraction of C3H5(OH)3 in a solution of 36 g of water and 46 g of glycerine is :
a) 0.46
b) 0.36
c) 0.20
d) 0.40
Answer:
c) 0.20
![]()
Question 6.
The molality of a urea solution in which 0.0100 g of urea, [(NH2)2CO] is added to 0.3000 dm3 of water at STP is
a) 0.555 m
b) 5.55 × 10-4
c) 33.3 m
d) 3.33 × 10-2 m
Answer:
b) 5.55 × 10-4
Question 7.
15 grams of methyl alcohol is dissolved in 35 grams of water. What is the mass percentage of methyl alcohol in solution?
a) 30%
b) 50%
c) 70%
d) 75%
Answer:
a) 30%
Question 8.
A 3.5 molal aqueous solution of methyl alcohol (CH3OH) is supplied. What is the mole fraction of methyl alcohol in the solution?
a) 0.100
b) 0.059
c) 0.086
d) 0.050
Answer:
b) 0.059
Question 9.
In which mode of expression of concentration of a solution remains independent of temperature?
a) Molarity
b) Normality
c) Formality
d) Molality
Answer:
d) Molality
Question 10.
Calculate the molarity of pure water (d = 1 g/L)
a) 555 M
b) 5.55 M
c) 55. 5 M
d) None
Answer:
c) 55. 5 M
![]()
Question 11.
Calculate the quantity of sodium carbonate (anhydrous) required to prepare 250 ml solution
a) 2.65 grams
b) 4.95 grams
c) 6.25 grams
d) None of these
Answer:
a) 2.65 grams
Question 12.
Find the molality of H2SO4 solution whose specific gravity is 1.98 g ml-1 and 95 % by volume H2SO4
a) 7.412
b) 8.412
c) 9.412
d) 10.412
Answer:
c) 9.412
Question 13.
Calculate molality of 1 liter solution of 93 % H2SO4 by volume. The density of solution is 1.84 g ml-1
a) 9.42
b) 10.42
c) 11.42
d) 12.42
Answer:
b) 10.42
Question 14.
Calculate the molality and mole fraction of the solute in aqueous solution containing 3.0 g of urea per 250 gm of water (Mol.wt. of urea = 60).
a) 0.2 m, 0.00357
b) 0.4 m, 0.00357
c) 0.5 m, 0.00357
d) 0.7 m, 0.00357
Answer:
a) 0.2 m, 0.00357
Question 15.
Calculate normality of the mixture obtained by mixing 100 ml of 0.1 N HCl and 50 ml of 0.25 N NaOH solution.
a) 0.0467 N
b) 0.0367 N
c) 0.0267 N
d) 0.0167 N
Answer:
d) 0.0167 N
![]()
Question 16.
300 ml 0.1 M HCl and 200 ml of 0.03 M H2SO4 are mixed. Calculate the normality of the resulting mixture
a) 0.084 N
b) 0.84 N
c) 2.04 N
d) 2.84 N
Answer:
a) 0.084 N
Question 17.
What weight of oxalic acid (H2C2O4.2H2O) is required to prepare, 1000mL of N/10 solution?
a) 9.0 g
b) 12.6 g
c) 6.3 g
d) 4.5 g
Answer:
c) 6.3 g
Question 18.
Which of the following units is useful in relating concentration of solution with its vapour pressure?
a) Mole fraction
b) Parts per million
c) Mass percentage
d) Molality
Answer:
a) Mole fraction
Question 19.
The pressure under which liquid and vapour can co-exist at equilibrium is called the
a) Limiting vapour pressure
b) Real vapour pressure
c) Normal vapour pressure
d) Saturated vapour pressure
Answer:
b) Real vapour pressure
Question 20.
CO(g) is dissolved in H2O at 30°C and 0.020 atm. Henry’s law constant for this system is 6.20 × 104 atm. Thus, mole fraction of CO(g) is
a) 1.72 × 10-7
b) 3.22 × 10-7
c) 0.99
d) 0.01
Answer:
b) 3.22 × 10-7
![]()
Question 21.
H2S gas is used in qualitative analysis of inorganic cations. Its solubility in water at STP is 0.195 mol kg-1. Thus, Henry’s law constant ( in atm raolaT1) for H2S is
a) 2.628 × 10-4
b) 5.128
c) 0.185
d) 3.826 × 103
Answer:
b) 5.128
Question 22.
Which of the following is correct for a solution showing positive deviations from Raoult’s law?
a) ∆V = +ve, ∆H = + ve
b) ∆V = -ve, ∆H = – ve
c) ∆V = + ve, ∆H = -ve
d) ∆V = – ve, ∆H = +ve
Answer:
a) ∆V = +ve, ∆H = + ve
Question 23.
If liquids A and B form an ideal solution
a) The entropy of mixing is zero
b) The Gibbs free energy is zero
c) The Gibbs free energy as well as the entropy of mixing are each zero
d) The enthalpy of mixing is zero
Answer:
d) The enthalpy of mixing is zero
Question 24.
Water and ethanol form non – ideal solution with positive deviation from Raoult’s law. This solution, will have vapour pressure
a) equal to vapour pressure of pure water
b) less than vapour pressure of pure water
c) more than vapour pressure of pure water
d) less than vapour pressure of pure ethanol
Answer:
c) more than vapour pressure of pure water
Question 25.
Which of the following is less than zero for ideal solutions?
a) ∆Hmix
b) ∆V
c) ∆Gmix
d) ∆Smix
Answer:
c) ∆Gmix
![]()
Question 26.
Which of the following shows negative deviation from Raoult’s law?
a) CHCl3 and CH3COCH3
b) CHCl3 and C2H5OH
c) C6H5CH3 and C6H6
d) C6H6 and CCl4
Answer:
a) CHCl3 and CH3COCH3
Question 27.
Given at 350 K, P°A = 300 torr and P°B = 800 torr, the composition of the mixture having a normal boiling point of 350 K is :
a) XA = 0.08
b) XA = 0.06
c) XA = 0.04
d) XA = 0.02
Answer:
a) XA = 0.08
Question 28.
In mixture A and B, components show – ve deviation as :
a) ∆Vmix is + ve
b) A – B interaction is weaker than A – A and B – B interaction
c) ∆Hmix is + ve
d) A – B interaction is stronger than A – A and B – B interaction
Answer:
d) A – B interaction is stronger than A – A and B – B interaction
Question 29.
If liquid A and B form ideal solution, then:
a) ∆Vmix is = 0
b) ∆Vmix = 0
c) ∆Gmix =0, ∆Smix = 0
d) ∆Smix = 0
Answer:
b) ∆Vmix = 0
Question 30.
Which liquid pair shows a positive deviation from Raoult’s law ?
a) Acetone – chloroform
b) Benzene – methanol
c) Water – nitric acid
d) Water – hydrochloric acid
Answer:
b) Benzene – methanol
![]()
Question 31.
For A and B to form an ideal solution which of the following conditions should be satisfied ?
a) ∆Hmixing =0
b) ∆Vmixing =0
c) ∆Smixing =0
d) All three conditions mentioned above
Answer:
d) All three conditions mentioned above
Question 32.
Two liquids are mixed together to form a mixture which boils at same temperature, and their boiling point is higher than the boiling point of either of them so they shows.
a) no deviation from Raoult’s law
b) positive, deviation from Raoult’s law
c) negative-deviation from Raoult’s law
d) positive or negative deviation from Raoult’s law depending upon the composition
Answer:
c) negative-deviation from Raoult’s law
Question 33.
Molal elevation constant of liquid is:
a) the elevation in b.p. which would be produced by dissolving one mole of solute in 1oo g of solvent
b) the elevation of b.p. which would be produced by dissolving 1 mole solute in 10 g of solvent
c) elevation in b.p. which would be produced by dissolving 1 mole of solute in 1000g of solvent
d) none of the above
Answer:
c) elevation in b.p. which would be produced by dissolving 1 mole of solute in 1000g of solvent
Question 34.
The vapour pressure of pure liquid solvent is 0.50 atm. When a non – volatile solute B is added to the solvent, its vapour pressure drops to 0.30 atm. Thus, mole fraction of the component B is
a) 0.6
b) 0.25
c) 0.45
d) 0.75
Answer:
a) 0.6
Question 35.
The mass of a non – volatile solute (molecular mass = 40) which should be dissolved in 114 g octane to reduce its vapour pressure to 80 % will be
a) 20 g
b) 30 g
c) 10 g
d) 40 g
Answer:
c) 10 g
![]()
Question 36.
The vapour pressure of pure liquid solvent A is 0.80 atm. When a non – volatile substance B is added to the solvent, its vapour pressure drops to 0.60 atm. Mole fraction of the component B in the solution is:
a) 0.50
b) 0.25
c) 0.75
d) 0.40
Answer:
b) 0.25
Question 37.
18 g of glucose (C6H12O6) is added to 178.2 g of water. The vapour pressure of water for this aqueous solution at 100°C is :
a) 752.40 torr
b) 759.00 torr
c) 7.60 torr
d) 76.00 torr
Answer:
a) 752.40 torr
Question 38.
Calculate the vapour pressure of a solution at 100°C containing 3 g of cane sugar in 33 g of water, (at wt. C = 12, H = 1, O = 16)
a) 760 mm
b) 756.90 mm
c) 758.30 mm
d) None of these
Answer:
b) 756.90 mm
Question 39.
Lowering of vapour pressure due to a solute in 1 molal aqueous solution at 100°C is
a) 13.44 mm Hg
b) 14.12 mm Hg
c) 31.2 mm Hg
d) 35.2 mm Hg
Answer:
a) 13.44 mm Hg
Question 40.
The vapour pressure of a dilute aqueous solution of glucose is 750 mm Hg at 373 K. The mole fraction of the solute is
a) \(\frac{1}{76}\)
b) \(\frac{1}{7.6}\)
c) \(\frac{1}{38}\)
d) \(\frac{1}{10}\)
Answer:
a) \(\frac{1}{76}\)
![]()
Question 41.
When 3 g of a nonvolatile solute is dissolved in 50 g of water, the relative lowering of vapour pressure observed is 0.018 Nm-2. Molecular weight of the substance is
a) 60
b) 30
c) 40
d) 120
Answer:
a) 60
Question 42.
Elevation in boiling point of a molar (1M) glucose solution (d = 1.2 gmL-1) is
a) 1.34 Kb
b) 0.98 Kb
c) 2.40 Kb
d) Kb
Answer:
b) 0.98 Kb
Question 43.
Given, H2O (l) ⇌ H2O (g) at 373 K, ∆H° = 8.31 kcal mol-1. Thus, boiling point of 0.1 molal sucrose solution is
a) 373. 52 K
b) 373.052 K
c) 373.06 K
d) 374.52 K
Answer:
c) 373.06 K
Question 44.
A solution of 0.450 g of urea (mol. Wt. 60) in 22.5 g of water showed 0.170°C of elevation in boiling point. Calculate the molal elevation constant of water.
a) 0.17°C
b) 0.45°C
c) 0.51°C
d) 0.30°C
Answer:
c) 0.51°C
Question 45.
At higher altitudes, water boils at temperature < 100°C because
a) temperature of higher altitudes is low
b) atmospheric pressure is low
c) the proportion of heavy water increases
d) atmospheric pressure becomes more
Answer:
b) atmospheric pressure is low
![]()
Question 46.
Which aqueous solution exhibits highest boiling point?
a) 0.015 M glucose
b) 0.01 M KNO3
c) 0.015 M urea
d) 0.01 M Na2SO4
Answer:
d) 0.01 M Na2SO4
Question 47.
A solution of urea in water has boiling point of 100.15°C. Calculate the freezing point of the same solution if Kf and Kb for water are 1.87 K kg mol-1 and 0.52 K kg mol-1 respectively
a) – 0.54°C
b) – 0.44°C
c) – 0.64°C
d) – 0.34°C
Answer:
a) – 0.54°C
Question 48.
Which will have largest ∆Tb?
a) 180 g glucose in 1 kg water
b) 342 g sucrose in 1,000 g water
c) 18 g glucose in 100 g water
d) 65 g urea in 1kg water
Answer:
d) 65 g urea in 1kg water
Question 49.
An aqueous solution of glucose boils at 100.01°C. The molal elevation constant for water is 0.5 K mol-1 kg. The number of molecules of glucose in the solution containing 100 g of water is
a) 6.023 × 1023
b) 12.046 × 1022
c) 12.046 × 1020
d) 12.046 × 1023
Answer:
c) 12.046 × 1020
Question 50.
The latent heat of vaporization of water is 9700 cal/mole and if the b.p. is 100°C, ebullioscopic constant of water is
a) 0.513°C
b) 1.026°C
c.) 10.26°C
d) 1.832°C
Answer:
a) 0.513°C
![]()
Question 51.
If for a sucrose solution elevation in boiling point is 0.1 °C then what will be the boiling point of NaCl solution for same molal concentration
a) 0.1°
b) 0.2°C
c) 0.08°C
d) 0.01°C
Answer:
b) 0.2°C
Question 52.
The molal boiling point constant for water is 0.513°C kg mol-1. When 0.1 mole of sugar is dissolved in 200 ml of water, the solution boils under a pressure of one atmosphere at
a) 100.513°C
b) 100.0513°C
c) 100.256°C
d) 101.025°C
Answer:
c) 100.256°C
Question 53.
The boiling point of 0.1 m K4[Fe(CN)6] is expected to be (Kb for water = 0.52 K kg mol’1)
a) 100.52°C
b) 100.10°C
c) 100.26°C
d) 102.6°C
Answer:
c) 100.26°C
Question 54.
The value of Kf for the water is 1.86K Kg mole-1, calculated from glucose solution. The value of Kf for water calculated for NaCl solution will be :
a) = 1.86
b) < 1.86
c) > 1.86
d) zero
Answer:
a) = 1.86
Question 55.
The amount of urea to be dissolved in 500 cc of water (Kf = 1.86) to produce a depression of 0.186°C in the freezing point is :
a) 9 g
b) 6 g
c) 3 g
d) 0.3 g
Answer:
c) 3 g
![]()
Question 56.
Freezing point of an aqueous solution is – 0.186°C. Elevation of boiling point of the same solution is if Kb = 0.512 K molality-1 and Kf= 1.86 K molality-1
a) 0.186°C
b) 0.0512°C
c) 0.092°C
d) 0.237°C
Answer:
b) 0.0512°C
Question 57.
What should be the freezing point of aqueous solution containing 17 g of C2H5OH in 1000 g of water (Kf for water = 1.86 deg kg mol-1)?
a) – 0.69°C
b) 0.34°C
c) 0.0°C
d) – 0.34°C
Answer:
a) – 0.69°C
Question 58.
The freezing point of equimolal aqueous solution will be highest for:
a) C6H5NH3Cl
b) Ca(NO3)2
c) La(NO3)2
d) C6H12O6
Answer:
d) C6H12O6
Question 59.
Cryoscopic constant of a liquid
a) is the decrease in freezing point when 1 g of solute is dissolved per kg of the solvent
b) is the decrease in the freezing point when 1 mole of solute is dissolved per kg of the solvent
c) is the elevation for 1 molar solution
d) is a factor used for calculation of depression in freezing point
Answer:
b) is the decrease in the freezing point when 1 mole of solute is dissolved per kg of the solvent
Question 60.
Which of the following solution will have highest freezing point?
a) 2 M NaCl solution
b) 1.5 M AlCl3 solution
c) 1 M Al2(SO4)3 solution
d) 3 M Urea solution
Answer:
d) 3 M Urea solution
![]()
Question 61.
0.48 g of a substance is dissolved in 10.6 g of C6H6. The freezing point of benzene is lowered by 1.8°C. what will be the mol.wt. of the substance (Kf for benzene = 5)
a) 250.2
b) 90.8
c) 125.79
d) 102.5
Answer:
c) 125.79
Question 62.
Which of the following aqueous molal solution have highest freezing point?
a) Urea
b) Barium chloride
c) Potassium bromide
d) Aluminium sulphate
Answer:
a) Urea
Question 63.
What weight of NaCl is added to one liter of water so that ∆Tf/Kf = 1?
a) 5.85 g
b) 0.585 g
c) 0.0585 g
d) 0.0855 g
Answer:
c) 0.0585 g
Question 64.
A solution of glucose (C6H12O6) is isotonic With 4 g of urea (NH2 – CO – NH2) per liter of solution. The concentration of glucose is :
a) 4 g/L
b) 8 g/L
c) 12 g/L
d) 14 g/L
Answer:
c) 12 g/L
Question 65.
A 5% solution of cane sugar (molar mass = 342) is isotonic with 1% of a solution of unknown solute. The molar mass of unknown solute in g/mol is
a) 136.2
b) 171.2
c) 68.4
d) 34.2
Answer:
c) 68.4
![]()
Question 66.
The weight of urea dissolved in 100 ml solution which produce an osmotic pressure of 20.4 atm, will be
a) 5 g
b) 4 g
c) 3 g
d) 6 g
Answer:
a) 5 g
Question 67.
In the phenomenon of osmosis, the membrane allow passage of _________.
a) Solute only
b) Solvent only
c) Both solute and solvent
d) None of these
Answer:
b) Solvent only
Question 68.
A 5.8% (wt./vol.) NaCl solution will exert an osmotic pressure closest to which one of the following:
a) 5.8% (wt./vol.) sucrose solution
b) 5.8% (wt./vol.) glucose solution
c) 2 molal sucrose solution
d) 1 molal glucose solution
Answer:
c) 2 molal sucrose solution
Question 69.
Osmotic pressure of a sugar solution at 24°C is 2.5 atmospheres. Determine the concentration of the solution in gram mole per liter.
a) 0.0821 moles/liter
b) 1.082 moles/liter
c) 0.1025 moles/liter
d) 0.0827moles/liter
Answer:
c) 0.1025 moles/liter
Question 70.
What is the freezing point of a solution that contains 10.0g of glucose C6H12O6 in 100 g of H2O? Kf = 1.86° C/m.
a) – 0.186°C
b) + 0.186°C
c) – 0.10°C
d) – 1.03°C
Answer:
d) – 1.03°C
![]()
Question 71.
The order of osmotic pressure of equimolar solutions of BaCl2, NaCl and glucose will be:
a) BaCl2 > NaCl > glucose
b) NaCl > BaCl2 > glucose
c) glucose > BaCl2 > NaCl
d) glucose > NaCl > BaCl2
Answer:
a) BaCl2 > NaCl > glucose
Question 72.
The wt. of urea dissolved in 100 ml solution which produce an osmotic pressure of 20.4 atm, will be
a) 5 g
b) 4 g
c) 3 g
d) 6 g
Answer:
a) 5 g
Question 73.
A compound MX2 has observed and normal molar masses 65.6 and 164 respectively. Calculate the apparent degree of ionization of MX2:
a) 75%
b) 85%
c) 65%
d) 25%
Answer:
a) 75%
Question 74.
The freezing point of 0.2 molal K2SO4 is – 1.1°C. Calculate van’t Hoff facor and percentage degree of dissociation of K2SO4. Kf for water is 1.86°
a) 97.5
b) 90.75
c) 105.5
d) 85.75
Answer:
a) 97.5
Question 75.
For 0.1M solution, the colligative property will follow the order
a) NaCl > Na2SO4 > Na3PO4
b) NaCl > Na2SO4 ≈ Na3PO4
c) NaCl < Na2SO4 < Na3PO4
d) NaCl < Na2SO4 = Na3PO4
Answer:
c) NaCl < Na2SO4 < Na3PO4
![]()
Question 76.
PH of a 0.1M monobasic acid is found to be 2. Hence its osmotic pressure at a given temp. T K is
a) 0.1 RT
b) 0.11 RT
c) 1.1 RT
d) 0.01 RT
Answer:
b) 0.11 RT
Question 77.
Which has the highest boiling point?
a) 0.1 m Na2SO4
b) 0.1 m Al(NO3)3
c) 0.1 m MgCl2
d) 0.1 m C6H12O6 (glucose)
Answer:
b) 0.1 m Al(NO3)3
Question 78.
Aluminium phosphate is 100% ionized in 0.01 molal aqueous solution. Hence ∆Tb/ Kb is:
a) 0.01
b) 0.015
c) 0.0175
d) 0.02
Answer:
d) 0.02
Question 79.
1.0 molal aqueous solution of an electrolyte X3Y2 is 25% ionized. The boiling point of the solution is (Kb for H2O = 0.52 K kg/mol)
a) 373.5 K
b) 374.04 K
c) 377.12 K
d) 373.25 K
Answer:
b) 374.04 K
Question 80.
The freezing point of 0,05 m solutions of a non – electrolyte in water is
a) -1.86 °C
b) -0.93°C
c)-0.093°C
d) 0.93°C
Answer:
c)-0.093°C
![]()
Question 81.
For an ideal solution containing a non – volatile solute, which of the following expression is correctly represented?
a) ∆Tb = Kb × m
b) ∆Tb = Kb × M
c) ∆Tb = Kb × 2m
d) ∆Tb = Kb × 2M
Where m is the molality of the solution and Kb is molal elevation constant.
Answer:
a) ∆Tb = Kb × m
Question 82.
If 5.85 g of NaCl are dissolved in 90 g of water, the mole fraction of NaCl is
a) 0.1
b) 0.2
c) 0.3
d) 0.0196
Answer:
d) 0.0196
Question 83.
What will be the molarity of a solution containing 5g of sodium hydroxide in 250 ml solution?
a) 0.5
b) 1.0
c) 2.0
d) 0.1
Answer:
a) 0.5
Question 84.
If 5.85 g of NaCl (molecular weight 58.5) is dissolved in water and the solution is made up to 0.5 liter, the molarity of the solution will be
a) 0.2
b) 0.4
c) 1.0
d) 0.1
Answer:
a) 0.2
Question 85.
To prepare a solution of concentration of 0.03 g/ml of AgNO3, what amount of AgNO3 should be added in 60ml of solution
a) 1.8
b) 0.8
c) 0.18
d) None of these
Answer:
a) 1.8
![]()
Question 86.
How many g of dibasic acid (mol.wt. 200) should be present in 100ml of its aqueous solution to give decinormal strength?
a) 1 g
b) 2 g
c) 10 g
d) 20 g
Answer:
a) 1 g
Question 87.
The molarity of a solution of Na2CO3 having 10.6 g/500 ml of solution is
a) 0.2 M
b) 2 M
c) 20 M
d) 0.02 M
Answer:
a) 0.2 M
Question 88.
Molecular weight of glucose is 180, A solution of glucose which contains 18 g per liter is
a) 2 molal
b) 1 molal
c) 0.1 molal
d) 18 molal
Answer:
c) 0.1 molal
Question 89.
0.5 M of H2SO4 is diluted from lliter to 10 liters, normality of resulting solution is
a) 1 N
b) 0.1 N
c) 10 N
d) 11 N
Answer:
b) 0.1 N
Question 90.
An aqueous solution of glucose is 10% in strength. The volume in which 1 g mole of it is dissolved will be
a) 18 liters
b) 9 liters
c) 0.9 liters
d) 1.8 liters
Answer:
d) 1.8 liters
![]()
Question 91.
When 1.80 g glucose dissolved in 90 g of H2O, the mole fraction of glucose is
a) 0.00399
b) 0.00199
c) 0.0199
d) 0.998
Answer:
b) 0.00199
Question 92.
A 5 molar solution of H2SO4 is diluted from 1 liter to 10 liters. What is the normality of the solution?
a) 0.25 N
b) 1 N
c) 2N
d) 7 N
Answer:
b) 1 N
Question 93.
Normality of 2 M sulphuric acid is
a) 2 N
b) 4 N
c) N/2
d) N/4
Answer:
b) 4 N
Question 94.
What is the molarity of H2SO4 solution, that has a density 1.84 g/cc at 35°C and Contains solute 98% by weight
a) 4.18 M
b) 8.14 M
c) 18.4 M
d) 18 M
Answer:
c) 18.4 M
Question 95.
Which of the following is a colligative property?
a) Osmotic pressure
b) Boiling point
c) Vapour pressure
d) Freezing point
Answer:
a) Osmotic pressure
![]()
Question 96.
The vapour pressure of benzene at a certain temperature is 640 mm of Eg. A non – volatile and non – electrolyte solid weighing 2.175 g is added to 39.08 g of benzene. The vapour pressure of the solution is 600 mm of Hg. What is the molecular weight of solid substance?
a) 49.50
b) 59.6
c) 69.5
d) 79.8
Answer:
c) 69.5
Question 97.
The average osmotic pressure of human blood is 7.8 bar at 37°C. What is the concentration of an aqueous NaCl solution that could be used in the Mood stream?
a) 0.16 mol/L
b) 0.32 mol/L
c) 0.60 mol/L
d) 0.45 mol/L
Answer:
b) 0.32 mol/L
Question 98.
The osmotic pressure in atmospheres of 10% solution of cane sugar at 69°C is
a) 724
b) 824
c) 8.21
d) 7.21
Answer:
c) 8.21
Question 99.
The molal boiling point constant for water is 0.513°C kg mol-1. When 0.1 mole of sugar is dissolved in 200 ml of water, the solution boils under a pressure of one atmosphere at
a) 100.513°C
b) 100.0513°C
c) 100.256°C
d) 101.025°C
Answer:
c) 100.256°C
Question 100.
The freezing point of a solution prepared from 1.25 g of a non – electrolyte and 20 g of water is 271.9 K. If molar depression constant is 1.8 K mole-1 then molar mass of the solute will be
a) 105.7
b) 106.7
c) 115.3
d) 93.9
Answer:
a) 105.7
![]()
Question 101.
Osmotic pressure of 0.1 M solution of NaCl and Na2SO4 will be
a) same
b) osmotic pressure of NaCl solution will be more than Na2SO4 solution
c) osmotic pressure of Na2SO4 solution will be more than NaCl
d) osmotic pressure of NaSO4 will be less than that of NaCl solution
Answer:
c) osmotic pressure of Na2SO4 solution will be more than NaCl
Question 102.
At 25 °C the highest osmotic pressure is exhibited by 0.1 M solution of
a) CaCl2
b) KCl
c) Glucose
d) Urea
Answer:
a) CaCl2
Question 103.
Azeotropic mixture of HCl and water has
a) 84% HCl
b) 22.2% HCl
c) 63 % HCl
d) 20.2 HCl
Answer:
d) 20.2 HCl
Question 104.
The boiling point of water (100°C) becomes 100.25°C, if 3 grams of a nonvolatile solute is dissolved in 200 ml of water. The molecular weight of solute is (Kb for water is 0.6 K Kg mol-1)
a) 12.2 g mol-1
b) 15.4 g mol
c) 17.3 g mol-1
d) 20.4 g mol
Answer:
c) 17.3 g mol-1
![]()
II. Very short question and answer(2 Marks):
Question 1.
Define solution.
Answer:
A solution is homogeneous mixture of two or more substances, consisting of atom, ions or molecules. The constituent of the homogeneous mixture present in a lower amount is called the solute, and the one present in larger amount is called the solvent. For example, when a small amount of NaCl dissolved in water.
Question 2.
What is saturated solution?
Answer:
A saturated solution is one that contains the maximum amount of a solute that can dissolve in a solvent at a specific temperature.
For example, the solubility of NaCl in 100 g of water at 20°C is 36 g but at other temperatures, or in other solvents, is different.
Question 3.
What is unsaturated solution?
Answer:
An unsaturated solution is the one that contains less amount of solute than its capacity to dissolve.
Question 4.
What is Supersaturated solution?
Answer:
A super saturated solution is one that contains more dissolved solute than the saturated solution. It is generally not stable and eventually the dissolved solute will separate as crystals.
Question 5.
What is mass percentage?
Answer:
The mass percentage of a component in a solution is the mass of the component present in 100 g of the solution.
Mass percentage of component = \(\frac{\text { Mass of the component in the solution } \times 100}{\text { Total mass of the solution }}\)
![]()
Question 6.
What is parts per million (ppm)?
Answer:
If the amount of solute in solution is very much less, then the concentration is expressed as parts per million (ppm).
Parts per million (ppm) = \(\frac{\text { Mass of the solute }(\mathrm{mg})}{\text { Mass of the solvent }} \times 10^{6}\)
Question 7.
What is Molarity?
Answer:
Molarity (symbol M) is defined as the number of moles of solute present in a liter of solution. The units of molarity are moles per liter (mol L-1) or moles per cubic decimeter (mol dm-3)
Molarity(m) = \(\frac{\text { Number of ntoles of solute }}{\text { Volume of solution in liter }}\)
Question 8.
What is Non – ideal solution?
Answer:
The solutions which do not obey Raoult’s law over the entire range of concentration, are called non – ideal solutions. For a non – ideal solution, there is a change in the volume and enthalpy upon mixing, i.e.
∆Hmixing ≠ 0 & ∆Vmixing ≠ 0.
Question 9.
State Raoult’s law.
Answer:
According to Raoult’s law, the vapor pressure of solvent over the solution is equal to the product of its vapor pressure in pure state and its mole fraction.
PA = P°A XA or
P = P°AXA
Question 10.
Define boiling point.
Answer:
The boiling point of a liquid is the temperature at which its vapour pressure becomes equal to the atmospheric pressure (1 atm).
![]()
Question 11.
Define freezing point.
Answer:
Freezing point is defined as “the temperature at which the solid and the liquid states of the substance have the same vapour pressure”.
Question 12.
What is Osmotic pressure?
Answer:
Osmotic pressure can be defined as “the pressure that must be applied to the solution to stop the influx of the solvent (to stop osmosis) through the semi permeable membrane”.
Question 13.
State Dalton’s law.
Answer:
According to Dalton’s law of partial pressure the total pressure in a closed vessel will be equal to the sum of the partial pressures of the individual components.
Question 14.
What is elevation of boiling point? Give it.
Answer:
The temperature difference between the solution and pure solvent is called elevation of boiling point,
∆Tb = T – T°
Unit of ∆Tb is K Kg mole-1.
![]()
III. Short Question and answers(3 Marks):
Question 1.
What is mole fraction?
Answer:
Mole fraction, x, of solute is defined as the ratio of the number of moles of the components divided by the total number of the moles of all the component present in the solution.
Mole fraction of the solute xsolvent = \(\frac{\text { Moles of the component }}{\text { total number of moles of all the component in solution }}\)
Question 2.
What is volume percentage?
Answer:
It is defined as the volume of the component present in 100mL of the solution. If Va is a volume of solute and Vb is volume of solvent, then
Vollume percentage of solute = \(\frac{V_{a} \times 100}{V_{a}+V_{b}}\)
Question 3.
What are the advantages of using standard solution?
Answer:
- The error due to weighing the solute can be minimized by using concentrated stock solution that requires large quantity of solute.
- We can prepare working standards of different concentrations by diluting the stock solution, which is more efficient since consistency is maintained.
- Some of the concentrated solutions are more stable and are less likely to support microbial growth than working standards used in the experiments.
Question 4.
Limitations of Henry’s law.
Answer:
Henry’s law is applicable at moderate temperature and pressure only. Only the less soluble gases obeys Henry’s law. The gases reacting with the solvent do not obey Henry’s law.
Example:
Ammonia or HCl reacts with water and hence does not obey this law.
NH3 + H2O ⇌ NH4+ + OH–
Question 5.
What is Van’t Hoff factor?
Answer:
It is defined as the ratio of the actual molar mass to the abnormal (calculated) molar mass of the solute. Here, the abnormal molar mass is the molar mass calculated using the experimentally determined colligative property.
i = \(\frac{\text { Normal (actual) molar mass }}{\text { obseved (abnormal) molar mass }}\)
![]()
Question 6.
What is ideal solution?
Answer:
An ideal solution is a solution in which each component i.e. the solute as well as the solvent obeys the Raoult’s law over the entire range of concentration.
For an ideal solution,
- There is no change in the volume on mixing the two components (solute & solvents) (∆Vmixing =0).
- There is no exchange of heat when the solute is dissolved in solvent (∆Hmixing =0).
- escaping tendency of the solute and the solvent present in it should be same as in pure liquids.
Example:
benzene & toluene; h-hexane & n-heptane; ethyl bromide & ethyl iodide; chlorobenzene & bromobenzene.
Question 7.
What are colligative properties?
Answer:
“ The properties of the solutions which depend only on the number of solute particles but not on the nature of the solute are called colligative properties”. The following four colligative properties are very important.
- Relative lowering of vapor pressure (∆P)
- Elevation of boiling point (∆Tb)
- Depression of freezing point (∆Tb)
- Osmotic pressure (π)
Question 8.
What are the significances of Osmotic pressure?
Answer:
Unlike elevation of boiling point (for 1 molal solution the elevation in boiling point is 0.512°C for water) and the depression in freezing point (for 1 molal solution the depression in freezing point is 1.86°C for water), the magnitude of osmotic pressure is large.
The osmotic pressure can be measured at room temperature enables to determine the molecular mass of bio molecules which are unstable at higher temperatures. Even for a very dilute solution the osmotic pressure is large.
Question 9.
0.24 g of a gas dissolves in 1L of water at 1.5 atm pressure. Calculate the amount of dissolved gas when the pressure is raised to 6.0 atm at constant temperature?
Solution:
Psolute = KH Xsolute in solution
At pressure 1.5 atm,
P1 = KHX1 …………..(1)
At pressure 6.0 atm,
p2 = KHX2 …………….(2)
Dividing equation (1) by (2)
From equation = \(\frac{P_{1}}{P_{2}}=\frac{X_{1}}{X_{2}}\)
There fore
\(\frac{0.24 \times 6.0}{1.5}\) = 0.96 g/L
Question 10.
Why the carbonated drinks are stored in a pressurized container?
Answer:
The carbonated beverages contain carbon dioxide dissolved in them. To dissolve the carbon dioxide in these drinks, the CO2 gas is bubbled through them under high pressure. These containers are sealed to maintain the pressure. When we open these containers at atmospheric pressure, the pressure of the CO2 drops to the atmospheric level and hence bubbles of CO2 rapidly escape from the solution and show effervescence. The burst of bubbles is even more noticeable, if the soda bottle in warm condition.
![]()
Question 11.
An aqueous solution of 2% non volatile solute exerts a pressure of 1.004 bar at the boiling point of the solvent. What is the molar mass of the solute when P°A is 1.013 bar?
Solution:
\(\frac{\Delta P}{P_{A}^{0}}=\frac{W_{B} \times M_{B}}{M_{B} \times W_{A}}\)
In a 2 % solution weight of the solute is 2 g and solvent is 98g
∆P = Psolution – P°A = 1.013 – 1.004 bar
= 0.009 bar
MB = \(\frac{\mathrm{P}_{\mathrm{A}}^{0} \times \mathrm{W}_{\mathrm{B}} \times \mathrm{M}_{\mathrm{A}}}{\Delta \mathrm{P} \times \mathrm{W}_{\mathrm{A}}}\)
MB = 2 × 18 × 1.013/(98 × 0.009) = 41.3 g mol-1
Question 12.
Ethylene glycol (C2H6O2) can be at used as an antifreeze in the radiator of a car. Calculate the temperature when ice will begin to separate from a mixture with 20 mass percent of glycol in water used in the car radiator. Kf for water = 1.86K Kg mol-1 and molar mass of ethylene glycol is 62 g mol-1.
Solution:
Weight of solute (W2) = 20 mass percent of solution means 20 g of ethylene glycol
Weight of solvent (water) W1 = 100 – 20 = 80 g
∆Tf = Kf m
= \(\frac{\mathrm{K}_{\mathrm{f}} \times \mathrm{W}_{2} \times 1000}{\mathrm{M}_{2} \times \mathrm{W}_{1}}=\frac{1.86 \times 20 \times 1000}{62 \times 80}\)
= 7.5 K
The temperature at which the ice will begin to separate is the freezing of water after the addition of solute i.e 7.5 K lower than the normal freezing point of water (273 – 7.5 K) = 265.5 K
Question 13.
At 400 K 1.5 g of an unknown substance is dissolved in solvent and the solution is made to 1.5 L. Its osmotic pressure is found to be 0.3 bar. Calculated the molar mass of the unknown substance.
Solution :

Question 14.
The depression in freezing point is 0.24 K obtained by dissolving 1 g NaCl in 200g water. Calculate van’t – Hoff factor. The molar depression constant in 1.86 K Kg mol-1
Solution :
Molar mass of solute = \(\frac{1000 \times K_{f} \times \text { mass of } N a C l}{\Delta T_{f} \times \text { mass of solvent }}\)
= \(\frac{1000 \times 1.86 \times 1}{0.24 \times 200}\)
= 38.75 g mol-1
Theoretical molar mass of NaCl is = 58.5 g mol-1
i = \(\frac{\text { Theoretical molar mass }}{\text { Experimental molar mass }}\)
= \(\frac{58.5}{38.75}\) = 1.50
![]()
IV. Long question and answers(5 Marks):
Question 1.
Explain the factors including the solubility of solute?
Answer:
Factors influencing the solute:
The solubility of a solute generally depends on the nature of the solute and the solvent in which it is dissolved. It also depends on the temperature and pressure of the solution.
Nature of solute and solvent:
Sodium-chloride, an ionic compound, dissolves readily iff a polar solvent such as water, but it does not dissolve in non polar-organic solvents such as benzene or toluene. Many organic compounds dissolve -readily in organic solvents and do not dissolve in water. Different gases dissolve in water to different extents: for example, ammonia is more soluble than oxygen in water.
Effect of temperature:
Solid solute in liquid solvent:
Generally, the solubility of a solid solute in a liquid solvent increases with increase in temperature. When the temperature is increased, the average kinetic energy of the molecules of the solute and the solvent increases. The increase in kinetic energy facilitates the solvent molecules to break the intermolecular attractive forces that keep the solute molecules together and hence the solubility increases.
When a solid is added to a solvent, it begins to dissolve. i.e. the solute leaves from the solid state (dissolution). After some time, some of the dissolved solute returns back to the solid state (recrystallisation). If there is excess of solid present, the rate of both these processes becomes equal at a particular stage. At this stage an equilibrium is established between the solid solute molecules and dissolved solute molecules.
Solute(solid) ⇌ Solute(dissolved)
According to Le-Chatelier principle, if the dissolution process is endothermic, the increase in temperature will shift the equilibrium towards left i.e solubility increases, for an exothermic reaction, the increase in temperature decreases the solubility. The solubilities, of ammonium nitrate, calcium Chloride, ceric sulphate nano-hydrate, and sodium chloride in water at different temperatures are given in the following graph.
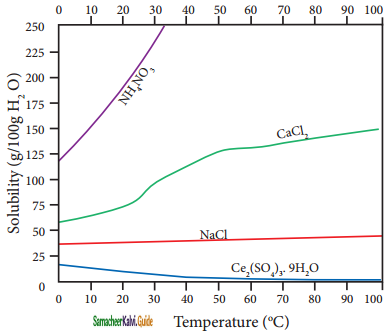
Plot of solubility versus temperature for selective compounds
The following conclusions are drawn from the above graph:
1. The solubility of sodium chloride does not vary appreciably as the maximum solubility is achieved at normal temperature. In fact, there is only 10 % increase in solubility between 0° to 100 °C.
2. the dissolution process of ammonium nitrate is endothermic, the solubility increases steeply with increase in temperature.
3. In the case of ceric sulphate, the dissolution is exothermic and the solubility decreases with increase in temperature.
4. Even though the dissolution of calcium chloride is exothermic, the solubility increases moderately with increase in temperature. Here, the entropy factor also plays a significant role in deciding the position of the equilibrium.
Gaseous solute in liquid solvent:
In the case of gaseous solute in liquid solvent, the solubility decreases with increase in temperature. When a gaseous solute dissolves in a liquid solvent, its molecules interact with solvent molecules with weak intermolecular forces. When the temperature increases, the average kinetic energy of the molecules present in the solution also increases.
The increase in kinetic energy breaks the weak intermolecular forces between the gaseous solute and liquid solvent which results in the release of the dissolved gas molecules to the gaseous state. Moreover, the dissolution of most of the gases in liquid solvents is an exothermic process, and in such processes, the increase in temperature decreases the dissolution of gaseous molecules.
![]()
Question 2.
Explain vapour pressure of liquid in liquids binary solution?
Answer:
Now, let us consider a binary liquid solution formed by dissolving a liquid solute ‘A’ in a pure solvent B in a closed vessel. Both the components A and B present in the solution would evaporate and an equilibrium will be established between the liquid and vapour phases of the components A and B.
The French chemist Raoult, proposed a quantitative relationship between the partial pressures and the mole fractions of two components A & B, which is known as Raoult’s Law. This law states that “in the case of a solution of volatile liquids, the partial vapour pressure of each component (A & B) of the solution is directly proportional to its mole fraction”.
According to Raoult’s law,
PA ∝ xA
PA = k xA
when xA = 1, k = P°A
where p°A is the vapour pressure of pure component A’ at the same temperature. Therefore,
PA = P°A xA
Similarly, for component ‘B’
PB =P°B xB
xA and xB are the mole fraction of the components A and B respectively.
According to Dalton’s law of partial pressure the total pressure in a closed vessel will be equal to the sum of the partial pressures of the individual components.
Hence,
Ptotal = PA + PB
Substituting the values of PA and PB from equations in the above equation,
Ptotal = XA P°A + XBP°B
We know that XA + XB = 1 or XA = 1 – XB
Therefore,
Ptotal = (1 – XB)P°A + XBP°B
Ptotal = P°A + XB(P°B – P°A)
The above equation is of the straight¬line equation form y = mx + c. The plot of Ptotalversus xB will give a straight line with (P°B – PA) as slope and P°A as the y intercept.
Let us consider the liquid solution containing toluene (solute) in benzene (solvent).
The variation of vapour pressure of pure benzene and toluene with its mole fraction is given in the graph.
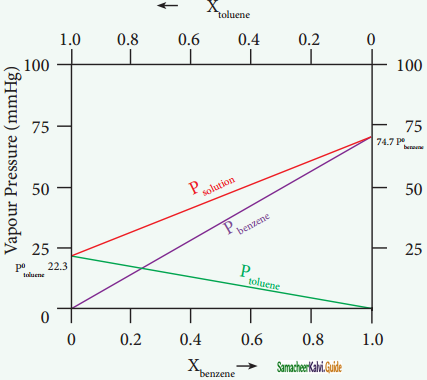
Solution of benzene in toluene obeying Raoult’s law
The vapour pressures of pure toluene and pure benzene are 22.3 and 74.7 mmHg, respectively. The above graph shows, the partial vapour pressure of the pure components increases linearly with the increase in the mole fraction of the respective components. The total pressure at any composition of the solute and solvent is given by the following straight line (represented as red line) equation.
PSolution = P°toluene + Xbenzene(Pbenzene – Ptoluene)
Question 3.
Explain Raoult’s law for the binary solution of Non-volatile solutes in liquids?
Answer:
When a nonvolatile salute js dissolved in a pure solvent, the vapour pressure of the pure solvent will decrease. In such solutions, the vapour pressure of the -Solution will depend only on the solvent ‘molecules as the solute is nonvolatile.
For example, when sodium chloride is added to the water, the vapor pressure of the salt solution is lowered. The vapour pressure of the solution is determined by the number of molecules of the solvent present in the surface at any time and is proportional to the mole fraction of the solvent.
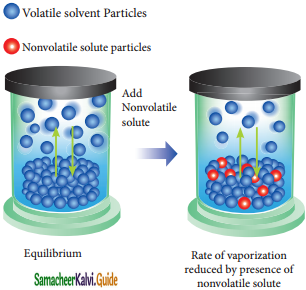
Rate of vapourization reduced by presence of nonvolatile solute.
Psolution ∝ XA
Where XA is the mole fraction of the solvent
Psolution = kXA
When XA = 1, k = P°solvent
(P°solvent is the partial pressure of pure solvent)
PSolution = P°Solvent
\(\frac{P_{\text {solution }}}{P_{\text {solvent}}^{a}}\) = XA
1 – \(\frac{p_{\text {Solution }}}{p_{\text {Solvent }}^{0}}\) = 1 – XA
\(\frac{p_{\text {Solvent }}^{o}-p_{\text {Solurion }}}{P_{\text {Solvent }}^{0}}\) = XB
Where XB is the fraction of the solute
(∴ xA + xB = 1, XB = 1 – XA)
The above expression gives the relative lowering of vapour pressure. Based on this expression, Raoult’s Law can also be stated as “the relative lowering of vapour pressure of an ideal solution containing the nonvolatile solute is equal to the mole fraction of the solute at a given temperature”.
![]()
Question 4.
What is ideal solution? Write special features and characters of ideal solution.
Answer:
An ideal solution is a solution in which each component i.e. the solute as well as the solvent obeys the Raoult’s law over the entire range of concentration. Practically no solution is ideal over the entire range of concentration. However, when the concentration of solute is very low, the dilute solution behaves ideally.
If the two components present in the solution (A and B) are identical in size, structure, and having almost similar intermolecular attractive forces between them (i.e. between A-A, B-B, and B-A) and then the solution tends to behave like an ideal solution.
For an ideal solution:
(i) there is no change in the volume on mixing the two components, (solute & solvents). (∆Vmixing = 0)
(ii) there is no exchange of heat when the solute is dissolved in solvent (∆Hmixing = 0).
(iii) escaping tendency of the solute arid the solvent present in it should be same as in pure liquids.
Example:
benzene & toulene; n-hexane & n-heptane; ethyl bromide & ethyl iodide; chlorobenzene & bromobenzene.
Question 5.
Explain Non – ideal solution with strong positive deviation.
Answer:
The solutions which do not Raoult’s law over the entire range, of concentration, are called non-ideal solutions. For a non-ideal solution, there is a change in the volume and enthalpy upon mixing, i.e.
∆Hmixing ≠ 0 &, ∆Vmixing ≠ 0. The deviation of the non-ideal solutions from the Raoult’s law can either be positive or negative.
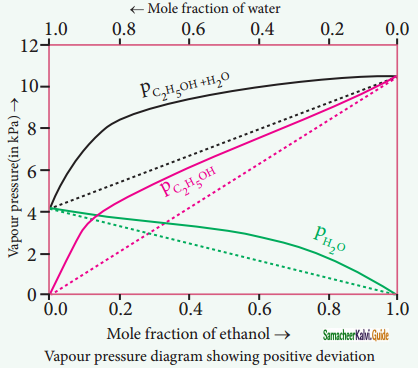
Non-ideal solutions – positive deviation from Rauolt’s Law:
The nature of the deviation from the Rauolt’s law can be explained in terms ©f the intermolecular interactions between solute and solvent (B). Consider a case in which the intermolecular attractive forces between A and B are weaker than those between the molecules of A (A-A) and molecules of B (B-B).
The molecules present in such a solution have a greater tendency to escape from the solution when compared to the ideal solution formed by A and B, in which the intermolecular attractive forces (A-A, B-B, A-B) are almost similar. Consequently, the vapour pressure of such non-ideal solution increases and it is greater than the sum of the vapour pressure of A and B as predicted by the Raoult’s law. This type of deviation is called positive deviation.
Here, PA > p°A XA and pB > P°B XB
Hence Ptotal > p°A XA + PB XB
Let us understand the positive deviation by considering a solution of ethyl alcohol and water. In this solution the hydrogen bonding interaction between ethanol and water is weaker than those hydrogen bonding interactions amongst themselves (ethyl alcohol-ethyl alcohol and water-water interactions).
This results in the increased evaporation of both components from the aqueous solution of ethanol. Consequently, the vapour pressure of the solution is greater than the vapour pressure predicted by Raoults law. Here, the mixing process is endothermic i.e. ∆Hmixing > 0 and there will be slight increase in volume(∆Vmixing > 0).
Example:
Ethyl alcohol cyclohexane, Benzene & acetone, Carbon tetrachloride & chloroform, Acetone & ethyl alcohol, Ethyl alcohol & water.
![]()
Question 6.
Explain Non – ideal solution with strong negative deviation.
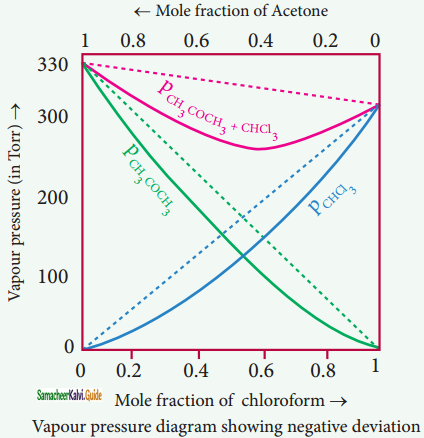
Answer:
Let us consider a case where the attractive forces between solute (A) and solvent t (B) are stronger – thtSSar intermolecular attractive forces between the individual components (A – A & B – B). Here, the escaping tendency of A and B will be lower when compared with an ideal solution formed by A and B. Hence, the vapour pressure of such solutions will be lower than the sum of the vapour pressure of A and B. This type of deviation is called negative deviation. For the negative deviation,
PA < P°A XA and PB < P°B XB.
Let us consider a solution of phenol and aniline. Both phenol and aniline form hydrogen bonding interactions amongst themselves. However, when mixed with aniline, the phenol molecule forms hydrogen bonding interactions with aniline, which are stronger than the hydrogen bonds formed amongst themselves.
Formation of new hydrogen bonds considerably reduce the escaping tendency of phenol and aniline from the solution. As a result, the vapour pressure of the solution is less and there is a slight decrease in volume (∆Vmixing < 0) on mixing. During this process evolution of heat takes place i.e. ∆Hmixing < 0 (exothermic)
Example:
Acetone + chloroform, Chloroform + diethyl ether, Acetone + aniline, Chloroform + Benzene.
Question 7.
Explain the factors responsible for deviation from Raoult’s law.
Answer:
Factors responsible for deviation from Raoult’s law:
The deviation of solution from ideal behavior is attributed to the following factors.
i) Solute-solvent interactions:
For an ideal solution, the interaction between the solvent molecules (A-A), the solute molecules (B-B) and between the solvent & solute molecules (A-B) are expected to be similar. If these interactions are dissimilar, then there will be a deviation from ideal behavior.
ii) Dissociation of solute:
When a solute present in a solution dissociates to give its constituent ions, the resultant ions interact strongly with the
solvent and cause deviation from Raoult’s law.
For example, a solution of potassium chloride in water deviates from ideal behavior because the solute dissociates to give K and Cl ion which form strong ion-dipole interaction with water molecules.
KCl(s) + H2O (l) → K+(aq)+ Cl–(aq)
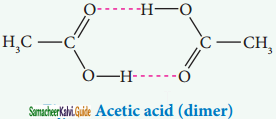
iii) Association of solute:
Association of solute molecules can also cause deviation from ideal behaviour. For example, in solution, acetic acid exists as a dimer by forming intermolecular hydrogen bonds, and hence deviates from Raoult’s law.
iv) Temperature:
An increase in temperature of the solution increases the average kinetic energy of the molecules present in the solution which causes decrease in the attractive force between them. As result, the solution deviates from ideal behaviour.
v) Pressure:
At high pressure the molecules tend to stay close to each other and therefore there will be an increase in their intermolecular attraction. Thus, a solution deviates from Raoult’s law at high pressure.
vi) Concentration:
If a solution is sufficiently dilute there is no pronounced solvent-solute interaction because the number of solute molecules are very low compared to the solvent. When the concentration is increased by adding solute, the solvent-solute interaction becomes significant. This causes deviation from the Raoult’s law.
![]()
Question 8.
How would you determine the molar mass of solute from Tb?
Answer:
The elevation of boiling point is directly proportional to the concentration of the solute particles.
∆Tb ∝ m
m is the concentration of solution expressed in molality.
∆Tb = Kbm
Where
Kb = molal boiling point elevation constant or Ebullioscopic constant.
∆Tb = \(\frac{K_{b} \times W_{B} \times 1000}{M_{B} \times W_{A}}\)
Mb = \(\frac{\mathrm{K}_{\mathrm{b}}}{\Delta \mathrm{Tb}} \times \frac{\mathrm{W}_{\mathrm{B}} \times 1000}{\mathrm{~W}_{\mathrm{A}}}\)
Question 9.
How would you determine the molar mass of solute from T?
Answer:
1f the solution is prepared by dissolving
WB g of solute in WB g of solvent, then the molality is,
m = \(\frac{\text { Number of moles of solute } \times 1000}{\text { weight of solvent in grams }}\)
Number of moles of solute = \(\frac{W_{B}}{M_{B}}\)
Where, MB = molar mass of the solute
Therefore,
m = \(\frac{\mathrm{W}_{\mathrm{B}} \times 1000}{\mathrm{M}_{\mathrm{B}} \times \mathrm{W}_{\mathrm{A}}}\)
∆Tf = \(\frac{K_{f} \times W_{B} \times 1000}{M_{B} \times W_{A}}\)
Molar mass can be calculated using
MB = \(\frac{\mathrm{Kb} \times \mathrm{W}_{\mathrm{B}} \times 1000}{\Delta \mathrm{T}_{\mathrm{b}} \times \mathrm{W}_{\mathrm{A}}}\)
![]()
Question 10.
How would you determine the molar mass of solute form A?
Answer:
According to van’t Hoff equation π = cRT
c = \(\frac{n}{V}\)
Here, n = number of moles of solute dissolved in ‘V’ liter of the solution.
Therefore,
π = \(\frac{n}{V}\)RT
πV = nRT
If the solution is prepared by dissolving WBg of nonvolatile solute in WA g of solvent,
then the number of moles ‘n’ is,
n = \(\frac{\mathrm{W}_{\mathrm{B}}}{\mathrm{M}_{\mathrm{B}}}\)
since, MB = molar mass of the solute
Substituting the ‘n’ value, we get,
π = \(\frac{\mathrm{W}_{\mathrm{B}}}{\mathrm{V}} \frac{\mathrm{RT}}{\mathrm{M}_{\mathrm{B}}}\)
MB = \(\frac{W_{B}}{V} \frac{R T}{\pi}\)
From the above equation molar mass of the solute can be calculated.