TN State Board 11th Chemistry Important Questions Chapter 10 Chemical Bonding
Question 1.
State Octet rule.
Answer:
“The atoms transfer or share electrons so that all atoms involved in chemical bonding obtain 8 electrons in their outer shell (valence shell)”.
Question 2.
Write the Lewis structure for
(i) HCl O4:
Step 1:
Draw the skeletal structure.

Step 2:
Total number of valence electrons = 1 × 1 (hydrogen) + 1 × 7 (chlorine) + 4 × 6 (oxygen)
= 1 + 7 + 24 = 32 = 16 pairs
Step 3:
Draw single bonds between all atoms.
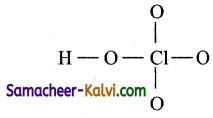
Five single bonds mestas 10 electrons are used for bonding. The remaining (32 – 10) = 22 electrons are to be distributed among all atoms so that their octet is complete.
Step 4:
Distribute the 11 electron pairs starting from oxygen.
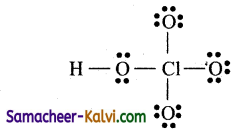
Step 5:
Check whether all atoms have 8 electrons around them.
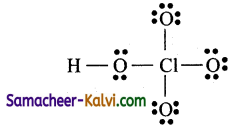
![]()
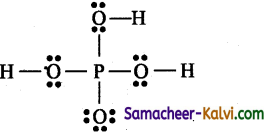
(ii) H3PO4
Answer:
Step 1:
Draw the skeletal structure. Hydrogen should always be placed at terminal position.

Step 2:
Draw single bonds between the atoms.
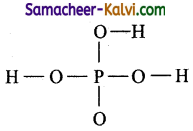
Step 3:
Total number of valence electrons = 1 × 5(P) + 4 × 6(O)1 + 3 × 1(H) = 5 + 24 + 3 = 32 = 16 pairs
Of the 32 electrons, 14 electrons (7 pairs) are used for covalent bond. The remaining electrons 32-14 = 18 (9 pairs) have to be distributed among all atoms so that they complete their octet.
Step 4:
The 18 electrons (9 pairs) are distributed among all atoms starting from oxygen.
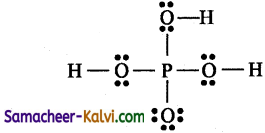
Step 5:
Check all atom have a complete octet.
The correct Lewis structure is
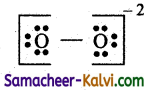
(iii) O2-2
Answer:
Step 1:
Draw the skeletal structure.
O O
Step 2:
Total number of valence electrons = 2 × 6 (oxygen) + 2 (-2 ve charge) = 12 + 2 = 14
Step 3:
Draw single bonds between the two oxygen atoms.
O — O
i.e., of the 14 electrons, 2 are used for bond formation.
The remaining number of electrons is 14 – 2 = 12
Step 4:
These 12 electrons (6 pairs) are distributed on the oxygen atom as lone pair, so that their octet is complete.
(iv) H3O+
Answer:
Step 1:
Draw the skeletal structure.
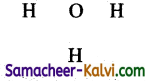
Step 2:
Total number of valence electrons = 3 × 1 (hydrogen) + 1 × 6 (oxygen) – 1(+ve charge)
= 3 + 6 – 1 = 8
Step 3:
Draw single bonds between the atoms.
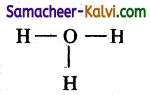
i.e., out of 8 e1ecrons, 6 electrons are used for bonding. The remaining number of electrons is two.
Step 4:
The two electrons are distributed on the oxygen atom as lone pair so that its octet is complete.
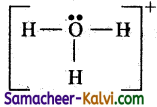
Step 5:
Check all atoms have an octet and hydrogen has 2 electrons. Hence the Lewis structure is
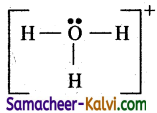
![]()
Question 3.
Explain the term formal charge with an example.
Answer:
The formal charge for the atom in a molecule or ion is the charge calculated for that atom based on the Lewis structure of the molecule or ion by using the equation.
Formal charge of the atom in a molecule = number of valence electrons in free atoms – (number of lone pair or non bonding electrons + \(\frac{1}{2}\) number of bonding electrons)
Formal charge = Nv – \(\left(\mathrm{N}_{l}+\frac{\mathbf{N}_{b}}{2}\right)\)
eg: 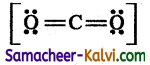
Formal charge on carbon = Nv – \(\left(\mathrm{N}_{l}+\frac{\mathbf{N}_{b}}{2}\right)\)
= 4 – \(\left[0+\frac{8}{2}\right]\) = 0
Formal charge on oxygen = 6 – \(\left[4+\frac{4}{2}\right]\) = 0 (for both oxygen)
Question 4.
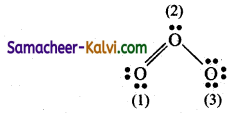
Calculate the formal charge on each oxygen atom inO3 molecule.
Answer:
The Lewis structure is
Formal charge (FC) = Nv – \(\left(\mathrm{N}_{l}+\frac{{\mathrm{N}}_{b}}{2}\right)\)
Nv – Number of valence electron of atom in its isolated state.
Nl – Number of electro us present as lone pairs around the atom in the Lewis structure.
Nb – Numbe of electrons present in bonds around the atõm (bond pairs) in the Lewis structure.
For oxygen atom (1):
Nv = 6; Nl = 4; Nb = 4
FC = 6 – \(\left[4+\frac{4}{2}\right]\)
= 6 – 6 = 0.
For oxygen atom (2):
Nv = 6; Nl = 2; Nb = 6
FC = 6 – \(\left[4+\frac{4}{2}\right]\)
=6 – 6 = 0
For oxygen atom (3):
Nv = 6; Nl = 6; Nb = 2
FC = 6 – \(\left(6+\frac{2}{2}\right)\)
= 6 – 7 = – 1
![]()
Question 5.
Calculate the formal charge on
(i) Sulphur in HSO4– ion
(ii) Chlorine in HClO4
Answer:
(i) The Lewis structure for HSO4– is

Formal charge on Sulphur atom:
Nv = 6; Nl = 0; Nb = 8
Formal charge = 6 – (0 + \(\frac{8}{2}\))
= 6 – 4 = + 2
Formal charge on sulphur in HSO4– is +2.
(ii) The Lewis structure for HC104 is
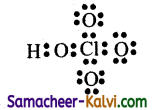
Formal charge on Chlorine atom:
Nv = 7; Nl = 0; Nb = 8
Formal charge – 7 – (0 – \(\frac{8}{2}\)) = 7 – 4 = +3
Formal charge on chlorine in HClO4 is +3.
![]()
Question 6.
Write foimal charges on atoms in
(i) Carbonate ion
(ii) Nitrite ion
(iii) Carbon dioxide.
Answer:
(i) Carbonate ion:
The Lewis structure for Carbonate ion is
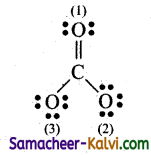
Formal charge on Carbon atom:
Nv = 4; Nl= 0; Nb = 8
Formal charge = 4 – (0 – \(\frac{8}{2}\)) = 4 – 4 = 0
Formal charge on double bonded oxygen atom;
Nv = 6; Nl = 4; Nb = 24 .
Formal charge = 6 – (4 + \(\frac{4}{2}\)) = 6 – 6 = 0
Formal charge on single bonded oxygen atom:
Nv = 6; Nl = 6; Nb = 2
Formal charge = 6 – (6 + \(\frac{2}{2}\)) = 6 – 7 = -1
Thus, formal charge on carbon atom = 0
Double bonded oxygen atom = 0
Single bonded oxygen atom = -1
(ii) The Lewis structure for Nitrite ion is (NO2–)
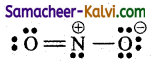
Formal charge on nitrogen atom:
Nv = 5; Nl = 2; Nb = 6
Formal charge = 5 – (2 + \(\frac{6}{2}\)) = 5 – 5 = 0
Formal charge on double bonded oxygen atom:
Nv = 6; Nl = 4; Nb = 2
Formal charge = 6 – (6 + \(\frac{2}{2}\)) = 6 – 6 = 0
Formal charge on single bonded oxygen atom:
Nv = 6; Nl = 6; Nb = 2
Formal charge = 6 – (6 +\(\frac{2}{2}\)) = 6 – 7 = -1
(iii) The Lewis structure for C02 is
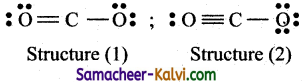
![]()
Question 7.
What are electron deficient compounds? Give an example for an electron deficient molecule. Write its Lewis structure. Find the formal charge on the central atom.
Answer:
Molecules in which the central atom has only six electrons and two short of its octet are called electron deficient compounds.
eg: Boron tri fluoride BF3
The Lewis structure is
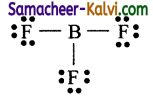
Formal charge on Boron:
Nv = 3; Nl = 0; Nb = 6 .
Formal charge = 3 – (0 + \(\frac{6}{2}\)) = 3 – 3 = 0
Question 8.
Give two examples of molecules with expanded valence shells.
Answer:
In molecules such as sulphur hexafluoride (SF6), phosphorous pentachloride (PCl5) the central atom has more than eight valence electrons around them. Here the central atom can accommodate additional electron pairs by using outer vacant d orbitals. In SF6 the central atom sulphur is surrounded by six bonding pair of electrons or twelve electrons.
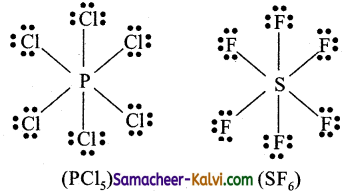
Question 9.
What are odd electron molecules? Give an example.
Answer:
There are number of stable molecules in which double bonds are formed by sharing of an odd number of electrons i.e., one, three, five etc., between two bonded»atoms. The bonds of these type are called odd electron molecules. In these bonds also, octet rule is violated.
eg:
He2+ ion, O2 molecule, nitric oxide, nitrogen dioxide molecules are examples of three electron bonds.
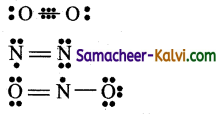
![]()
Question 10.
Mention the conditions for the two atoms to form ionic bond.
Answer:
(i) The electronegativity difference between the two combining atoms should be large.
(ii) The least electro negative atom should have lower ionization enthalpy and the other atom should have higher electron gain enthalpy.
(iii) The lattice energy of formation should be high.
Question 11.
Explain the formation of potassium chloride.
Answer:
The electronic configuration of potassium and chlorine are
Potassium (K): [Ar] 4s1
Chlorine (Cl) : [Ne] 3s2, 3p5
Potassium has one electron in its valence shell and chlorine has seven electron in its valence shell. By loosing one electron potassium attains the inert gas electronic configuration of argon and becomes a unipositive cation (K+) and chlorine accepts this electron to become uninegative chloride ion (Cl–) there by attaining the stable electronic configuration of argon. These two ions combine to form an ionic crystal in which they are held together by electrostatic attractive force.
Question 12.
Define Lattice energy.
Answer:
The energy released when the requisite number of gaseous positive and negative ions combine to form one mole of an ionic compound is known as lattice energy.
![]()
Question 13.
Explain the formation of coordinate covalent bond with an example.
Answer:
In the formation of coordinate covalent bond, one of the combining atom donates a pair of electrons (also known as donor atom) to an electron deficient acceptor atom and these atoms share their pair of electrons.
For example,In boron tri fluoride, boron atom is electron deficient and in ammonia the nitrogen has a lone pair of electron. In the formation of an adduct BF3 NH3, the nitrogen acts as a donor atom and boron acts as an acceptor atom. The bond formed between nitrogen and boron is a coordinate covalent bond.
Question 14.
Define the following (i) bond length (ii) bond angle (iii) bond order.
Answer:
(i) Bond length:
The distance between the nuclei of the two covalently bonded atoms is called bond length.
(ii) Bond angle:
The angle made by two covalent bonds is known as bond angle.
(iii) Bond order:
The number of bonds formed between the two bonded atoms in a molecule is called the bond order.
Question 15.
Define average bond enthalpy of’a bond. Explain with an example.
Answer:
The arithmetic mean of the bond energy values of the same type of bonds is considered as average bond enthalpy. For example in water, there are two OH bonds present and the energy needed to break them are not same.
H2O (g) → H(g) + OH(g) ∆H1 502 kJ mol-1
OH(g) → H(g) + 0(g) ∆H2 = 427 kJ mol-1
The average bond enthalpy of OH bond in water = \(\frac{502+427}{2}\) = 464.5 kJ mol-1
![]()
Question 16.
Mention the characteristics of resonance.
Answer:
(i) The contributing structures do not have real existence. Only resonance hybrid has the real existence.
(ii) As a result of resonance, the bond lengths become equal.
(iii) The resonance hybrid has the lowest energy and hence morè stable than the contributing structure.
(iv) Greater the resonance energy, greater is the stability of the molecule.
(v) Greater the number’ of qanonical forms, with nearly the same energy, greater is the stability of the molecule.
Question 17.
Write the resonance structures of the following:
(i) CO2
(ii) Carbonate ion (CO3-2)
(iii) Sulphur trioxide (SO3)
(iv) Nitrate ion (NO3–)
(v) Carbon monoxide(CO)
(vi) Nitrous acid (HNO2)
(vii) Sulphate ion (SO4-2)
Answer:
(i) CO2
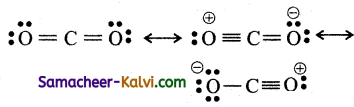
(ii) Carbonate ion (CO3-2)
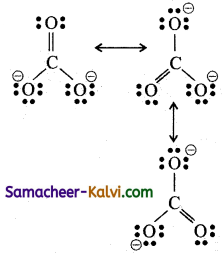
(iii) Sulphur trioxide (SO3)
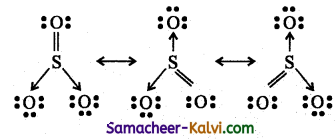
(iv) Nitrate ion (NO3–)
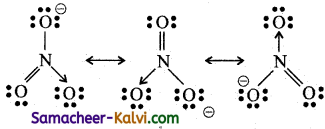
(v) Carbon monoxide(CO)
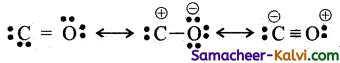
(vi) Nitrous acid (HNO2)
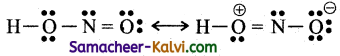
(vii) Sulphate ion (SO4-2)
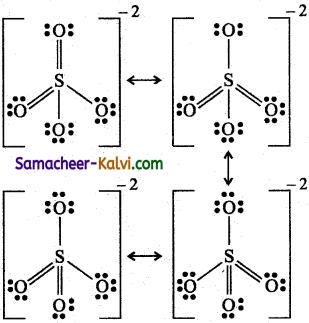
![]()
Question 18.
Define Resonance energy.
Answer:
The difference in energy between the most stable canonical structure and that of resonance hybrid is called resonance energy.
Question 19.
Diatomic molecules like H2, O2 etc have zero dipole moment. Explain why?
Answer:
In these diatomic molecules, the shared pair of electrons lie in between the nucleus of the two bonded atom. Hence, no separation of charges occur, i.e., these molecules have zero dipole moment.
Question 20.
Give examples for non polar molecule.
Answer:
Molecules which have a fixed value of dipole moment are known as non polar molecules, eg: HF, HCl, CO, NO etc…
![]()
Question 21.
Explain the formation of a polar covalent bond with an example.
Answer:
A polar covalent bond is formed between two atoms having a large difference in their electronegativities. In such a ease, the highly electronegative atom attracts the shared pair of electrons towards itself. As a result, a partial negative charge is developed on the electronegative atom arid a partial positive charge is developed on the electropositive atom, eg: Hδ+ – Fδ-. Thus apolarity is created between the two atoms. This type of covalent bond is known as polar covalent bond.
Question 22.
Explain why the dipole moment of carbon dioxide is zero.
Answer:
In CO2, the dipole moments of two polar bonds (CO) are equal in magnitude but have opposite direction. Hence, the net dipole moment of the CO2 is, p = µ1 + µ2
= µ1 + (-µ1) = 0.
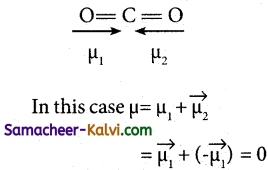
Question 23.
Water has a dipole moment of 1.85 D. Explain.
Answer:
Incase of water net dipole moment is the vector sum of µ1 + µ2 as shown.
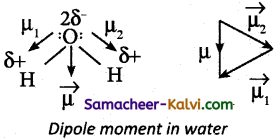
Dipole moment in water is found to be 1.85D.
![]()
Question 24.
How will you determine the extent of ionic character in a covalent bond?
Answer:
The extent of ionic character in a covalent bond can be related to the electro negativity difference to the bonded atoms. In a typical polar molecule, Aδ+ – Bδ–, the electronegativity difference (χA – χB) can he used to predict the percentage of ionic character as follows.
If the electronegativity difference (χA – χB)> is equal to 1.7, then the bond A – B has 50% ionic – character if it is greater than 1.7, then the bond A – B has more than 50% ionic character and if it is lesser than 1.7, then the bond A – B has less than 50% ionic character.
Question 25.
Explain the term polarization.
Answer:
In an ionic compound, there is an electrostatic attractive force, between the cation and anion. The positively charged cation attracts the valence electrons of anion while repelling the nucleus. This causes a distortion in the electron cloud of the anion and its electron density drifts towards the cation, which results in some sharing of the valence electrons between these ions. Thus, a partial covalent character is developed between them. This phenomenon is called polarization.
Question 26.
SnCl2 is ionic but SnCl4 is covalent. Explain.
Answer:
The size of Sn+4 is smaller than Sn+2. Hence, polarization or distortion of electrons charge cloud of chloride ion is more. Hence SnCl4 is covalent.
![]()
Question 27.
Among NaCl, MgCl2, AlCl3, AlCl3 is covalent, while others are ionic. Explain.
Answer:
Greater the charge of the ion greater is its polarisability. Hence greater is its covalent character. Among the cation, Al+3 ion has a smaller size and greater charge. Hence it could polarise the chloride ion to a greater extent and so AlCl3 is covalent.
Question 28.
Lithium chloride is more covalent than sodium chloride. Explain why?
Answer:
The size of the Li+ ion is smaller than Na+ ion. Hence the polarizing power of Li+ ion is more, i.e., it polarizes the chloride ion more that of Na+ ion. Hence LiCl is covalent.
Question 29.
Lithium iodide is more covalent than Lithium chloride. Explain why?
Answer:
Lithium iodide is more covalent than lithium chloride as the size of I– is larger than the Cl–. Hence I– will be more polarised than Cl– by the cation, Li+.
![]()
Question 30.
CuCl is more covalent than NaCl. Explain why?
Answer:
Cu+ ion is smaller in size, compared to that of Na+ ion and has 3s2 3p6 3d10 configuration. According to Fajan’s rule, cations having ns2 np6 nd10 configuration exercise greater polarising power. Therefore Cu+ ion polarizes chloride ion to a greater thafi Na+ ion. Hence, CuCl is covalent while NaCl is ionic.
Question 31.
The shape of SF4 is see saw and that of XeF2 is linear. Explain on the basis of VSEPR theory.
Answer:
Shape of SF4:
The Lewis structure for SF4 is
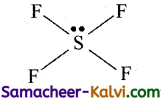
Sulphur atom has 5 electron pairs in it. i.e., 4 bond pairs and one lone pair. Hence it belongs to AB3L4. The expected shape is see-saw.
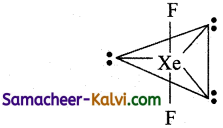
Shape of XeF2:
The Lewis structure for XeF2 is
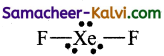
It has 3 lone pair and 2 bond pair. According to VSEPR theory, this belongs to AB2L3 type which has linear geometry.
![]()
Question 32.
Based on the VSEPR theory, predict the shapes of the following molecules,
(i) NH3
(ii) H2O
(iii) CIF3
Answer:
(i) Shape of NH3 molecule:
The Lewis structure for NH3 is
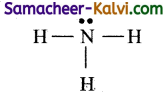
It has 3 bond pair and one lone pair of electron. According to VSEPR theory, this belongs to AB3L type. The l.p -l.p repulsion is greater than b.p – b.p repulsion. The shape is pyramidal with the bond angle 107°. ’
(ii) Shape of water molecule:
The Lewis structure for water molecule is
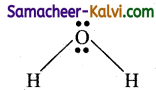
It has 2 bond pair and 2 lone pairs of electrons, According to VSEPR theory, this belongs to AB3L2 type, i.e., the shape is V shaped.
(iii) Shape of CIF3 molecule:
The Lewis structure for CIF3 is
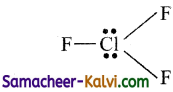
It has three bond pairs and one lone pair of electrons. The l.p – l.p repulsion is greater than b.p – b.p (or) b.p – l.p repulsions. Minimum repulsion between the electron pairs, when the lone pairs are at equatorial position, i.e., it has T shape.
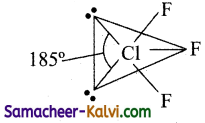
The lone pairs are present in equatorial positions and the bond pairs in axial position. According to VSEPR theory, this belongs to AB3L2 type, i.e., it has T shape.
Question 33.
Explain the salient features of valence bond theory.
Answer:
(i) This theory explains the formation of a covalent bond.
(ii) A covalent bond is formed by the overlapping of two atomic orbitals of the bonded atom.
(iii) For the formation of the strong covalent bond, the following conditions are to be met.
(a) The overlapping atomic orbital must contain one impaired electron and possess almost equal energy.
(b) In the region of maximum overlap the two electrons get paired.
(c) Greater the extent of overlap, stronger is the covalent bond formed.
(d) For maximum overlap, atomic orbital (except ‘s’ orbital) must approach along the same inter-nuclear axis.
![]()
Question 34.
Explain the formation of hydrogen molecule based on valence bond theory.
Answer:
Electronic configuration of hydrogen atom is lx1. During the formation of H2 molecule, the »lx orbitals of two hydrogen atoms containing one unpaired electron overlap with each other along the intemuclear axis. As the orbitals overlap with each other, the electrons get paired. This overlap is called x-x overlap. Such axial overlap results in the formation of a o- covalent bond.
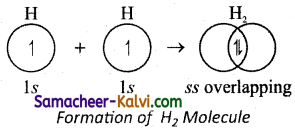
Question 35.
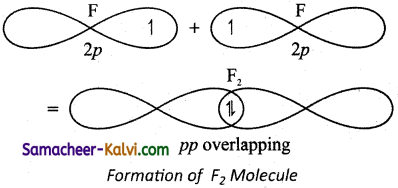
Explain the formation of fluorine molecule based on valence bond theory.
Answer:
Valence shell electronic configuration of fluorine atom : 2s2 2px2, 2py2, 2pz1
When the half filled pz orbitals of two fluorine overlaps along the z-axis, a a- covalent bond is formed between them.
![]()
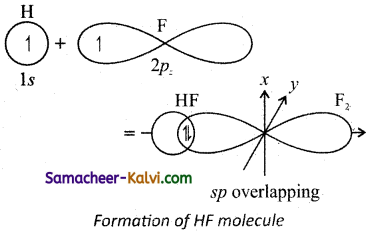
Question 36.
Explain the formation of (i) hydrogen fluoride and (ii) oxygen based on valence bond theory.
Answer:
Electronic configuration of hydrogen atom is 1s1
(i) Valence shell electronic configuration of fluorine atom:
2s2 2px2, 2py2, 2pz1. When half filled 1s orbital of hydrogen linearly overlaps with a half filled 2pz orbital of fluorine, a σ – covalent bond is formed between hydrogen and fluorine.
(ii) Valence shell electronic configuration of oxygen atom:2×2 2p2, 2py\ 2pzl
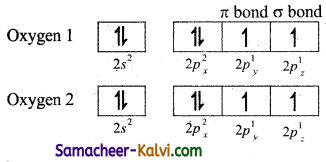
When the half filled pz orbitals of two oxygen overlaps along the z-axis (considering molecular axis as z axis), a o-covalent bond is formed between them. Other two half filled py orbitals of two oxygen atoms overlap laterally (sideways) to form a 7i-covalent bond between the oxygen atoms. Thus, in oxygen molecule, two oxygen atoms are connected by two covalent bonds (double bond). The other two pair of electrons present in the 2s and 2px orbital do not involve in bonding and remains as lone pairs on the respective oxygen.
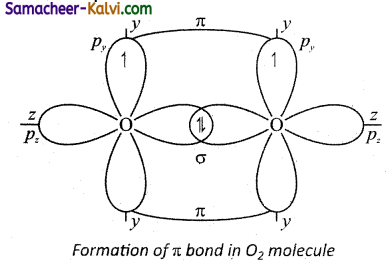
![]()
Question 37.
Briefly explain the shape of BeCl2 molecule based on hybridisation.
Answer:
The electronic configuration of Be is 1s2 2s2 and that of chlorine is 1s2 2s2 2p6 3s2 3p5.
Electronic configuration of Be in ground state:
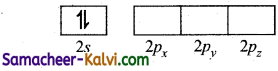
To form two covalent bonds, two atomic orbitals with one unpaired electron must be available.
Electronic configuration of Be in excited state:

To explain the equivalence of two Be — Cl bonds, one ‘2s’ and one ‘2p’ orbitals undergo sp hybridisation.
Electronic configuration of Be in hybridised state:
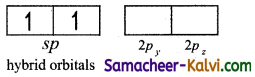
The two sp hybrid orbital orient in space in opposite direction making an angle of 180°. These sp hybrid orbitals overlap with 2pz orbital of chlorine and form two sigma bond.
Formation of BeCl2:
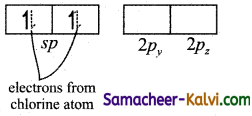
Thus the shape is linear. Cl – Be – Cl
Question 38.
Explain the shape of BF3 molecule based on hybridisation.
Answer:
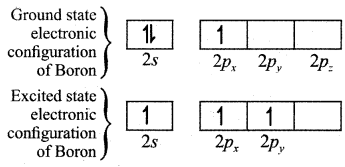
(i) In the ground state boron has only one unpaired electron in the valence shell. In order to form three covalent bonds with fluorine atoms, three unpaired electrons are electrons in the 2s orbital is promoted to the 2py orbital in the excite state. In boron, the s orbital and two p orbitals (px and py) in the valence shell hybridses, to generate three equivalent sp2 orbitals as shown in the Figure.

(ii) These three orbitals lie in the same xy plane and the angle between any two orbitals is equal to 120°.
(iii) The three sp2 hybridised orbitals of boron now overlap with the 2pz orbitals of fluorine (3 atoms). This overlap takes place along the axis as shown below.
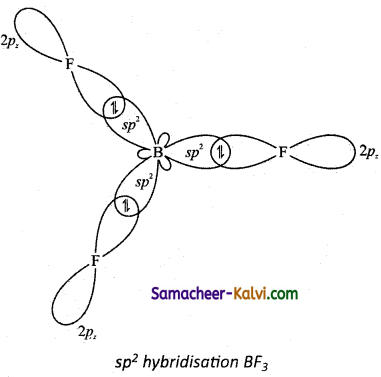
(iv) Formation of BF3 molecules:
![]()
Question 39.
Explain the sp3 hybridisation taking the formation of methane as example.
Answer:
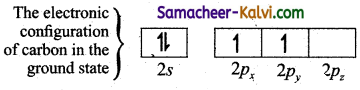
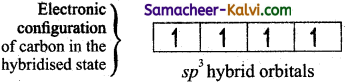
In order to form four covalent bonds with the four hydrogen atoms, one of the paired electrons in the 2s orbital of carbon is promoted to its 2pz orbital in the excite state.configuration of carbon in the excited stateThe one 2s orbital and the three 2p orbitals of carbon mixes to give four equivalent sp2 hybridised orbitals. The angle between any two sp3 hybridised orbitals is 109°28′.of carbon in the ?
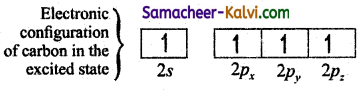
The one 2s orbital and the three 2p orbitais of carbon mixes to give four equivalent sp3 hybridised orbitais. The angle between any two sp3 hybridised orbitais is 109°28′.
The 1s orbitals of the four hydrogen atoms overlap linearly with the four sp3 hybridised orbitals of carbon to form four C – H σ – bonds in the methane molecule, as shown below.
Formation of CH4 molecule:
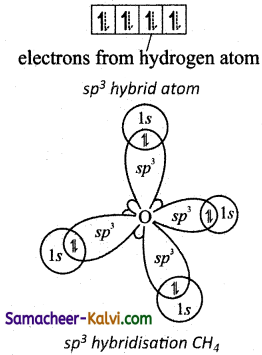
![]()
Question 40.
Explain the hybridisation in PCl5.
Answer:
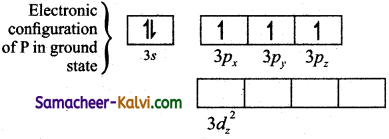
To form five covalent bonds one of the 2s electron is produced to vacant 3dz2 orbital.
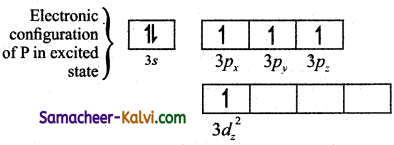
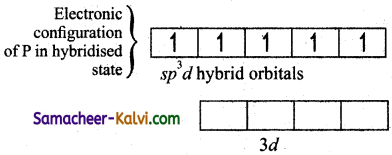
The orbital geometry of sp3 hybridised orbitals is trigonal bi-pyramidal as shown in the figure.
The 3pz orbitals of the five chlorine atoms linearly overlap along the axis with the five sp2d hybridised orbitals of phosphorous to form the five P – Cl σ – bonds, as shown below.
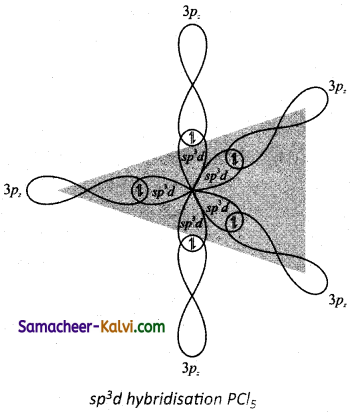
Formation of PCl5 molecule:
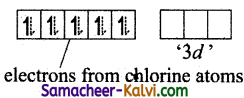
![]()
Question 41.
Explain the sp3d2 hybridisation with an example.
Answer:
(i) In sulphur hexafluoride (SF6) the central atom sulphur extend its octet to undergo sp3d2 hybridisation to generate six sp2cf hybridised orbitals which accounts for six equivalent S – F bonds. The ground state electronic configuration of sulphur is [Ne] 3s2 3px2 3py1 3pz1.
(ii) 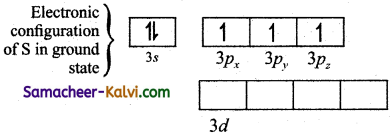
(iii) One electron each from 3s orbital and 3p orbital of sulphur is promoted to its two vacant 3d orbitals (dz2 and dx2 – y2) in the excite state.
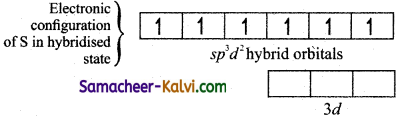
(iv) A total of six valence orbitals from sulphur (one 35 orbital, three 3p orbitals and two 3d orbitals) mixes to give six equivalent sp3d2 hybridised orbitals. The orbital geometry is octahedral as shown in the figure.
(v) 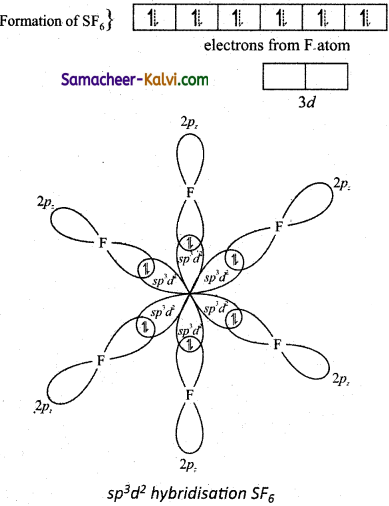
(vi) The six sp3d2 hybridised orbitals of sulphur overlaps linearly with 2pz orbitals of six fluorine atoms to form the six S – F bonds in the sulphur hexafluoride molecule.
![]()
Question 42.
Explain the salient features of molecular orbital theory.
Answer:
(i) When atoms combines to form molecules, their individual atomic orbitals lose their identity and forms new orbitals called molecular orbitals.
(ii) The shapes of molecular orbitals depend upon the shapes of combining atomic orbitals.
(iii) The number of molecular orbitals formed is the same as the number of combining atomic orbitals. Half the number of molecular orbitals formed will have lower energy than the corresponding atomic orbital, while the remaining molecular orbitals will have higher energy.
The molecular orbital with lower energy is called bonding molecular orbital and the one with higher energy is called antibonding molecular orbital. The bonding molecular orbitals are represented as σ (Sigma), π (pi), δ (delta) and the corresponding antibonding orbitals are denoted as σ*, π* and δ*.
(iv) The electrons in a molecule are accommodated in the newly formed molecular orbitals. The filling of electrons in these orbitals follows Aufbau’s principle, Pauli’s exclusion principle and Hund’s rule as in the case of filling of electrons in atomic orbitals.
(v) Bond order gives the number of covalent bonds between the two combining atoms. The bond order of a molecule can be calculated using the following equation,
Bond order = \(\frac{\mathrm{N}_{b}-\mathrm{N}_{a}}{2}\)
Where Nb = Total number of electrons present in the bonding molecular orbitals.
Na = Total number of electrons present in the antibonding molecular orbitals and A bond order of zero value indicates that the molecule doesn’t exist.
![]()
Question 43.
Explain the formation of bonding and antibonding molecular orbitals in terms of linear combination of atomic orbitals.
Answer:
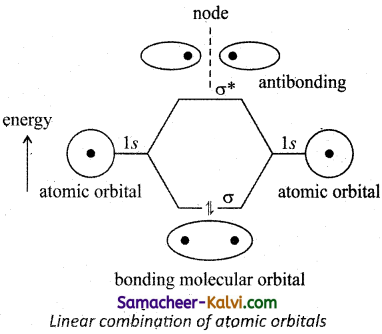
The wave function for molecular orbitals are obtained by linear combination of atomic orbitals.
(i) Two atomic orbitals represented by the wave function ψA and ψB with comparable energy, combines to form two molecular orbitals.
One is bonding molecular orbital(ψbonding) and the other is antibonding molecular orbital(ψantibonding). The wave functions for these two molecular orbitals can be obtained by the linear combination of the atomic orbitals ψA and ψB as below.
ψbonding = ψA + ψB
ψantibonding = ψA – ψB
(ii) The formation of bonding molecular orbital can be considered as the result of constructive interference of the atomic orbitals and the formation of antibonding molecular orbital can be the result of the destructive interference of the atomic orbitals.
Bonding molecular orbitals have lower energy compared to that of the atomic orbitals and antibonding molecular orbitals have higher energy compared to the atomic orbitals, node
![]()
Question 44.
Bring out the difference between q molecular orbital and it molecular orbital.
Answer:
| σ molecular orbital | π molecular orbital |
| It is formed by head to head overlap of atomic orbitals along inter nuclear axis. | It is formed by the sideways overlap of atomic orbitals perpendicular to inter nuclear axis. |
| The extent of overlap is maximum | The extent of overlap is minimum. |
| It leads to the formation of a strong covalent bond. | It leads to the formation of a weak covalent bond. |
Question 45.
Draw the energy level diagrams indicating the energy of the molecular orbitals.
Answer:
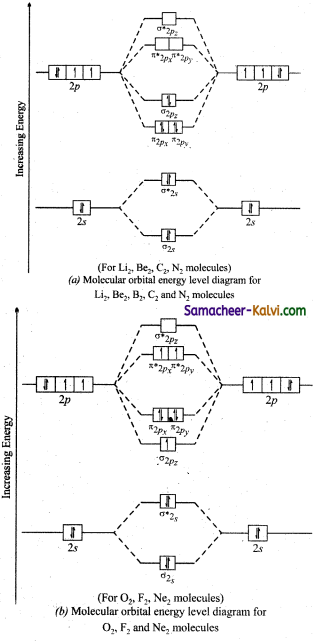
![]()
Question 46.
Draw the Molecular orbital diagram for
(i) H2 molecule
(ii) Li2 molecule
(iii) B2 molecule. Find their bond order. Indicate whether they are paramagnetic or diamagnetic. Answer:
(i) Molecular orbital diagram of hydrogen molecule (H2)
Electronic configuration of H atom 1s1
Electronic configuration of H2 molecule σ1s2
Bond order = \(\frac{\mathrm{N}_{b}-\mathrm{N}_{a}}{2}=\frac{2-0}{2}\) = 1
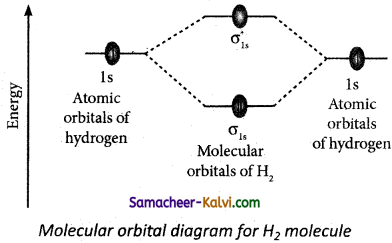
(ii) Molecular orbital diagram of lithium molecule (Li2)
Electronic configuration of Li atom 1s2 s1
Electronic configuration of Li2 molecule σ1s2, σ1s*2, σ2s2
Bond order = \(\frac{\mathrm{N}_{b}-\mathrm{N}_{a}}{2}=\frac{4-2}{2}\) = 1
Molecule has no unpaired electrons hence it is diamagnetic.
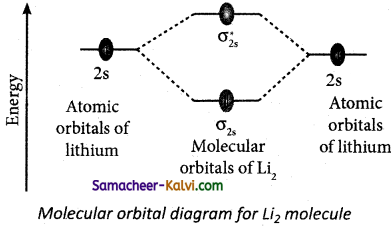
(iii) Molecular orbital diagram of boron molecule (B2)
Electronic configuration of B atom 1s2 2s2 2P1
Electronic configuration of B2 molecule σ1s2, σ*1s2, σ2s2, σ*2s2, π2py1, π2pz1
Bond order = \(\frac{\mathrm{N}_{b}-\mathrm{N}_{a}}{2}=\frac{6-4}{2}\) = 1
Molecule has two unpaired electrons hence it is paramagnetic.
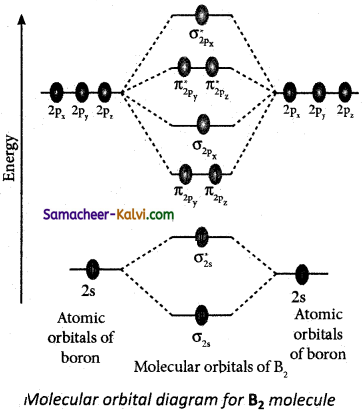
![]()
Question 47.
Give a brief account of metallic bonding.
Answer:
The force that keeps the atoms of the metal in a metallic crystal is known as metallic bond.
According to the theory of Drude and Lorentz, metallic bond is due to the positive charged metal ions and the free electrons produced by the ionisation of the metal atom. The free electrons are shared by all the ions in the crystal. These free electrons are uniformly distributed around the metal ions.
According to Molecular orbital theory the atomic orbitals of the metal combine to form molecular orbitals, which are-so close in energy to each other as to form a bond. The lowest lying empty bond is called conduction bond and the outer most filled bond is called valence bond.
(i) In metals, the energies of conduction bond and valence bond are close to each other, that there is no gap between them.
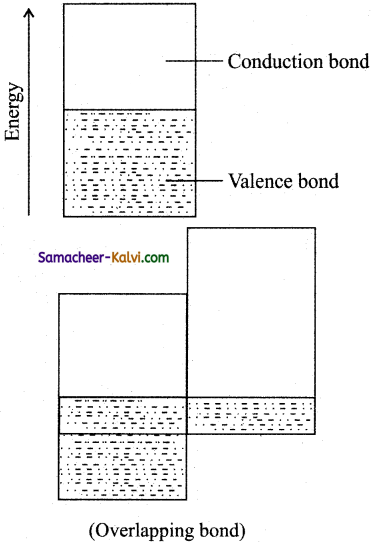
Thus electrons pass easily from valence bond to conduction bond when a potential difference is applied. This is the reason why metals are good conductors of electricity.
(ii) Metals are also good conductors of heat. This is due to excitation of electrons from the valence bond to conduction bond on heating (thermal excitation).
![]()
Choose the correct answer:
Question 1.
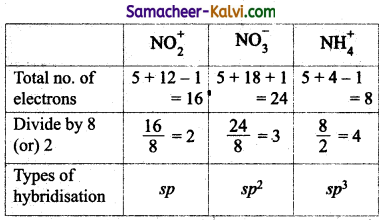
The types of hybrid orbitals of nitrogen in NO2+, NO3– and NH4+ respectively are j expected to be:
(a) sp, sp3 and sp2
(b) sp, sp2 and sp3
(c) sp2, sp and sp3
(d) sp2, sp3 and sp
Answer:
(b) sp, sp2 and sp3
Hint:
Question 2.
In NO3– ion the number of bond pairs and lone j pairs of electrons mid nitrogen atoms are:
(a) 2, 2
(b) 3, 1
(c) 1, 3
(d) 4, 0
Answer:
(d) 4, 0
Hint:
The Lewis structure of NO3– is
Thus it has 4 bond pair and no lone pair.
![]()
Question 3.
In which of the following central atom is sp2 hybridised?
(a) BH4–
(b) NH2–
(c) CO3-2
(d) H3O+
Answer:
(c) CO3-2
Hint:
In BH4–, NH2– and H3O+, the central atom in sp3 hybridised.
Step 1:
For CO3-2, the total no. of electron = 4+18 + 2 = 24
Step2:
Divide 24 by 8: The quotient (Q) = 3 and no remainder.
x = 3 or In CO3-2, C is sp2 hybridised.
Question 4.
Which molecule / ions, of the following does not contain unpaired electrons?
(a) N2+
(b) O2
(c) O2-2
(d) B2
Ans.
(c) O2-2
Hint:
The molecular orbital configuration of
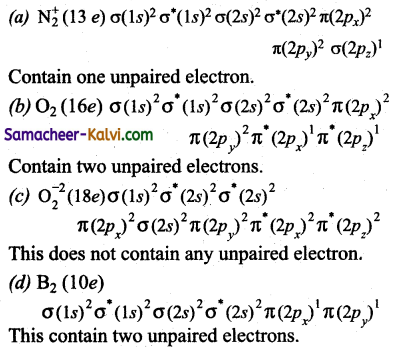
![]()
Question 5.
The H – H baond energy is 43.6 kJ mol-1 and Cl – Cl band energy is 242 kJ mol-1. H – Cl bond energy is 431 kJ mol-1
Choose the correct statement:
(a) The H – H bond length is the shortest among the molecules given.
(b) The H – Cl bond length is the shortest among the molecules given.
(c) The Cl – Cl bond length is the shortest among the molecules given.
(d) The H – H bond length is the highest among the molecules given.
Answer:
(a) The H – H bond length is the shortest among the molecules given.
Hint:
If bond enthalpy increases, bond length decreases.
Question 6.
The correct order of polarising power of the cations is:
(a) Li+ > Na+ > K+ > Rb+ > Cs+
(b) Cs+ > Rb+ > K+ > Na+ > Li+
(c) Li+ > K+ > Na+ > Rb+ > Cs+
(d) K+ > Li+ > Rb+ > Na+ > Cs+
Answer:
(a) Li+ > Na+ > K+ > Rb+ > Cs+
Hint:
When the size of the cation is smaller than the other cations with the same charge the smaller cation causes greater extent of polarisation.
![]()
Question 7.
Which of the following represents the correct bond order?
(a) O2 > O2– > O2+
(b) O2– < O2 < O2+
(c) O2– > O2 > O2+
(d) O2 > O2– > O2+
Answer:
(b) O2– < O2 < O2+
Hint:
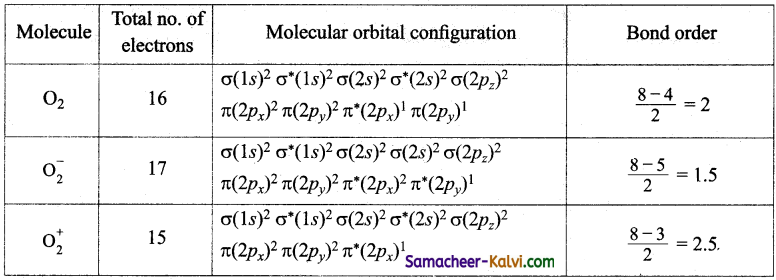
Question 8.
Which of the following has the most covalent j character?
(a) LiCl
(b) CsCl
(c) RbCl
(d) KCl
Answer:
(a) LiCl
Hint:
A small cation can polarise the large anion to a greater extent. Greater the extent of polarisation, greater is the covalent characters.
![]()
Question 9.
A molecule processing dipole moment is:
(a) CH4
(b) H2O
(c) BF3
(d) CO2
Answer:
(b) H2O
Hint:
In all other cases, the bond moments cancel each other and have zero dipole moment.
Question 10.
The type of overlapping of atomic orbitals involved in the formation of lithium hydride is:
(a) s – s overlap
(b) s – p overlap
(c) p – p overlap
(d) p – d overlap
Answer:
(a) s – s overlap
Hint:
The ‘2 s’ orbital of lithium and ‘1s’ orbital of hydrogen, each containing one unpaired electron overlap and form a sigma bond.
![]()
Question 11.
Which of the following species contain three bond pairs and one lone pair around the central atom?
(a) H2O
(b) BF3
(c) NH2–
(d) PCl3
Answer:
(d) PCl3
Hint:

Question 12.
Which of the following is a polar molecule?
(a) BF3
(b) SF4
(c) SeF4
(d) FeF4
Answer:
(b) (b) SF4
Hint:
SF4 has a distorted geometry due to the presence of a lone pair of electrons and hence polar. All the others are symmetrical and have zero dipole moment.
![]()
Question 13.
Which of the following pairs of ions are isoelectronic and isostructural?
(a) SO3-2, NO3–
(b) CIO3–, SO3-2
(c) CO3-2, SO3-2
(d) CrO3–, CO3-2
Answer:
(b) CIO3–, SO3-2
Hint:
SO3-2 – sp3 – pyramidal: 16 + 24 + 2 = 42e
CIO3– – sp3 – pyramidal: 17 + 24+ 1 = 42e
CO3-2 – sp2 – Triangular planar: 6 + 24 + 2 = 32e
NO3– – sp2 – Triangular planar: 7 + 24 + 1 = 32e
Question 14.
Some of the properties of the two species, NO3 and H3O+ are described below: Which one of them is correct?
(a) Dissimilar in hybridisation for the central atom with the different structures.
(b) so structural with the same hybridisation for the central atony
(c) so structural with the different hybridisation for the central, atom.
(d) Similar in hybridisation for the central atom with the different structures.
Answer:
(a) Dissimilar in hybridisation for the central atom with the different structures.
Hint:
NO3–: 5 + 18 + 1 = 24
\(\frac{24}{8}\) = 3 = sp2 hybridisation plane triangular structure.
H3+: 3 + 6 – 1 = \(\frac{8}{2}\) = 4 = sp3 hybridisation square pyramidal.
Thus hybridisation as well as structure are different.
![]()
Question 15.
The O – N – O bond angle is maximum in:
(a) NO3–
(b) NO2–
(C) NO2
(d) NO2+
Answer:
(d) NO2+
Hint:
NO3– has sp2 hybridisation and three resonating structures.
Hence the bond angle is 120°
NO2+ has no unshared electron. It has one bond pairs of electrons in two directions
![]()
The shape is linear with bond angle 120°.
NO2 has one unshared electron. Whereas NO2– has one unshared electron pair. Hence in N2– the repulsions on the bond pairs are more and the angle is less.
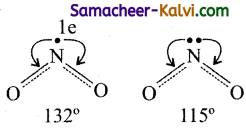
Question 16.
Assertion:
LiCl is covalent whereas NaCl is ionic.
Reason :
Greater the size of the- cation, greater is its polarising power.
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
Correct statement of reason: Smaller the size of the cation, greater is its polarising power.
![]()
Question 17.
Assertion:
The H — S — H bond angle in H2S is closer to 90° but H — O — H bond angle in water is 104.5°
Reason :
l.p – l.p repulsion is stronger in H2S than in H2O.
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
Hint:
Correct Explanation: Bond pair—Bond pair repulsions are greater in water than that in H2S because ‘O’ atom is smaller than ‘S’ atom.
Question 18.
Assertion:
NO3– ion is planar while NH3 is pyramidal.
Reason :
N in NO3– is sp2 hybridised and in NH3 it is sp3 hybridised.
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
![]()
Question 19.
Assertion:
The resonance hybrid is more stable than any of the contributing structures.
Reason :
The contributing structures contain the same number of unpaired electrons and have real existence.
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
Correct statement of reason: The contributing structures contain the same number of unpaired electron but do not have . real existence.
Question 20.
Assertion:
Molecular nitrogen is less reactive than molecular oxygen.
Reason :
The bond length of N2 is shorter than that of O2
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation for assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a) Both assertion and reason are true but reason is the correct explanation of assertion.
![]()
Question 21.
Select the odd man out from the following: The pair of molecules having identical geometry is:
(a) BCl3, PCl3
(b) BF3, NF3
(c) CCl4, CH4
(d) CHCl3, CH3Cl
Answer:
(c)
Hint:
CCl4 and CH4 have four bond pairs and have tetrahedral geometry whereas BCl3 is planar but PCl3 is pyramidal. BF3 is planar but NF3 is pyramidal. Both CHCl3 and CH3Cl have sp3 hybridisation but have different geometries because CHCl3 has three polar C — Cl bonds while in CH3Cl has only one C — Cl polar covalent bond.
Question 22.
In homonuclear molecules which of the following set of orbitals are degenerated?
(a) σ2s and σ1s
(b) π2px and π2py
(c) π2px and σ2pz
(d) σ2pz and π*2px
Answer:
(b) π2px and π2py
Question 23.
Among the following choose the one which have 2 bond pair and two lone pair of electrons on the central atom.
(a) BeCl2 and HgCl2
(b) CH4 and CCl4
(c) H2O and OF2
(d) PCl5 and AgF5
Answer:
(c) H2O and OF2
![]()
Question 24.
Which of the following pairs of compounds that cannot form hydrogen bond?
(a) HCl and HF
(b) H2O and HF
(c) CO2 and H2O
(d) CO2 + H2O2
Answer:
(d) CO2 + H2O2
Question 25.
Assertion:
Ionic compounds are non volatile.
Reason :
Intermolecular forces in these compounds are weak.
(a If both assertion and reasons are true and reason is the correct explanation of the assertion.
(b) If both assertion and reasons are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(c) If assertion is true but reason is false.
Question 26.
Assertion:
LiCl is predominantly a covalent compound.
Reason :
Electronegativity difference between Li nd Cl is too small.
(a) If both assertion ánd reasons are true and reason is the correct explanation of the assertion.
(b) If both assertion and reasons are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but rason is false.
(d) If both assertion and reason are false.
Answer:
(c) If assertion is true but rason is false.
Hint:
Electronegativity difference between Li and Cl is quite large covalent compound is formed due to high ionisation energy of Li.
![]()
Question 27.
Assertion:
Overall electron affinity to form O-2 is negative.
Reason :
First electron affinity of oxygen is negative while the second electron affinity is positive. The former is greater in magnitude than the latter.
(a) If both assertion and reasons are true and reason is the correct explanation of the assertion.
(b) If both assertion and reasons are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(a) If both assertion and reasons are true and reason is the correct explanation of the assertion.
Question 28.
Assertion:
O2 and N2– have the same bond order.
Reason :
O2 and N2– have the same number of electrons and same molecular orbital configuration.
(a) If both assertion and reasons are true and reason is the correct explanation of the assertion.
(b) If both assertion and reasons are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(c) If assertion is true but reason is false.
Hint:
In O2, σ(2pz) is filled first before π(2px) and π(2py) while in N2– π(2px) and π(2py) are filled first.
![]()
Question 29.
Assertion:
Na2SO4 is soluble in water while BaSO4 is insoluble.
Reason :
Lattice energy of BaSO4 exceeds its hydration energy.
(a) If both assertion and reasons are true and reason is the correct explanation of the assertion.
(b) If both assertion and reasons are true but reason is not the correct explanation of the assertion.
(c) If assertion is true but reason is false.
(d) If both assertion and reason are false.
Answer:
(a) If both assertion and reasons are true and reason is the correct explanation of the assertion.
Question 30.
Which of the following statement is incorrect regarding bonding molecular orbitals?
(a) Bonding molecular orbitals posses less energy than atomic orbitals from which they are formed.
(b) Bonding molecular orbitals have low electron densities between the two nuclei.
(c) Every electron in bonding molecular orbitals contributes to attraction between the atoms.
(d) They are formed when the lobes of combining atomic orbitals have the same sign.
Answer:
(b) Bonding molecular orbitals posses less energy than atomic orbitals from which they are formed.
Question 31.
Mark the incorrect statement in the following.
(a) The bond order in the species O2, O2+ and O2– decreases as O2+ > O2 > O2–.
(b) The bond energy in a diatomic molecule always increases when an electron is lost.
(c) Electrons in antibonding molecular orbitals contribute to repulsion between two atoms.
(d) With increase in bond order, bond length decreases and bond strength increases.
Answer:
(b) The bond energy in a diatomic molecule always increases when an electron is lost.
![]()
Question 32.
Choose the correct statement with regard to oxygen molecule.
(a) It is diamagnetic with no unpaired electrons.
(b) It is diamagnetic with two unpaired electrons.
(c) It is paramagnetic with two unpaired electrons.
(d) It is paramagnetic with no unpaired electrons.
Answer:
(c) It is paramagnetic with two unpaired electrons.
Question 33.
Polarisation is the distortion of the shape of an anion by an adjacently placed cation. Which j of the following statements is correct?
(a) Maximum polarisation is brought about by a cation of high charge.
(b) Maximum polarisation is brought about j by a cation of low radius.
(c) A huge cation is likely to bring about a lai c . degree of polarisation.
(d) Polarising power of a cation is less than that of an anion.
Answer:
(a) Maximum polarisation is brought about by a cation of high charge.
![]()
Question 34.
Which of the following statements is correct?
(a) HCl is covalent both in aqueous solution and in the gaseous state.
(b) HCl is covalent in the gaseous state but ionic in aqueous solution.
(c) HCl is ionic both in the gaseous state and in aqueous solution.
(d) None of the above.
Answer:
(b) HCl is covalent in the gaseous state but ionic in aqueous solution.
Question 35.
Match the entities of column I with appropriate entities of column II.
| Column I (molecule/ ion) | Column II (shape) |
| (i) SnCl<sub>2</sub> | (A) linear |
| (ii) CO<sub>3</sub><sup>-2</sup> | (B) V – shape (bent) |
| (iii) HgCl<sub>2</sub> | (C) Trigonal pyramidal |
| (iv) H<sub>3</sub>O<sup>+</sup> | (D) Triangular planar |
(a) (i) – (A), (ii) – (B), (iii) – (D), (iv) – (C)
(b) (i) – (D), (ii) – (C), (iii) – (A), (iv) – (B)
(c) (i) – (B), (ii) – (D), (iii) – (A), (iv) – (C)
(d) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)
Answer:
(c) (i) – (B), (ii) – (D), (iii) – (A), (iv) – (C)
![]()
Question 36.
Match the entities of column I with appropriate entities of column II.
| Column I (molecule/ ion) | Column II (Bond order) |
| (i) O2+ | (A) 1.5 |
| (ii) O2 | (B) 1.0 |
| (iii) O2– | (C) 2.5 |
| (iv) O2-2 | (D) 2.0 |
(a) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)
(b) (i) – (D), (ii) – (C), (iii) – (B), (iv) – (A)
(c) (i) – (A), (ii) – (B), (iii) – (D), (iv) – (C)
(d) (i) – (C), (ii) – (A), (iii) – (B), (iv) – (D)
Answer:
(a) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)