TN State Board 11th Chemistry Important Questions Chapter 12 Basic Concepts of Organic Reactions
Question 1.
Explain with example, homolytic and heterolytic cleavage of a covalent bond.
Answer:
(i) Homolytic cleavage:
In this type of cleavage a covalent bond breaks symmetrically such that each of the bonded atom retains one electron.
eg: 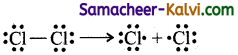
(ii) Heterolytic cleavage:
In this type of cleavage, a covalent bond breaks unsymmetrically such that one of the bonded atoms retains the bond pair of electrons.
eg: 
![]()
Question 2.
Explain the formation of tetra-butyl carbonium ion from tert butyl bromide.
Answer:
The carbon-bromine (— C — Br) bond is k polar covalent bond in tert-butylbromide due to greater electronegativity of bromine compound to that of carbon, i.e., As a result, the bromine atom acquires a slight negative charge and carbon atom a slight positive charge.
The — C — Br bond undergoes heterolytic fission, during a substitution reaction to form . tert-butyl carbocation which is more stable.
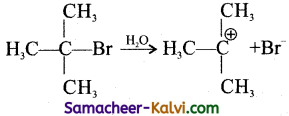
Question 3.
Give an example for the formation of carbanion.
Answer:
Carbanions are negatively charged carbon atom in an organic compound formed by the heterolytic cleavage of a C — H bond.
eg: 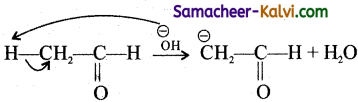
Carbanions are reaction intermediates formed during organic reactions.
![]()
Question 4.
Explain the hybridization of carbon in carbocation.
Answer:
The carbon atom in a carbocation has a positive charge, and is sp2 hybridised. Hence, it has a planar structure. It is formed during an organic reaction as an intermediate.
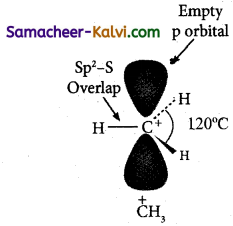
In the above structure, the three C — H bonds are formed by the overlap of the sp2 hybrid orbital of the carbon atom and Is orbital of hydrogen atom, each having one unpairedelectrons. The unhybridised ‘p’ orbital lies above and below the plane. The angle between H — C — H bond is 120°.
Question 5.
What is the shape of carbanion?
Answer:
The negatively charged carbon (carbanion) is sp3 hybridised. The hybrid orbitals are directed towards the comers of a tetrahedron. Three hybrid orbitals are involved in the formation of three sigma bonds with other atoms. While the fourth hybrid orbital contain lone pair of electrons. The lone-pair, bond-pair repulsion results in a pyramidal shape for the carbanion.
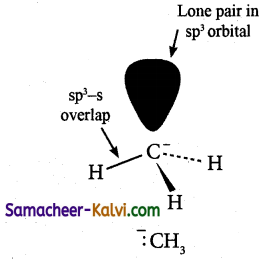
![]()
Question 6.
Briefly mention the types of electron movements which are used to explain organic reactions.
Answer:
There are three types of electron movement viz.,
Type 1:
A lone pair to a bonding pair:
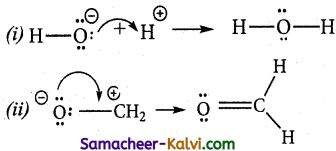
Type 2:
A bonding pair to a lone pair:
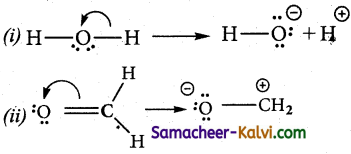
Type 3:
A bonding pair to an another bonding pair:
![]()
Question 7.
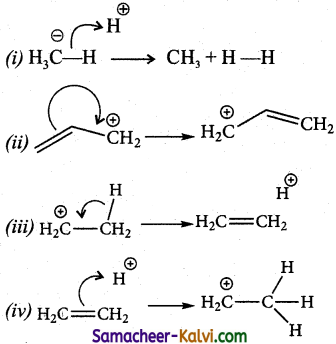
What is a free radical? How is it formed? Indicate the type of hybridization in an alkyl free radical.
Answer:
(i) Free radicals are neutral species.
(ii) They are formed by the homolytic fission of a covalent bond in such a way that each bonded atom retains one of the bond pair of electrons.
eg: H – H → H. + H.
H3C – H → H3C . + .H
(iii) The carbon atom in an alkyl free radical is sp2 hybridised. An alkyl free radical may be either planar or pyramidal. The odd electron present in the unhybridised ‘p’ orbital.
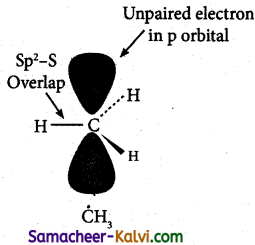
Question 8.
Explain inductive effect with an example.
Answer:
(i) Inductive effect is defined as the change in the polarisation of a covalent bond – due to the presence of adjacent bonds atoms or groups in the molecule. This is a permanent phenomenon.
(ii) The C – Cl bond in ethyl chloride is a polar covalent bond because chlorine is more electronegative than carbon. As a result, Chlorine acquires a small negative charge and the carbon atom acquires small
positive charge.

The positively charged carbon atom (1), draws the shared pair of electrons between itself and C2. This type of polarization effect is called inductive effect.
(iii) The electron displacement in inductive effect is permanent.
(iv) Groups which draws electrons away from the carbon chain but towards the substituents are said to exert -I effect.
eg: F, Cl, COOH, NO2, NH2.
(v) If the electron displacement is towards the carbon chain and away from the substituent, it is known as +1 effect. The groups which cause +I effect are CH3O–, C2H5O–, COO– etc…
![]()
Question 9.
Explain how the electromeric effect explains the nucleophilic addition in a carbonyl compound.
Answer:
The carbon-oxygen bond in a carbonyl group contains a σ and π bond. When a nucleophile approaches the carbonyl compound, the pi (π) electron are shifted towards the oxygen atom there by creating a positive charge on the carbon atom, which forms a new bond between the electron deficient carbon atom .and the nucleophile.
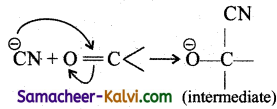
The electro-positive part of the hueleophile forms a bond with the oxygen atom to form the addition product.
Question 10.
Write the resonance structure for 1, 3 butadiene.
Answer:
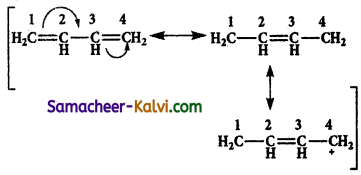
![]()
Question 11.
Explain +M (positive mesomeric) effect with an example.
Answer:
+M (positive mesomeric) effect arises, when the π electron shift occurs away from the substituent but towards the conjugated system.
eg: 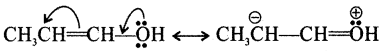
Generally, electron releasing substituents exert +M effect. In the above example, the lone pair of electrons on the oxygen atom, of the hydroxyl group through +M effect moves to the carbon atom which gains a positive charge.
Question 12.
Explain -M (negative mesomeric) effect with an example.
Answer:
When the n electrons shift towards the substituent but away from the conjugated system, it is known as -M (negative mesomeric) effect, eg:
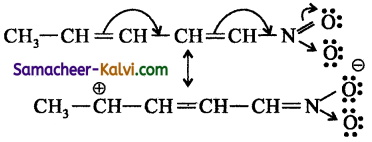
In this case, the nitro group (electron withdrawing group), through its -M effect pulls the π electron pair towards itself across the conjugated system, giving rise to the resonance structure as shown.
Electron withdrawing substituents like CHO, COOH, CN etc exert -M effect.
![]()
Question 13.
Draw the resonance structures of phenol and phenoxide ion.
Answer:
(i) In phenol, the lone pair of electrons on the oxygen atom of the phenolic group enters into conjugation with the benzene ring resulting in the following canonical structures.
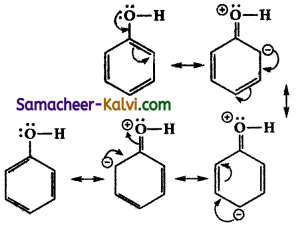
(ii) Phenoxide ion contains a negative charge on the oxygen atom attached to the benzene ring. This negative charge is dispersed throughout the benzene ring by resonance. This enhances the stability of phenoxide ion. The various canonical structures for phenoxide ion is
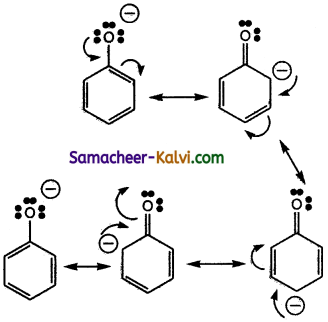
![]()
Question 14.
Account for the stability of the tertiary, secondary and primary carbocations based on hyper conjugation.
Answer:
The stability of the earbocation decreases in the order.
3° earbocation > 2° earbocation > 1° carbocation
Greater the number of alkyl groups attached to the carbon bearing positive charge, greater is number of the hyper conjugate structure.
Question 15.
Give an example for nucleophilic substitution reaction.
Answer:
In a nucleophilic substitution, a nucleophile (negatively charged reagent or a neutral molecule in which the central atom has a lone pair of electrons) attack a positively charged reactions site, and expel an atom or a group – from the substrate as a negative ion.
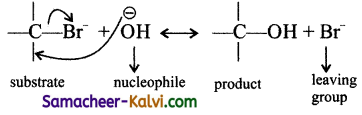
![]()
Question 16.
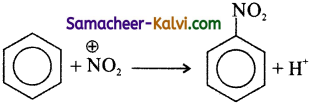
Give an example for electrophilic substitution reaction.
Answer:
Nitration of benzene, in the presence of a mixture of concentrated nitric acid and concentrated sulphuric acid gives nitro benzene. This is an example of electrophilic substitution reaction.
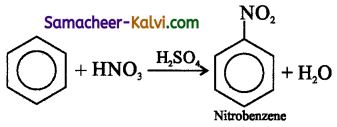
In this reaction, NO2+ ion (nitronium ion) acts an electrophile, attacks the electron rich carbon atom of the benzene molecule, and expel the hydrogen atom as H+ ion.
![]()
Question 17.
Explain free radical substitution reaction with an example.
Answer:
A free radical substitution reaction is one in which a free radical (reagent) replaces an atom or a group from the substrate molecule, with an odd electron (free radical). Chlorination of methane is an example of free radical substitution reaction.
![]()
In this reaction, the chlorine free radical, replaces hydrogen atom from CH4 as shown above.
Free radical substitution reactions occur through a chain reaction, which involves the following steps.
(i) initiation
(ii) propagation and
(iii) termination.
Question 18.
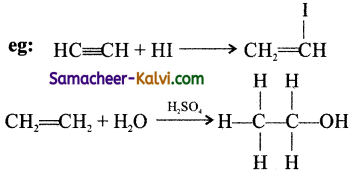
Give a brief account of addition reactions.
Answer:
Addition reactions are those in which the attacking species adds up to the substrate molecule to give a single product molecule. Usually, double or triple bonded compounds undergo addition reactions. In these reactions, a triple bond is converted to a double bond, and double bonds are converted to single bonds. For each pi(π) bond of the molecule, two sigma(σ) bonds are formed and the hybridisation of the carbon atom changes from sp to sp2 and sp2 to sp3.
![]()
Question 19.
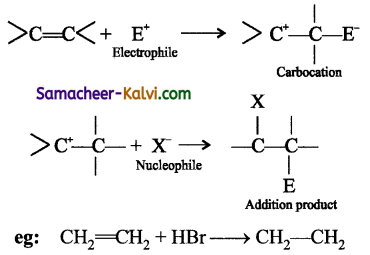
Give an example for electrophilic addition reaction.
Answer:
In an electrophilic addition reactions, an electrophile approaches, the double or triple bond in the first step forms a covalent bond with one of the carbon atoms resulting in the formation of a carbocation, which then takes nucleophile to result in the addition product.

Question 20.
Give an example for (i) free radical addition reaction, (ii) nucleophilic addition reaction.
Answer:
(i) Free radical addition reaction:
Photo chemically catalysed addition of chlorine to ethylene is an example of free radical addition reactions.
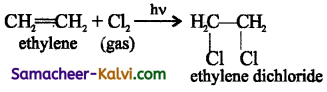
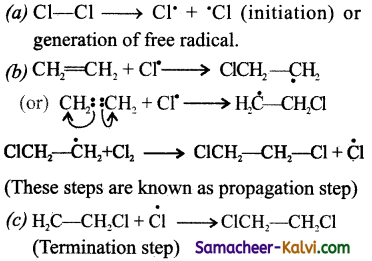
The mechanism involves the following steps:
(ii) Nucleophilic addition reaction:
When a nucleophile attacks an electron deficient reaction site, the reaction is known as a nucleophilic addition reaction. Aldehydes and ketones undergo nucleophilic addition reaction.
The carbonyl group in aldehydes and ketones gets polarised due to the presence of highly electronegative oxygen atom as shown below:
![]()
The nucleophile, attacks for the electron deficient carbon atom and forms an intermediate, which is further attacked by the electrophile on the negatively charged oxygen atom to form the addition product.
eg: Addition of HCN.to acetone.
![]()
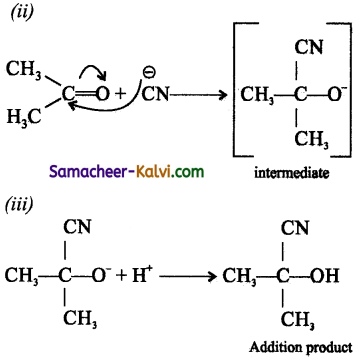
![]()
Question 21.
What are elimination reactions? Give an example.
Answer:
An elimination reaction is one which involves the loss of two atoms or groups from the same or adjacent carbon atom resulting in the formation of π bonds (double or triple). In an elimination reactions, the hybridised state of carbon get changed. Whenever an elimination reaction involves the formation of alkene, the sp3 hybridised state of carbon is changed to sp2 hybridised state.
eg: Base catalysed dehydrohalogenation of alkyl halide to give an alkene.
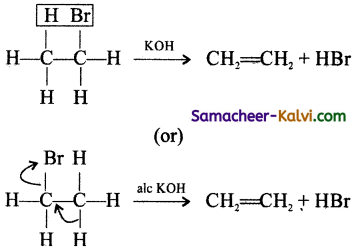
Question 22.
Write a short note on oxidation reduction reaction in organic chemistry.
Answer:
Organic reactions involving addition of oxygen to a substrate are known as oxidation reaction and these involve addition of hydrogen to the substrate is known as reduction reaction. They are brought about by oxidising and reducing agents. General oxidising agents used are acidified KMnO4, acidified K2Cr2O7, H2SO4 etc., Mostly used reducing agents are sodium and ethanol, Lithium aluminium hydride, Zn and HCl, Sn and HCl etc.
eg: Oxidation
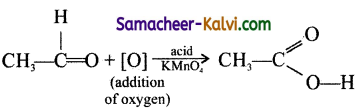
eg: Reduction
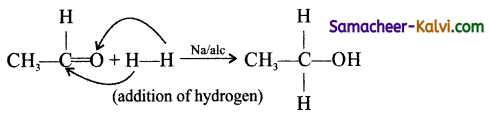
![]()
Question 23.
How will you convert
(i) an alcohol to aldehyde
(ii) an alcohol to Ketone
Answer:
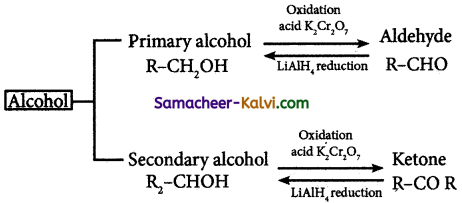
Question 24.
What are the various steps involved in the conversion of an alkyl cyanide to a carboxyl acid?
Answer:
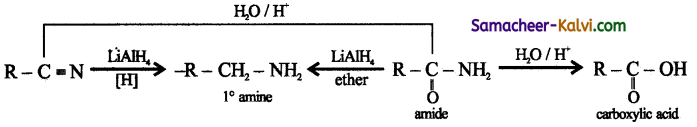
Question 25.
How will you convert (i) an alkyl halide to alcohol (ii) an alkene to alcohol (iii) an alkyl halide to alkene.
Answer:
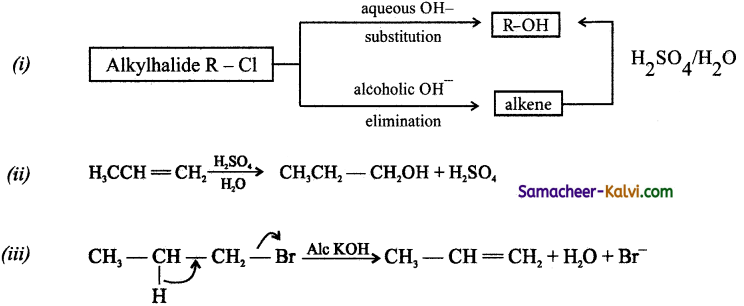
![]()
Choose the correct answer:
Question 1.
Homolytic fission of a covalent bond produces:
(a) cation
(b) anion
(c) both cation and anion
(d) free radical
Answer:
(d) free radical
Question 2.
In the compound, CH,C – Cl, the 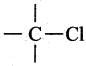 bond undergoes heterolytic fission. The species formed are:
bond undergoes heterolytic fission. The species formed are:
(a) ![]()
(b) ![]()
(c) ![]()
(d) both (a) and (b)
Answer:
(d) both (a) and (b)
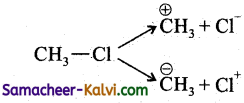
Hint:
depending on the energy supplied to break the bond.
![]()
Question 3.
Choose the correct statement:
(a) The carbon atom in an alkyl free radical is sp3 hybridised.
(b) The carbon atom in a carbocation is sp3 hybridised.
(c) The carbocation has a planar structure.
(d) The carbon atom in an alkyl carbanion is sp2 hybridised.
Answer:
(c) The carbocation has a planar structure.
Hint:
The carbon atom in an alkyl carbon cation is sp2 hybridised and has a planar structure.
Question 4.
Choose the correct statement:
The stability of:
(a) Carbocation is achieved by decreasing the positive charge on the carbon.
(b) The stability of the carbocation is achieved by increasing the positive charge on the carbon atom.
(c) Among (CH3)3 C+ and (CH3)2CH+, the latter is more stable.
(d) Both (a) and (c)
Answer:
(a) Carbocation is achieved by decreasing the positive charge on the carbon.
Hint:
The stability of carbocation is influenced by both resonance and inductive effects. An alkyl group has electron releasing inductive effect. An alkyl group attached to the positively charged carbon atom tends to release electrons towards that carbon atom.
In doing so, it reduces the positive charge, on the carbon. In other words, the positive charge gets dispersed as a result, the alkyl group becomes somewhat positively charged itself. The dispersal of charge stabilizes the cafbocation. More the number of alkyl groups, more is the stability of the carbonation.
![]()
Question 5.
Which of the following Carbocation would have the greatest stability?
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Answer:
(b) ![]()
Hint:
The lone pair of electrons present in s is present in 3p orbital, while these of N, O and F are present in 2p orbital. Further the carbon atom bearing the positive charge has an empty 2p orbital. Effective resonance stabilisation occurs between orbitals of similar sizes. Hence, least resonance stabilisation occurs between S and C atoms. Among N, O and F, Nitrogen is least electronegative. Hence most effective resonance stabilisation occurs between N and C.
Question 6.
In which of the following compounds, C – Cl bond ionization shall give most stable carbonium ion?
(a) 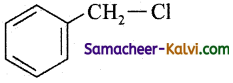
(b) O2NCH2 – CH2 – Cl
(c) 
(d) 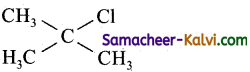
Answer:
(d) 
Hint:
It gives most stable tertiary carbonium ion.
![]()
Question 7.
The ascending order of stability of the carbanion:
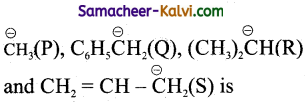
(a) P < R < S < Q
(b) R < P < S < Q
(c) R < P < Q < S
(d) P < R < Q < S
Answer:
(b) R < P < S < Q
Hint:
The stability of carbanion increases with -increase in ‘s’ Character of the carbon atom carrying the negative charge.
eg: 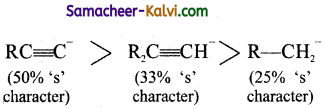
In general, the stability of various carbanions decrease in the order.
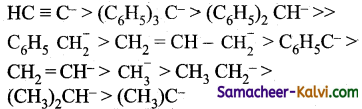
Question 8.
Arrange the carbanions:
(CH3)3C–, Cl3C–, (CH3)2 CH–, C6H5CH2– in order of their decreasing stability.
(a) (CH3)2CH– > Cl3C– > C6H5CH2– > (CH3)3C–
(b) Cl3C– > C6H5CH2– > (CH3)2CH– > (CH3)3 C–
(c) (CH3)3C– > (CH3)2CH– > C6H5CH2– > Cl3C–
(d) C6H5CH2– > Cl3C– > (CH3)3C– > (CH3)2CH–
Answer:
(b) Cl3C– > C6H5CH2– > (CH3)2CH– > (CH3)3 C–
Hint:
Due to -I effect of the three chlorine atoms Cl3C– is the most stable. This is followed by C6H5 CH2–, which is stabilised by resonance. Out of (CH3)3C– and (CH3)2CH–, (CH3)2 CH– is more stable due to + I effect.
![]()
Question 9.
The lone pair of electrons in a carbanion is present in:
(a) sp3 hybrid orbital
(b) sp2 hybrid orbital
(c) empty orbital
(d) none of the above
Answer:
(c) empty orbital
Question 10.
The relative stability of alkyl free radical is in the order:
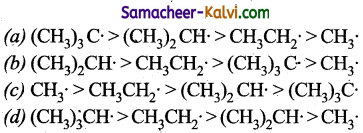
Answer:
(a) 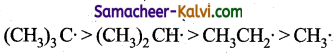
Hint:
The order of stability of free radicals can be explained on the basis of hyper conjugation. Greater the number of alkyl groups attached to the carbon atom carrying the odd electron, greater is the delocalisation of the electron and hence more stable the free radical.
![]()
Question 11.
Which of the following pairs of species are neutral electrophiles?
(a) NH3 and CI+
(b) CO2 and H3O+
(c) dichloro carbene (: CCl2) and proton
(d) carbon dioxide and dichloro carbene
Answer:
(d) carbon dioxide and dichloro carbene
Question 12.
Three sets of charged species are given. Which one of the following sets act as a nucleophile?
(a) H2O, AlCl3, H+
(b) AlCl3, H3O+, OH–
(c) CH3 NH2, CH3OH, I–
(d) CH3O–, CH3COO–, CH3+
Answer:
(c) CH3 NH2, CH3OH, I–
Hint:
CH3NH2 and CH3OH are neutral nucleophile, I– is negative nucleophile.
![]()
Question 13.
The relative stability of alkyl carbanion decreases in the order:
CH3 > 1° > 2 ° > 3°. This is explained by:
(a) inductive effect
(b) resonance effect
(c) both inductive and resonance effect
(d) hyper conjugative effect
Answer:
(a) inductive effect
Hint:
When an alkyl group is attached to a negatively charged carbon atom of the carbanion, it tends to release electrons towards that carbon. Thus it increases the electron density (- ve charge) on the carbon and destabilizes the carbanion. i.e., lesser the negative charge on the carbon atom, greater is its stability.
More the number of alkyl groups around the carbon atom carrying the negative charge, more is the destabilization and hence less stable the carbanion.
![]()
Question 14.
The group which show -I effect are:
(a) (CH3)3C–
(b) CH3–
(c) COO–
(d) SO3H
Answer:
(d) SO3H
Hint:
Higher the electronegativity of the substituent, greater is it’s -I effect.
Question 15.
Arrange the following groups in the decreasing order of -I effect.
NO2 (I), CHO (II), F (III), H (IV)
(a) CHO > F > H > NO2
(b) F > CHO > NO2 > H
(c) H > F > CHO > NO2
(d) NO2 > CHO > F > H
Answer:
(d) NO2 > CHO > F > H
Hint:
Higher the electronegativity of the substituent, greater is it’s -I effect.
![]()
Question 16.
Among the following which is most acidic?
(a) CH3COOH
(b) ClCH2 COOH
(c) Cl2CHCOOH
(d) Cl3CCOOH
Answer:
(d) Cl3CCOOH
Hint:
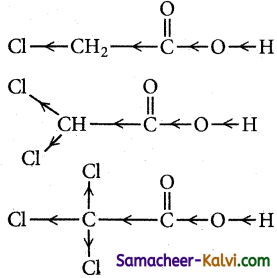
When a halogen atom is attached to the carbon which is nearer to the carboxylic acid group, its -I effect withdraws the bonded electrons towards itself and makes the ionisation of H+ easy. The acidity of various chloro acetic acid is in the following order. The strength of the acid increases with increase in the -I effect of the group attached to the carboxyl group.
Trichloro acetic acid > Dichloro acetic acid > Chloro acetic acid > acetic acid
Question 17.
Which does not represent the correct order of -I effect of the substituent?
(a) I < Cl < Br < F
(b) RS– < R2N– < RO–
(c) R2O+ < R2N+ > R2S
(d) R2O+ > RO– > C6H5O–
Answer:
(a) I < Cl < Br < F
Hint:
The -I effect of halogens follows the order I < Br < Cl < F.
![]()
Question 18.
In which of the following compounds, nucleophilic substitution reaction takes place at a faster rate?
(a) (CH3)3CF
(b) (CH3)3CCl
(c) (CH3)3CBr
(d) (CH3)3CI
Answer:
(a) (CH3)3CF
Hint:
When a highly electronegative atom such as halogen is attached to a carbon then it makes the C — X bond polar. In such cases the – I effect of halogen facilitates the attack of an incoming nucleophile at the polarised carbon, and hence increases the reactivity. The – I effect of halogen follow the order I < Br < Cl < F
Question 19.
The following order of acidity of the carboxylic acids
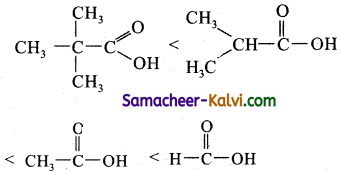
is due to
(a) + I Inductive effect
(b) – I Inductive effect
(c) Resonance effect
(d) Both inductive and resonance effect
Answer:
(a) + I Inductive effect
Hint:
The acidity of carboxylic acid to the ionisation of – COOH group as
RCOOH ⇌ RCOO– + H+
The + I effect of alkyl groups, increase the electron density around carboxyl carbon, which makes O – H bond stronger and the removal of proton more difficult. Greater the number of alkyl groups attached to the carbon atoms carrying the COOH group, lesser its acidity.
![]()
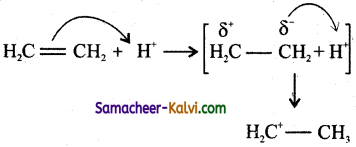
Question 20.
In which of the following, the electron displacement is due to +E electromeric effect?
(a) 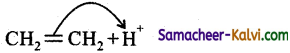
(b) ![]()
(c) 
(d) both (a) and (b)
Answer:
(a) 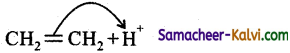
Hint:
If the electrons due to pi (π) bond are transferred to that atom of the double bond to which the reagent gets finally attached, the effect is called +E effect.
Question 21.
Which of the following is the most correct electron displacement for a nucleophile reaction to take place?
(a) 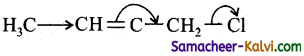
(b) 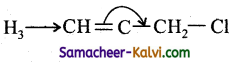
(c) 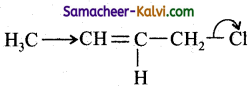
(d) 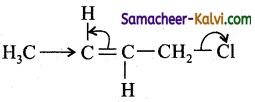
Answer:
(a) 
Hint:
In, nucleophilic displacement reactions the flow of electron occurs in such a way to break the C – Cl bond and expel Cl as Cl–.
![]()
Question 22.
Which of the following electron displacement refer to + R or + M effect?
(a) 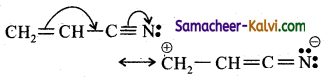
(b) 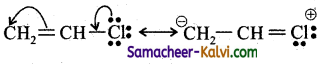
(c) 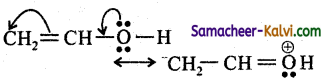
(d) both (b) and (c)
Answer:
(a) 
Hint:
Groups which withdraw electrons from the double bond or from a conjugated system towards themselves due to resonance are said to have +R or +M effect.
Question 23.
Resonance effect involves:
(a) Delocalisation of pi – electrons along a conjugated system.
(b) Delocalisation of n – electrons along a conjugated system.
(c) Delocalisation of π – electrons along a conjugated system.
(d) All the above
Answer:
(d) All the above
Hint:
Resonance effect involves delocalisation of non-bonding pi and sigma electrons (hyper conjugation) to the adjacent π bond.
![]()
Question 24.
1, 3 – butadiene has the structure 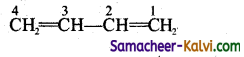
Choose the correct statement.
(1) The bonds between C1 – C2 and C3 – C4 are shorter than that of C2 – C3.
(2) The bond length between all the carbon atoms are the same.
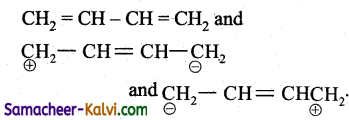
(3) 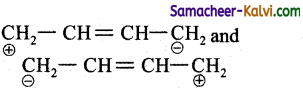 are the canonical structure of the compound.
are the canonical structure of the compound.
(4) The actual structure is a resonance hybrid of the contributing structures
(a) all
(b) 1, 3, 4
(c) 2, 3, 4
(d) 3, 4
Answer:
(c) 2, 3, 4
Hint:
1, 3 butadiene exhibits resonance. The actual structure is the resonance hybrid of all the contributing structures. Because of resonance all C — C bonds in the compound are equal.
Question 25.
The effect that makes 2, 3 dimethyl 2 butene more stable than 2-butene is:
(a) resonance
(b) hyper conjugation
(c) electromeric effect
(d) inductive effect
Answer:
(b) hyper conjugation
Hint:
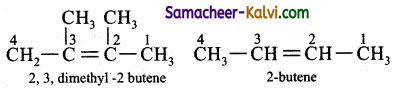
more hyper conjugative structures are possible for 2, 3 dimethyl 2 – butene than 2 butene. Hence it is more stable.
![]()
Question 26.
Which of the following compounds show hyper conjugation?
(a) CH2CHCl
(b) CH2CHCN
(c) CH3 CH = CH2
(d) all
Answer:
(c) CH3 CH = CH2
Hint:
In propene, the o-electrons of C – H bond of methyl group can be delocalised into the π – orbital of doubly bonded carbon as represented below.
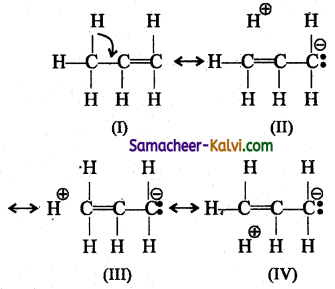
In the above structure the sigma bond is involved in resonance and breaks in order to supply electrons for delocalisation giving rise to 3 new canonical forms. In the contributing canonical structures: (II), (III) & (IV) of propene, there is no bond between an a-carbon and one of the hydrogen atoms. Hence the hyperconjugation is also known as “no bond resonance” or “Baker-Nathan effect”. The structures (II), (III) & (IV) are polar in nature.
(ii) Hyper conjugation effect is also observed when atoms / groups having lone pair of electrons are attached by a single bond, and in conjugation with a n bond. The lone pair of electrons enters into resonance and displaces 71 electrons resulting in more than one structure.
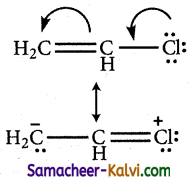
(iii) When electronegative atoms or group of atoms are in conjugation with a 71 -bond, they pull % – electrons from the multiple bond.
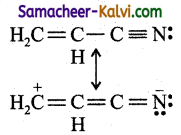
![]()
Question 27.
CH3 CH2 Br + aqueous KOH → CH3 CH2 OH + KBr. This is an example of:
(a) nucleophilic substitution reaction
(b) free, radical substitution reaction
(c) electrophilic substitution reaction
(d) nucleophilic addition reaction
Answer:
(a) nucleophilic substitution reaction
Hint:
![]() (nucleophilic)
(nucleophilic)
OH– replaces in CH3CH2 Br. Hence it is nucleophilic substitution reaction.
Question 28.
Which of the following is an example for an elimination reaction?
(a) CH3CH2Br + aq KOH → CH3CH2OH + KBr + H2O
(b) CH3CH2Br + alc. KOH → CH2 = CH2 + KBr + H2O
(c) CH3 – CHO ![]() CH3COOH
CH3COOH
(d) CH2 = CH2 + HBr → CH3CH2Br
Answer:
(b) CH3CH2Br + alc. KOH → CH2 = CH2 + KBr + H2O
Hint:
The hydrogen and bromine atom from adjacent carbon atoms are eliminated and a new double bond is created between the two carbon atoms.
![]()
Question 29.
Assertion:
Tertiary carbocations are generally formed more easily than primary carbocations.
Reason :
Hyper conjugation as well as inductive effect due to additional alkyl groups stabilise tertiary carbocations. .
(a) Both assertion and reason are true and the reason is the correct explanation of assertion.
(b) Both assertion and reason are true but the reason is not the correct explanation of assertion.
(c) Assertion is true but the reason is false.
(d) Both assertion and reason are false.
Answer:
(a) Both assertion and reason are true and the reason is the correct explanation of assertion.
Question 30.
Assertion:
Alkyl carbanion have pyramidal shape.
Reason :
The carbon atom carrying negative charge has an octet of electrons.
(a) Both assertion and season are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
Hint:
Correct Reason.
Because of lone pair – bond pair repulsion.
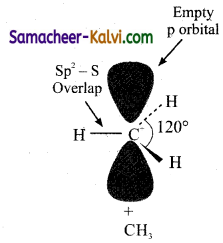
![]()
Question 31.
Assertion:
The Chlorine atom attached to 1 – Chloropropane exerts -I effect.
Reason:
Chlorine being more electronegative pulls the electron pair towards itself from the carbon atom to which it is attached.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are fals
Answer:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
Question 32.
Assertion:
Phenol is acidic because the phenoxide ion is more stabilized than phenol by resonance.
Reason:
The ‘OH’ group in phenol exerts -M effect.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
The ‘OH’ group in phenol exerts +M effect.
![]()
Question 33.
Assertion:
The compound propene CH3CH = CH2 gives three hyper conjugative structures.
Reason:
The pi electron in propene are involved in hyper conjugation.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
The 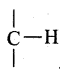 group, adjacent to C = C, is involved hyper conjugation (σ electrons).
group, adjacent to C = C, is involved hyper conjugation (σ electrons).
Question 34.
Among the following structures, the one which is not the resonating structures of others in:
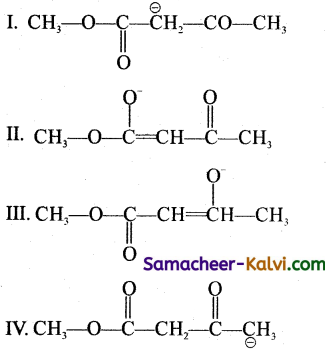
(a) I
(b) II
(c) III
(d) IV
Answer:
(d) IV
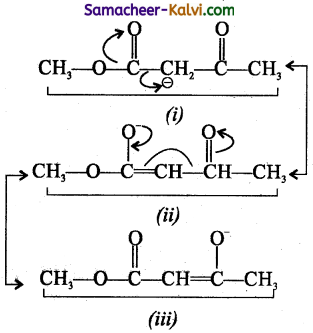
Hint:
Thus, IV is not the resonating structures of I, II, and III.
![]()
Question 35.
Which of the following compounds does not contain sp3 hybridised carbons?
(a) Cyclo alkanes
(b) Single chain alkanes
(c) Branched chain alkanes
(d) Benzene
Answer:
(d) Benzene
Question 36.
Out of the following, the one containing only nucleophiles is:
(a) AlCl3, BF3, NH3
(b) NH3, CN–, CH3OH
(c) AlCl3, NH2– , H2O
(d) RNH2, CX2, H–
Answer:
(b) NH3, CN–, CH3OH
![]()
Question 37.
In which of the following pairs of carbocations, the first carbocation is more stable than the second.
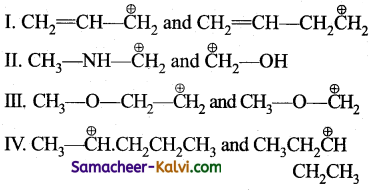
(a) II and III
(b) I, II and IV
(c) II and IV
(d) III and IV
Answer:
(b) I, II and IV
Question 38.
Among the following pairs, the one which will exhibit +I effect only?
(a) ![]()
(b) CO, COOH
(c) Cl and OH
(d) F and Cl
Answer:
(d) F and Cl
![]()
Question 39.
Which of the following will neither act as an electrophile nor a nucleophile.
(a) H+, OH–
(b) +NO2, Cl–
(c) ![]()
(d) CH3NH2, (CH3)2NH2
Answer:
(c) ![]()
Hint:
H3O+ has a lone pair of electrons but due to the presence of the positive charge it cannot donate the electron pair and have does not act as a nucleophile. has a lone pair of electrons but due to the presence of the positive charge it has 8 electrons in its valence shell of oxygen atom.
It cannot expand its valence shell beyond 8 electrons and hence, it cannot act an electrophile ![]() does not have a lone pair of electrons on the nitrogen atom. Hence it cannot act as a nucleophile. Similarly
does not have a lone pair of electrons on the nitrogen atom. Hence it cannot act as a nucleophile. Similarly ![]() also has 8 electrons around nitrogen atom. It also cannot expand its valence shell beyond 8 electrons and hence cannot act as electrophile.
also has 8 electrons around nitrogen atom. It also cannot expand its valence shell beyond 8 electrons and hence cannot act as electrophile.
Question 40.
Assertion (A):
In carbonyl compounds, in the presence of an attacking reagent, the carbonyl group is polarised as
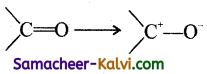
Reason (R):
Electromeric effect .involves the complete transfer of electrons of a multiple bond to one of the bonded atoms in the presence of an attacking reagent.
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false
(d) both assertion and reason are false
Answer:
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
![]()
Question 41.
Assertion (A):
Among the resonance structures of 1, 3 Butadiene, structure I is more stable, than structures II and III.
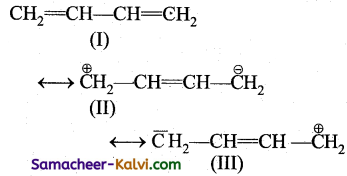
Reason (R):
Structure with greater number of covalent bonds contributes more towards resonance hybrid.
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false.
(d) both assertion and reason are false.
Answer:
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
Hint:
Since the formation of a bond is accompanied by release of energy, the structure (I) with two π bonds is more stable than II and III, which contain one π bond in each. Hence, structure (I) makes more contribution towards resonance hybrid than structure II and III.
Question 42.
Assertion (A):
The relative stability of carbocations follow the sequence:
tertiary > secondary > primary
Reason (R):
The order of reactivity of carbpcation follows the sequence
primary > secondary > tertiary
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false.
(d) both assertion and reason are false.
Answer:
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
Hint:
The relative stability of carbocation is .explained as the basis of hyper conjugation greater the number of α – hydrogen atoms, greater is the hyper conjugative structures and greater is its stability.
The order of reactivity is reverse of their stability.
![]()
Question 43.
Assertion (A):
The reaction, between an alkyl halide and sodium hydroxide is a nucleophilic substitution reaction.
Reason (R):
In all nucleophilic substitution is a stronger nucleophile displaces a weaker nucleophile.
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
(b) both assertion and reason are correct but ‘ reason is not the correct explanation of assertion.
(c) assertion is true but reason is false.
(d) both assertion and reason are false.
Answer:
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
Hint:
OH– is a stronger nucleophile and it displaces X– ion which is a weaker nucleophile.
Question 44.
Assertion (A):
All the carbon oxygen bonds in carbon dioxide have the same bond length of 1.15 Å.
Reason (R):
Carbon dioxide is a linear molecule.
(a) both assertion and reason are correct and reason is the correct explanation of the assertion.
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false.
(d) both assertion and reason are false.
Answer:
(b) both assertion and reason are correct but reason is not the correct explanation of assertion.
Hint:
The correct reason for assertion is the structure of carbon dioxide is a resonance hybrid of
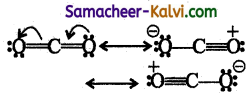
![]()
Question 45.
Which of the following statement is wrong with respect to carbanion?
(a) The carbon atom in a carbanion posses eight electrons in its valence shell.
(b) The carbon atom in a carbanion contain a lone pair of electrons in its one of the sp3 hybrid orbitals.
(c) The stability of carbanion be explained by inductive and resonance effects.
(d) Greater the negative charge on the carbon atom of the carbanion, the more stable it is.
Answer:
(d) Greater the negative charge on the carbon atom of the carbanion, the more stable it is.
Hint:
The correct statement is lesser the negative charge on the carbon atom of the carbanion more stable it is.
Question 46.
Consider the following reaction:
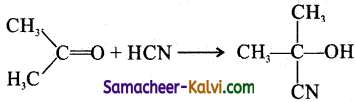
Choose the correct statement with regard to the above reaction.
I. The reaction is a nucleophilic substitutions reaction.
II. The reaction is a nucleophilic addition reaction.
III. The nucleophile is CN– ion.
IV. The nucleophile is N–C ion.
(a) I & III
(b) II & III
(c) II & IV
(d) I & IV
Answer:
(b) II & III
Hint:
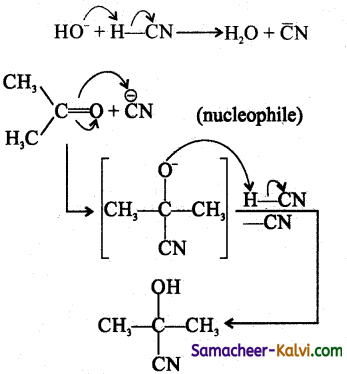
![]()
Question 47.
Choose the correct statement from among the following.
(a) The order of stability of carbocations is
(CH3)3C+ > (CH3)2 CH > +CH3CH2 > +CH3
(b) The carbocation are pyramidal in shape
(c) The stability of carbocations decreases in the order 1° > 2° > 3°
(d) In carbocation, bond pair of electrons are present in an unhybridised ‘p’ orbital
Answer:
(a) The order of stability of carbocations is
(CH3)3C+ > (CH3)2 CH > +CH3CH2 > +CH3
Question 48.
Choose the correct statement from the ‘ following, regarding eliminations reactions.
(a) Elimination reactions are these which involves the ion of two atoms or groups from the same or adjacent atoms of a substance to form a multiple bond.
(b) 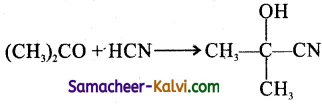 is an example of substitution reaction.
is an example of substitution reaction.
(c) The two atoms groups, of removed from two from the same atom of the molecule it is called p – elimination.
(d) If two atoms or groups, if removed from the adjacent carbon atoms, of a-molecule, it is called a – elimination.
Answer:
(a) Elimination reactions are these which involves the ion of two atoms or groups from the same or adjacent atoms of a substance to form a multiple bond.
![]()
Question 49.
Match the entities of column I with appropriate entities of column II
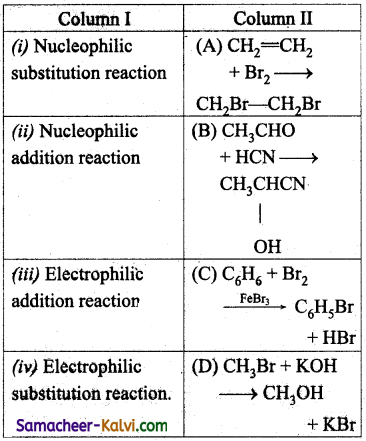
(a) (i) – (D), (ii) – (B), (iii) – (A), (iv) – (C)
(b) (i) – (B), (ii) – (C), (iii) – (D), (iv) – (A)
(c) (i) – (D), (ii) – (B), (iii) – (C), (iv) – (A)
(d) (i) – (A), (ii) – (B), (iii) – (D), (iv) – (C)
Answer:
(a) (i) – (D), (ii) – (B), (iii) – (A), (iv) – (C)
Question 50.
Match the entities of column I with appropriate entities of column II.
| Column I | Column II |
| (i) Neutral electrophile | (A) Nitronium ion |
| (ii) Positive electrophile | (B) Boron tri fluoride |
| (iii) Neutral nucleophile | (C) Carboxylate ion |
| (iv) Negative nucleophile | (D) Amines |
(a) (i) – (B), (ii) – (A), (iii) – (D), (iv) – (C)
(b) (i) – (A), (ii) – (C), (iii) – (B), (iv) – (D)
(c) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)
(d) (i) – (B), (ii) – (A), (iii) – (D), (iv) – (C)
Answer:
(a) (i) – (B), (ii) – (A), (iii) – (D), (iv) – (C)