TN State Board 11th Chemistry Important Questions Chapter 14 Haloalkanes and Haloarenes
Question 1.
Give an example each for:
(i) a primary alkyl halide
Answer:
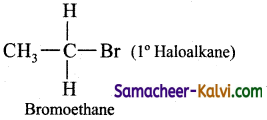
(ii) a secondary alkyl halide
Answer:
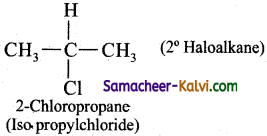
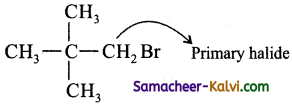
(iii) a tertiary alkyl halide
Answer:
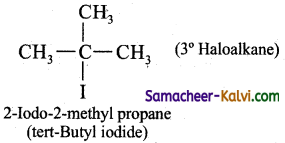
![]()
Question 2.
The common names of certain alkyl halides are given below. Write their structure and their IUPAC names.
(i) n-propyl fluoride
Answer:
Structure:CH3CH2CH2F
IUPAC Name: 1 -Fluoropropane
(ii) A-butyl chloride
Answer:
Structure: CH3 — CH2 — CH2 — CH2 — Cl
IUPAC Name: 1-Chlorobutane
(iii) sec-butyl chloride
Answer:
Structure: 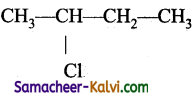
IUPAC Name: 2 – Chlorobutane
![]()
(iv) tert-butyl chloride
Answer:
Structure: 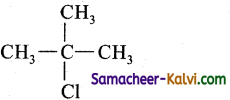
IUPAC Name: 2 – Chloro – 2 – methyl propane
(v) neo pentyl bromide
Answer:
Structure: 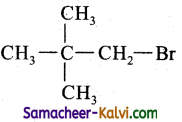
IUPAC Name: 1 – Bromo – 2, 2 – dimethyl propane
(vi) vinyl chloride
Answer:
Structure: CH2 = CH — Cl
IUPAC Name: Chloroethene
(vii) allyl bromide
Answer:
Structure: CH2 = CH — CH2 — Br
IUPAC Name: 3 – Bromopropene
![]()
Question 3.
Classify the following alkyl halides as primary, secondary and tertiary or vinyl / allyl halides.
(i) Bromoethane:
Answer:
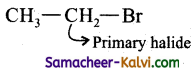
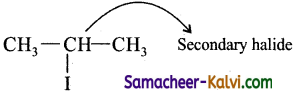
(ii) 2 – chloropropane:
Answer:
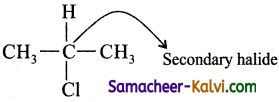
(iii) 2-iodo-2-methyl propane:
Answer:
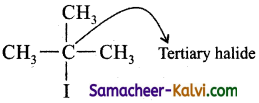
(iv) 2-fluoro propane:
Answer:
![]()
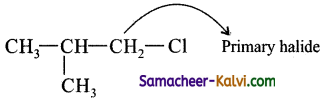
(v) 1 – chloro – 2 – methyl propane:
Answer:
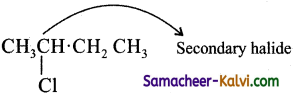
(vi) 2 – chloro butane
Answer:
(vii) 1-bromo-2, 2, dimethyl propane:
Answer:
(viii) Chloro ethane:
Answer:
CH2 = CH – Cl – Vinyl halide
(ix) 3-bromo propen e:
CH2 = CH — CH2 – Br – Allyl halide
![]()
Question 4.
Give examples for:
(i) a dichloroalkane
Answer:

1, 1, dichloroethane
(ii) a tribromoalkane.
Answer:
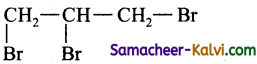
1, 2, 3 tribromopropane
Question 5.
Write the IUPAC names of the following:
(i) 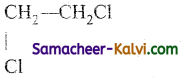
Answer:
1, 2 dichloroethane
(ii) CH3CHCl2
Answer:
1, 1 dichloroethane
(iii) 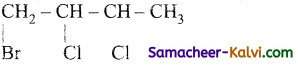
Answer:
1 – bromo – 2, 3, dichlorobutane
(iv) 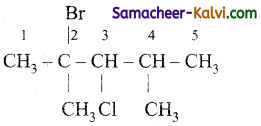
Answer:
2-bromo – 3 – chloro – 2, 4 dimethyl pentane
![]()
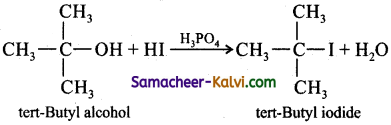
Question 6.
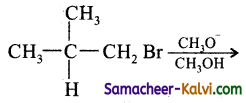
What is Lucas reagent? How does it react with tertiary butyl alcohol? Give equation for the reaction.
Answer:
Mixture of con.HCl and anhydrous ZnCl2 is called Lucas reagent.
Question 7.
Complete the following equations.
(i) CH3CH2OH (Ethanol) + PCl5 →
Answer:
CH3CH2Cl (Chloroethane) + POCl3 + HCl
(ii) 3 CH3CH2OH (Ethanol) + PCl3 →
Answer:
3 CH3CH2Cl (Chloroethane) + H3PO3
(iii) 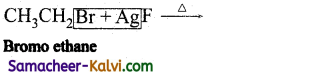
Answer:
CH3CH2I (Iodo ethane) + NaBr
(iv) 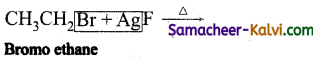
Answer:
CH3CH2F (Flouoroethane) + AgBr
(v) 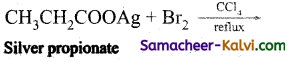
Answer:
CH3CH2Br (Bromoethane) + CO2 + AgBr
![]()
Question 8.
Give equations for: (i)Finkelstein reaction, (ii) Hunsdiecker reaction, (iii) Swartz reaction.
Answer:
(i) Finkelstein reaction:
Chloro or bromoalkane on heating with a concentrated solution of sodium iodide in dry acetone gives iodo alkanes. This reaction is called Finkelstein reaction, (SN2 reaction).

(ii) Hunsdiecker reaction:
Silver salts of fatty acids when refluxed with bromine in CCl4 gives bromo alkane.
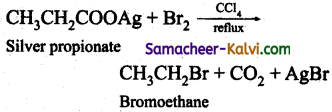
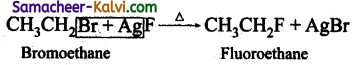
(iii) Swartz reaction:
Chloro or bromo alkanes on heating with metallic fluorides like AgF, SbF3 or Hg2F2 gives fluoroalkanes. This reaction is called Swarts reaaction.
![]()
Question 9.
Chlorination of methane gives different products. How are they Separated?
Answer:
Chlorination of methane gives different products,
(i) Chloromethane,
(ii) Dichloromethane,
(iii) Trichloro methane,
(iv) Tetraohloro methane which have differences in their boiling points. Hence, these can be separated by fractional distillation.
Question 10.
Identify the products, (major organic product)
(i) CH3CH = CH2 + HBr →
Answer:
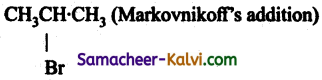
(ii) 
Answer:

(iii) 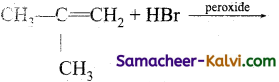
Answer:
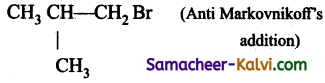
(iv) 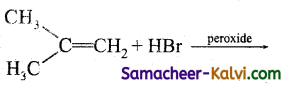
Answer:

![]()
Question 11.
Starting from propene, how will you prepare:
(i) 1 – bromopropane
Answer:
CH3 CH = CH2 + HBr ![]() CH3CH2CH2Br
CH3CH2CH2Br
(1 – Bromopropane)
(ii) 2 – bromopropane
Answer:
CH3 CH = CH2 + HBr → 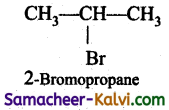
(iii) 1 – propanol
Answer:
CH3 CH = CH2 ![]() CH3CH2CH2Br
CH3CH2CH2Br ![]() CH3CH2CH2OH (1 – Propanol)
CH3CH2CH2OH (1 – Propanol)
(iv) propyl amine
Answer:
CH3 CH = CH2 + HBr ![]() CH3CH2CH2Br
CH3CH2CH2Br ![]() CH3CH2CH2NH2 (Propylamine)
CH3CH2CH2NH2 (Propylamine)
(v) propyl cyanide
Answer:
CH3 CH = CH2 + HBr ![]() CH3 CH2 CH2 Br
CH3 CH2 CH2 Br ![]() CH3CH2CH2CN (Propyl cyanide)
CH3CH2CH2CN (Propyl cyanide)
![]()
Question 12.
Identify the reagent used in the following conversions.
(i) CH3CH2OH ![]() CH3CH2I
CH3CH2I
Answer:
X = HI
(ii) 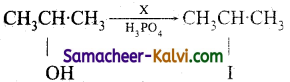
Answer:
X = HI
(iii) CH3CH2OH ![]() CH3CH2Cl
CH3CH2Cl
Answer:
X = PCl5 or PCl3or SOCl2
(iv) CH3CH2Br ![]() CH3CH2I
CH3CH2I
Answer:
X = NaI
(v) CH3CH2Br ![]() CH3CH2F
CH3CH2F
Answer:
X = AgF
Question 13.
Account for the following:
(i) Ethyl bromide has a higher boiling point than ethane.
Answer:
Haloalkanes have higher boiling point than those of corresponding alkanes because of the polarity of C – X bond. As a result, strong dipole-dipole attractions exist in alkyl halides. Such kind of attractions are absent in alkanes.
(ii) The boiling poinj of methyl halides decrease in the order CH3I > CH3Br > CH3Cl > CH3F
Answer:
This is because, with increase in size and mass of halogen atom, the magnitude of Vander Waals force of attraction increases.
(iii) The boiling point of CCl4 is greater than CCl3.
Answer:
With the increase in number of halogen atoms, the Vander Waals force of attraction increases. Hence, the alkyl halide which has more number of halogen atoms has a higher boiling point.
(iv) n – propyl chloride has a higher boiling point in methyl chloride.
Answer:
For the same halogen atom, boiling points of haloalkanes increase with the size of alkyl groups. Larger the size of the alkyl group, greater is the Vander Waals force of attraction.
![]()
Question 14.
Tertiary butyl chloride has the lowest boiling point compared to its isomers. Explain why?
Answer:
This is because of branching of the chain makes the molecule more compact and therefore decreases the surface area of haloalkanes and so the magnitude of Vander Waals force of attraction decreases, n – butyl chloride > isobutyl chloride > tert – butyl chloride.
Question 15.
Explain why?
(i) Ethyl bromide is insoluble in water.
Answer:
Ethyl bromide is insoluble in water because it cannot form hydrogen bonding with water.
(ii) The densities of haloalkanes are of the order RI > RBr > RCl > RF.
Answer:
The density increases with increasing number and atomic mass of halogen.
Question 16.
Name the reagents used to convert bromo ethane into:
(i) ethyl nitrate –
Answer:
KNO2
(ii) nitro ethane –
Answer:
Ag NO2
(iii) propane nitrite –
Answer:
KCN
(iv) ethyl carbyl amines –
Answer:
AgCN
![]()
(v) ethane thiol –
Answer:
NaSH or KSH
(vi) ethoxy ethane –
Answer:
Ag2O or C2H5ONa
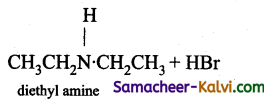
(vii) diethyl amine –
Answer:
C2H5 NH2
(viii) tri-ethyl amine –
Answer:
(C2H5)2NH
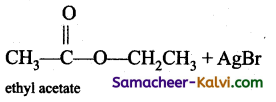
(ix) ethyl acetate –
Answer:
CH3COOAg
(x) 1 – butyne –
Answer:
HC ≡ C.Na
(xi) butane –
Answer:
Na/ether
(xii) ethene –
Answer:
alc.KOH
![]()
Question 17.
Give equations for the conversions in:
(i) CH3CH2Br + KNO2 →
Answer:
CH3CH2ONO + KBr
(ii) CH3CH2Br + AgNO →
Answer:
CH3CH2NO2 (nitroethane) + AgBr
(iii) CH3CH2Br + KCN →
Answer:
CH3CH2CN (propane nitrite) + KBr
(iv) CH3CH2Br + AgCN →
Answer:
CH3CH2CN (ethyl carbylatnines (or) ethyl isocyanide) + AgBr
![]()
(v) CH3CH2Br + NaSH →
Answer:
CH3CH2SH (Sodium ethoxide) + NaBr
(vi) 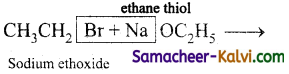 →
→
Answer:
CH3CH2OCH2CH3 (ethoxy ethane) + NaBr
(vii) 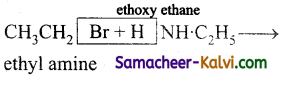 →
→
Answer:
(viii) 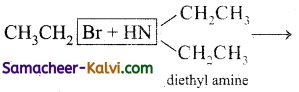 →
→
Answer:
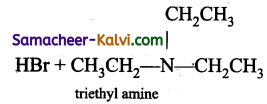
(ix) 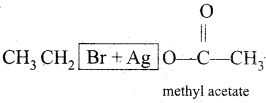 →
→
Answer:
(x) 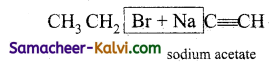 →
→
Answer:
NaBr + CH3CHC≡CH (1 – butyne)
(xi) 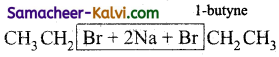 →
→
Answer:
2 NaBr + CH3CH2CH2CH3 (n – butane)
(xii) CH3CH2Br + alc KOH →
Answer:
CH2 = CH2 + KBr + H2O
![]()
Question 18.
What is ammonolysis? Why this method is not recommended for the preparation of mono alkyl amines?
Answer:
Reaction of an alkyl halide with alcoholic ammonia to give a mixture of amines is known as ammonolysis.
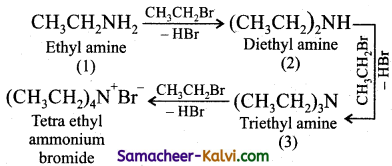
This method is not recommended because the product is a mixture of amines.
Question 19.
What are ambident nucleophiles? Give two examples.
Answer:
Nucleophiles which attack the nucleophilic sites from two sides are called ambident nucleophiles. For example, cyanide ion, CN– ion can attack the reaction site through carbon or nitrogen atom depending upon the reagent and reaction condition, eg: CN– and NO2–
Question 20.
When ethyl bromide is treated with KCN, the product is ethyl cyanide while, when treated with AgCN, the product is ethyl isocyanide. Explain why?
Answer:
When ethyl bromide is treated with KCN, the nucleophile is –CN (the negative charge on carbon) and hence ethyl cyanide is formed. KCN is an ionic compound. It ionizes to give –CN as a nucleophile.
whereas AgCN is a covalent compound. It gives –NC (negative charge on nitrogen) as a nucleophile. Hence ethyl isocyanide is formed.
![]()
Question 21.
Give equation for the reaction between ethyl bromide with
(i) KNO2 and
(ii) AgNO2 and write their nucleophiles.
Answer:
(i) 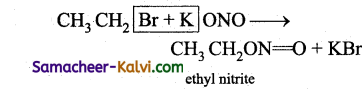
(ii) 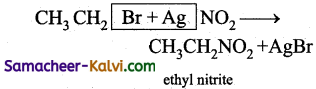
Nucleophiles:
If potassium nitrite is used as a reagent, the nucleophile is –ONO with the negative charge on the oxygen atom. Hence alkyl nitrite is formed. If silver nitrite is used as a reagent, –NO2 (the negative charge on nitrogen) is the nucleophile and the product is nitroalkane.
Question 22.
What is Williamson’s ether synthesis? Explain with an equation.
Answer:
Haloalkane, when boiled with sodium alkoxide gives corresponding ethers.
eg: This method can be,used to prepare mixed (unsymmetrical) ethers also.
CH3CH2Br (Bromoethane ) + NaOCH2CH3 (Sodiumethoxide) ![]() CH3CH2OCH2CH3 (diethylether) + NaBr
CH3CH2OCH2CH3 (diethylether) + NaBr
![]()
Question 23.
Briefly outline SN2 mechanism with an example.
Answer:
(i) The reaction follows second order kinetics and the rate equation is
rate = R2 [RX] [Nucleophile].
Generally primary alkyl halides undergo nucleophilic substitution reaction by SN2 mechanism.
(ii) This reaction involves the formation of a transition state in which both the reactant molecules are partially bonded to each other. The attack of nucleophile occurs from the back side (i.e., opposite to the side in which the halogen is attacked). The carbon at which substitution occurs has inverted configuration during the course of reaction just as an umbrella has tendency to invert in a wind storm. This inversion of configuration is called Walden inversion; after paul walden who first discovered the inversion of configuration of a compound in SN2 reaction.
(iii) When 2 – Bromo octane is heated with sodium hydroxide, 2 – Octanol is formed with inversion of configuration. (-) 2 – Bromo octane is heated with sodium hydroxide (+) 2 – Octanol is formed in which –OH group occupies a position opposite to what bromine had occupied,
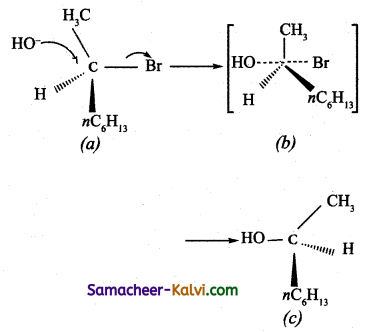
(a) (-) 2 – Bromo octane
(b) Transition State
(c) (+) 2 – Octanol (product)
![]()
Question 24.
Explain SN1 mechanism with an example.
Answer:
(i) The rate of the following SN1 reaction depends upon the concentration of
alkyl halide (RX) and is independent of the concentration of the nucleophile (OH–).
Hence Rate of the reaction = k [alkyl halide]
R — Cl + OH– → R — OH + Cl–
This SN1 reaction follows first order kinetics and occurs in two steps.
We understand SN1 reaction mechanism by taking a reaction between tertiary butyl bromide with aqueous KOH.
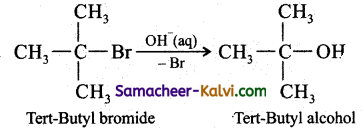
This reaction takes place in two steps as shown below:
Step-1:
Formation of carbocation: The polar C – Br bond breaks forming a carbocation and bromide ion. This step is slow and hence it is the rate determining step.
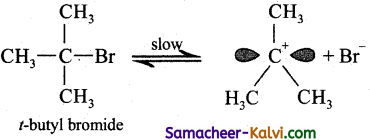
The carbocation has 2 equivalent lobes of the vacant 2p orbital, so it can react equally rapidly from either face.
Step-2:
The nucleophile immediately reacts with the carbocation. This step is fast and hence does not affect the rate of the reactions.
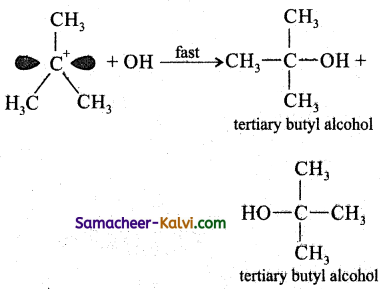
As shown above, the nucleophilic reagent OH– can attack carbocation from both the sides.
(ii) If haloalkane substrate is optically active then, the product obtained will be optically inactive racemic mixture. As nucleophilic reagent OH- can attack carbocation from both the sides, to form equal proportion of dextro and laevorotatory optically active isomers which results in optically inactive racemic mixture.
![]()
Question 25.
Explain Saytzeff ‘s Rule with an example.
Answer:
In a dehydrohalogenation reaction, the preferred product is that alkene which has more number of alkyl groups attached to the doubly bonded carbon (more substituted double bond is formed), eg:
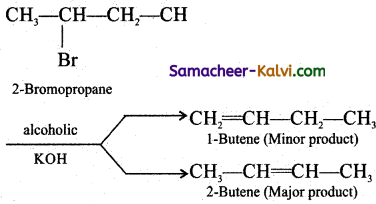
Question 26.
Explain E2 mechanism with an example.
Answer:
The rate of E2 reaction depends on the concentration of alkyl halide and base
Rate = k [alkyl halide] [base]
It is therefore, a second order reaction. Generally primary alkyl halide undergoes this reaction in the presence of alcoholic KOH. It is a one step process in which the abstraction of the proton from the |3 carbon and expulsion of halide from the a carbon occur simultaneously. The mechanism is shown below.
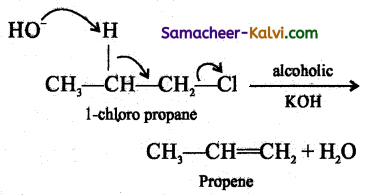
![]()
Question 27.
Explain E1 mechanism with an example.
Answer:
E1 mechanism means elimination unimolecular. It follows first order kinetics conversion oftert-butyl chloride to isobutylene by alcoholic potash is an exertic for E1 reaction. The mechanism is given below:
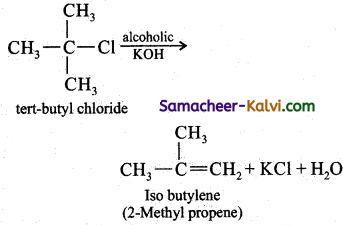
Step-1:
Heterolytic fission to yield a carbo – cation.
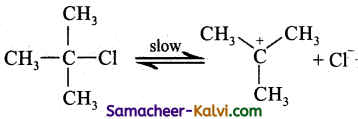
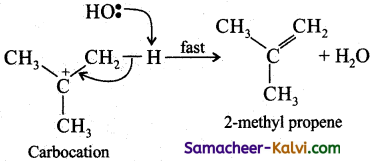
Step-2:
Elimination of a proton from the β – carbon to produce an alkene.
Question 28.
Complete the following equations:
(i) CH3CH2Br (Ethyl bromide) ![]()
Answer:
CH3CH2MgBr (Ethyl magnesium bromide)
(ii) CH3CH2Br (Ethyl bromide) + 2Li ![]()
Answer:
CH3CH2Li (Ethyl Lithium) + LiBr
(iii) 4CH3CH2Br (Ethyl bromide) + 4Na/Pb (Sodium-lead alloy) ![]()
Answer:
(CH3CH2)4 Pb (Tetra ethyl lead (TEL)) + 4 NaBr + 3 Pb
(iv) CH3CH2Br (Bromoethane) + H2 ![]()
Answer:
CH3 – CH3 (Ethane) + HBr
(v) CH3CH2I (lodoethane) + HI ![]()
Answer:
CH3 — CH3 (Ethane) + I2
![]()
Question 29.
Mention the uses of
(i) chloroform
(ii) iodoform
(iii) carbon tetrachloride.
Answer:
(i) Chloroform:
It is used as a solvent in pharmaceutical industry and for producing pesticides and drugs as an anesthetic. As a preservative for anatomical specimens.
(ii) Iodoform:
Iodoform is used as an antiseptic for dressing wounds.
(iii) Carbon tetrachloride:
Carbon tetrachloride is used as dry cleaning agent. It is used as a solvent for oils, fats and waxes. As the vapour of CCl4 is non-combustible, it is used under the name pyrene for extinguishing the fire in oil or petrol.
Question 30.
What are organo metallic compounds? Give example.
Answer:
Organo metallic compounds are organic compounds in which there is a direct carbon -metal bond.
eg: CH3 MgI – Methyl magnesium iodide
CH3CH2Mg Br – Ethyl magnesium bromide.
![]()
Question 31.
Briefly outline the nature of carbon-magnesium bond in alkyl magnesium halide.
Answer:
The carbon-magnesium bond in Grignard reagent is covalent but highly polar, The carbon atom is more, electronegative than magnesium. Hence, the carbon atom has partial negative charge and the magnesium atom has partial positive charge,
Rδ- ………. MgXδ+
Question 32.
Mention the uses of Grignard reagent.
Answer:
Grignard reagents are synthetically very useful compounds. These reagents are converted to various organic compounds like alcohols, carboxylic acids, aldehydes and ketones. The alkyl group being electron rich acts as a carbanion or a nucleophile. They would attack polarized molecules at a point of low electron density.
Question 33.
Using a suitable Grignard reagent, write equations for the synthesis of:
(i) Ethyl alcohol:
Answer:
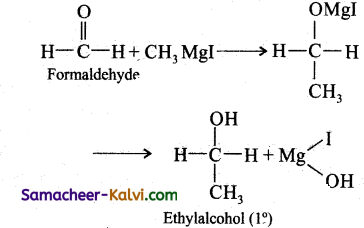
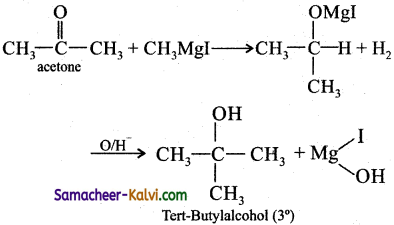
(ii) Tertiary butyl alcohol:
Answer:
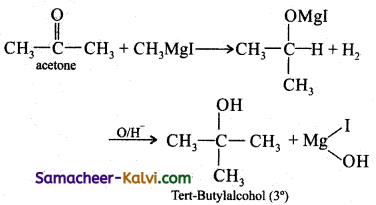
(iii) Acetaldehyde:
Answer:
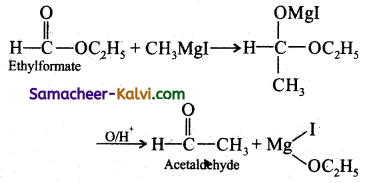
(iv) Ethyl methyl ether:
Answer:
CH3 – 0 – CH2Cl (Chloro dimethyl ether) + CH3MgI → CH3 – O – CH2CH3 (Ethyl methyl ether) + 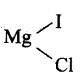
(v) Methane:
CH3MgI+HO – H → CH4 + MgI (OH)
CH3MgI + C2H5OH (Ethylalcohol) ![]() CH4 (Methane) + MgI(OC2H5)
CH4 (Methane) + MgI(OC2H5)
![]()
Question 34.
Briefly outline the nature of carbon-halogen (X) bond in haloarenes.
Answer:
In haloarenes the carbon atom is sp2 hybridised. The sp2 hybridised orbitals are shorter and holds the electron pair of bond more tightly. Halogen atom contains P – orbital with lone pair of electrons which interacts with π – orbitals of benzene ring to form extended conjugated system of π – orbitals. The delocalisation of these electrons give double bond character to C – X bond. The resonance structure of halobenzene is given as:
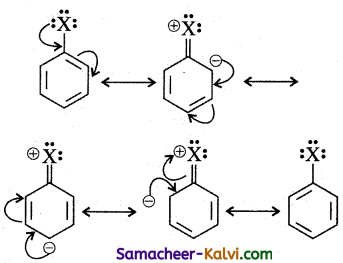
Due to this double bond character of C — X bond in haloarenes, the C — X bond is shorter in length and stronger than in haloalkanes.
![]()
Question 35.
Haloarenes undergo nucleophilic substitution reactions less readily compared to halo alkanes. Explain why?
Answer:
Because of resonance, the carbon-halogen bond gets a double bond character.
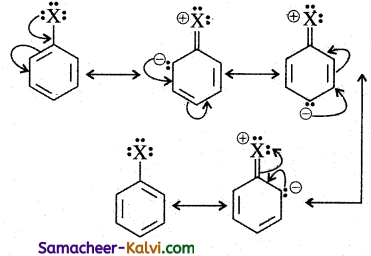
Thus, C — X bond is shorter in haloarenes compared to that of haloalkenes i.e., The halogen atom is more firmly held, by the benzene ring, breaking of C — X bond is difficult. Hence haloarenes undergo nucleophilic substitution reaction under drastic conditions.
Question 36.
How is chlorobenzene prepared from
(i) benzene
(ii) benzene diazonium chloride?
Answer:
(i) Chlorobenzene is prepared by the direct chlorination of benzene in the presence of lewis acid catalyst like FeCl3.
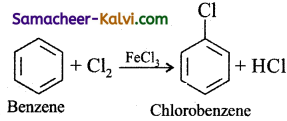
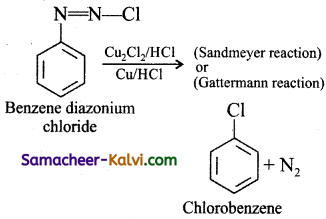
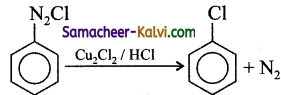
(ii) When aqueous solution of benzene diazonium chloride is warmed with Cu2Cl2 in HCl gives chlorobenzene.
![]()
Question 37.
How is chlorobenzene prepared by
(i) Sandmeyer reaction
(ii) Gattermann reaction?
Answer:
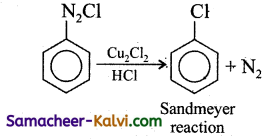
(i) Sandmeyer reaction:
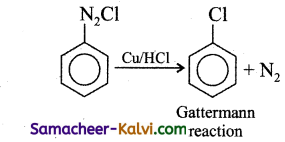
(ii) Gattermann reaction:
Question 38.
How is (i) iodobenzene and (ii) fluorobenzene prepared?
Answer:
(i) Iodobenzene is prepared by warming benzene diazonium chloride with aqueous KI solution.
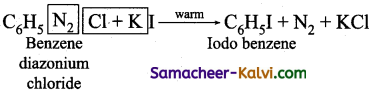
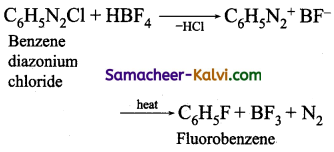
(ii) Fluorobenzene is prepared by treating benzene diazonium chloride with fluoro boric acid. This reaction produces diazonium fluoroborate which on heating produces fluorobenzene. This reaction is called Balz-schiemann reaction.
![]()
Question 39.
Give equation for Wurtz-Fittig reaction.
Answer:
Haloarenes reacts with haloalkanes when heated with sodium in ether solution to form alkyl benzene. This reaction is called Wurtz Fittig reaction.
C6H5Cl + 2 Na + ClC2H5 ![]() C6H5C2H5 + 2 NaCl
C6H5C2H5 + 2 NaCl
Question 40.
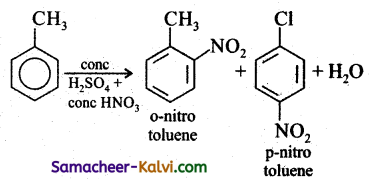
How are (i) benzene, (ii) ortho and para chloro toluene, (iii) ortho and para nitro toluene prepared from toluene.
Answer:
Toluene to benzene:
C6H5CH3 (chloro benzene) + 2(H) ![]() C6H6 (Benzene) + HCl
C6H6 (Benzene) + HCl
(ii) Ortho and para chloro toluene:
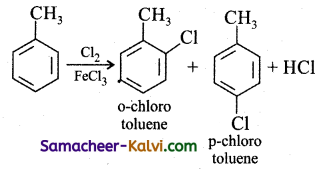
(iii) Ortho and paranitro toluene:
![]()
Question 41.
Mention the uses of chlorobenzene.
Answer:
(i) Chlorobenzene is used in the manufacture of pesticides like DDT.
(ii) It is used as high boiling solvent in organic synthesis.
(iii) It is used as fibre – swelling agent in textile processing.
Question 42.
What are ‘gem’ and vicinal dihalides? Give examples.
Answer:
In ‘gem’ dihalides, the halogen atoms attached to the same carbon atom.
eg: CH3CH2Cl2 – Ethylidene chloride (or) 1, 1 dichloro ethane.
In ‘vic’ dihalides, the halogen atoms are attached to adjacent carbon atoms.
eg: 
Question 43.
How will you prepare
(i) ethylidene chloride from acetaldehyde,
(ii) ethylidene chloride from acetylene,
(iii) ethylene dichloride from ethylene,
(iv) ethylene dichloride from ethylene glycol.
Answer:
(i) CH3CHO (Acetaldehyde) + PCl5 → CH3CHCl2 (Ethylidene dichloride) + POCl3
(ii) 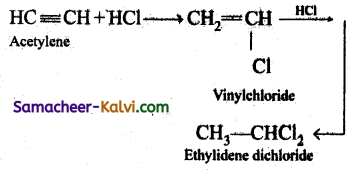
(iii) 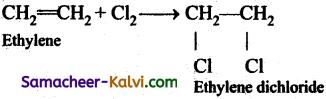
(iv) 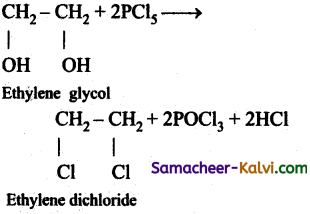
![]()
Question 44.
Give the chemical reaction that is used to distinguish ‘gem’ and ‘vic’ dihalides.
Answer:
Gem-Dihalides, on hydrolysis with aqueous KOH give an aldehyde or a ketone vic-Dihalides, on hydrolysis with aqueous KOH gives glycols.
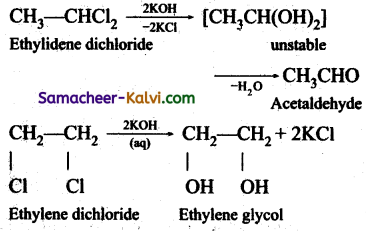
This reaction can be used to distinguish the gem- Dihalides and vie- Dihalides.
Question 45.
What is the action of zinc dust in methanol on ethylidene chloride and ethylene chloride? Give equation.
Answer:
Gem- Dihalides and vic – Dihalides on treatment with zinc dust in methanol give alkenes.
(i) CH3 — CHCl2 (Ethylidene dichloride) + Zn ![]() CH2 = CH2 (Ethylene) + ZnCl2
CH2 = CH2 (Ethylene) + ZnCl2
(ii) 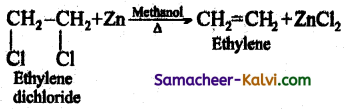
Question 46.
How is acetylene prepared from ethylidene chloride and ethylene chloride? Give equation.
Answer:
Gem-Dihalides and vic-Dihalides on treatment with alcoholic KOH give alkynes.
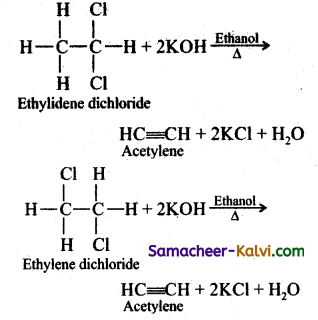
![]()
Question 47.
What is the action of (i) zinc and HCl (ii) H2/Ni on chloroform? Give equation.
Answer:
(i) Reduction of chloroform in the presence of Zn + HCl gives methylene chloride.
CHCl3 (chloroform) ![]() CH2Cl2 (methylene chloride) + HCl
CH2Cl2 (methylene chloride) + HCl
(ii) CHCl3 (chloroform) ![]() CH2Cl2 (methylene chloride) + HCl
CH2Cl2 (methylene chloride) + HCl
Question 48.
How is methylene chloride prepared from chloroform?
Answer:
Chloroform undergoes reduction with zinc and HC1 in the presence of ethyl alcohol to form methylene chloride.
CHCl3 (Methylene chloride) + 2(H) ![]() CH2Cl2 + HCl
CH2Cl2 + HCl
![]()
Question 49.
Complete the following by writing the structures of (A) and (B).
(i) 
Answer:
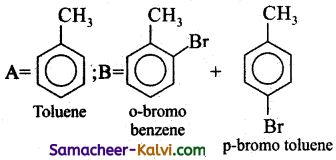
(ii) 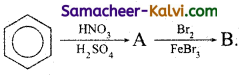
Answer:
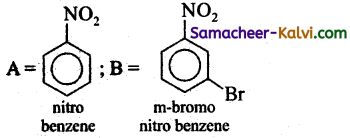
(iii) 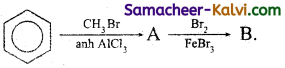
Answer:
(iv) 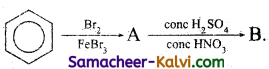
Answer:
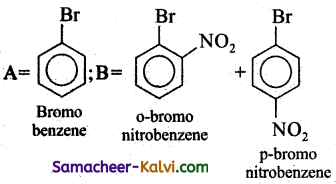
(v) 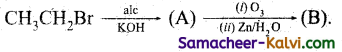
Answer:
A = CH2 = CH2 (ethylene)
B = Two molecules of HCHO (formaldehyde)
![]()
Question 50.
Mention the uses of methylene chloride.
Answer:
Methylene chloride is used as:
(i) Aerosol spray propellant.
(ii) Solvent in paint remover.
(iii) Process solvent in the manufacture of drugs. .
(iv) A metal cleaning solvent.
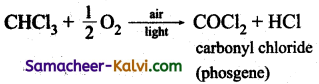
Question 51.
What is phosgene? How is it prepared?
Answer:
Phosgene is a poisonous gas (COCl2) and is prepared by the oxidation of chloroform in the presence of light and air.
![]()
Choose the correct answer:
Question 1.
The IUPAC name of the compound is:
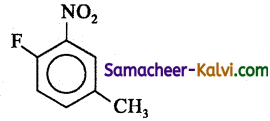
(a) 1 – fluoro – 4 – methyl – 2 – nitrobenzene
(b) 4 – fluoro – 1 – methyl – 3 – nitrobenzene
(c) 4 – methyl – 1 – fluoro – 2 – nitrobenzene
(d) 2 – fluoro – 5 – methyl – 1 – nitrobenzene
Answer:
(a) 1 – fluoro – 4 – methyl – 2 – nitrobenzene
Question 2.
In the addition of HBr to propene, the first step involves, the addition of
(a) H+
(b) Br–
(c) H.
(d) Br.
Answer:
(a) H+
Hint:
The reaction is electrophilic addition i.e., H+.
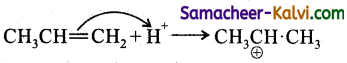
Question 3.
The intermediate during the addition of HCl to propene in the presence of a peroxide is:
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
Answer:
(b) ![]()
Hint:
Anti Markovnikoff‘addition is observed only with HBr. Therefore with HCl, even in the presence of peroxides, the reaction occurs through a more stable carbocation intermediate.
![]()
Question 4.
In the presence of peroxides hydrogen chloride and hydrogen iodide do not give anti Markovnikoff’s addition to alkenes because:
(a) both are highly ionic.
(b) one is oxidising and the other is reducing.
(c) one of the steps is endothermic in both cases.
(d) all the steps are endothermic in both reactions.
Answer:
(c) one of the steps is endothermic in both cases.
Hint:
Anti Markovnikoff’s addition is a free radical addition. The first step in generation of free radical, the second is a propagation step and the third is termination. The propagation step is
(i) X. + CH2 = CH — CH3 → XCH2 — CH — CH3
(ii) XCH2 — CH — CH3 + HX → XCH2CH2CH3 + X.
with X being bromine, both steps are. exothermic and hence peroxide effect is observed with HI and HCl, the step
(ii) is endothermic with HI, both steps are endothermic i.e., energy has to be supplied. Hence peroxide effect is not observed with HCl and HI.
Question 5.
Identify the set of reagent / reaction conditions X and Y in the following set of transformations. CH3CH2CH2Br ![]() Product
Product ![]()
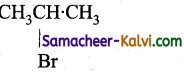
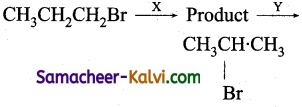
(a) X = dilute aqueous NaOH, 20°C.
Y = HBr/acetic acid, 20°C.
(b) X = cone, alcoholic NaOH, 80°C.
Y = HBr/acetic acid, 20°C.
(c) X = dilute aqueous NaOH, 20°C.
Y = Br2 / CHCl3 at 0°C.
(d) X- cone, aqueous NaOH, 80°C.
Y = Br2 / CHCl3, 0°C.
Answer:
(b) X = cone, alcoholic NaOH, 80°C.
Y = HBr/acetic acid, 20°C.
Hint:
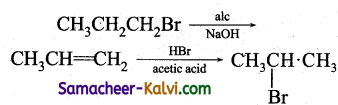
![]()
Question 6.
Predict the product (C) obtained in the following reactions of butyne-1.
CH3CH2 C ≡ CH + HCl → B ![]() C
C
(a) 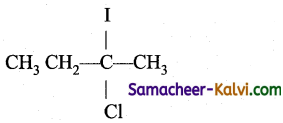
(b) 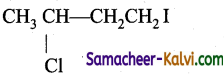
(c) 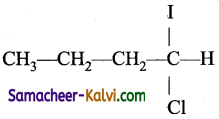
(d) 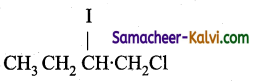
Answer:
(a) 
Hint:
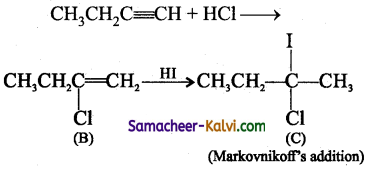
Question 7.
The reaction between toluene with chlorine in the presence of FeCl3 gives ‘X’ and the reaction in the presence of light gives ‘Y’. Thus ‘X’ and ‘Y’ are:
(a) X =benzyl chloride; Y = m – chlorotoluene
(b) X = benzal chloride; Y = o – chlorotoluene
(c) X = – m – chlorotoluene; Y = p – chlorotoluene
(d) X = o– and p– chlorotoluene;
Y = trichloromethyl benzene.
Answer:
(d) X = o– and p– chlorotoluene;
Y = trichloromethyl benzene.
Hint:
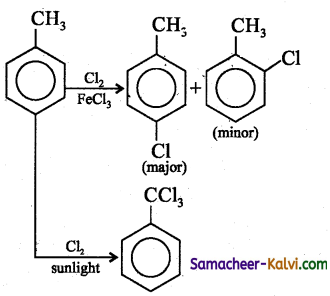
![]()
Question 8.
Which of the following in a nucleophilic substitution reaction?
(a) 2RX + 2Na → R – R + 2 NaX
(b) RX + H2 → RH + HX
(c) RX + Mg → RMgX
(d) RX + KOH → ROH + KX
Answer:
(d) RX + KOH → ROH + KX
Question 9.
For the following (a) I– (b) Cl– (c) Br–, the increasing order of nucleophilicity is:
(a) Br– < Cl– < I–
(b) I– < Br– < Cl–
(c) Cl– < Br– < I–
(d) I– < Cl– < Br–
Answer:
(c) Cl– < Br– < I–
Question 10.
Consider the following Bromides.
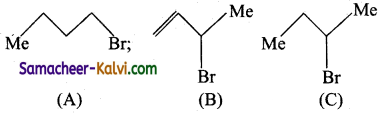
The correct order of SN1 reactivity is:
(a) C > B > A
(b) A > B > C
(c) B > C > A
(d) B > A > C
Answer:
(c) B > C > A
Hint:
The reactivity of SN1 reactions depends upon the stability of the intermediate carbocation. The stability of carbocations derived from A, B, C follows the order
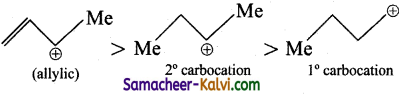
the reactivity follows the order B > C > A.
![]()
Question 11.
The major product formed in the following reaction is:
(a) 
(b) 
(c) 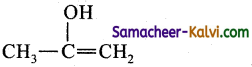
(d) 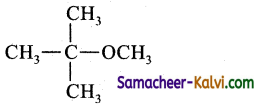
Answer:
(c) 
Hint:
Dehydrohalogenation. Strong bases like -OCH3 also used for the reaction.
Question 12.
The correct prder of increasing reactivity of C – X bond towards nucleophile in the following compounds are:
I. 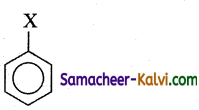
II. 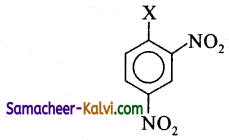
III. (CH3)3 C – X
IV. (CH3)2 CH – X
(a) III < II < I < IV
(b) I < II < IV < III
(c) II < III < I < IV
(d) IV < III < I < II
Answer:
(b) I < II < IV < III
Hint:
Alkyl halides are more reactive than aryl halides. Among alkyl halides 3° alkyl halide (III) are more reactive than 2° alkyl halide (IV). Among aryl halides having electron with drawing groups at ortho and para positions are more reactive than sirnplp aryl halides (I). Thus the overall reactivity increases in the order.
I < II < III < IV
![]()
Question 13.
The reagent used to convert ethyl bromide to ethyl fluoride is/are.:
(a) cone NaOH ; HF
(b) HF
(c) AgF
(d) F2
Answer:
(c) AgF
Hint:
CH3CH2Br + AgF → CH3CH2F + AgBr
Question 14.
Among the following, the alkyl halide which has the highest boiling point is:
(a) CH3Cl
(b) CH3CH2Cl
(c) CH3CH2CH2Cl
(d) CH3F
Answer:
(c) CH3CH2CH2Cl
Hint:
Between CH3Cl and CH3F, CH3Cl has a higher boiling point. Among CH3Cl, CH3CH2Cl, CH3CH3CH3Cl, the boiling point increases with increasing number of carbon atoms.
![]()
Question 15.
Choose the correct statement.
I. Among methyl chloride, ethyl chloride . and butyl chloride, methyl chloride has the least boiling point.
II. Among the methyl halides, methyl iodide has the highest boiling point.
III. Both I and II are correct.
IV. Both I and II are wrong.
(a) I
(b) II
(c) III
(d) IV
Answer:
(c) III
Hint:
(i) The boiling point and melting point of mono haloalkane increase with the increase in the number of carbon atoms.
(ii) The boiling point and melting point of haloalkanes decreases with respect to the halogen in the following order.
Question 16.
Which of the following are ambident nucleophiles? .
(a) –OH
(b) –SH
(c) –CN
(d) Br–
Answer:
(c) –CN
Hint:
–CN and CN– have two nucleophilic sites, where the carbon atom of the cyanide ion attacks the reaction site and CN– ion, the nitrogen atom attacks the reaction site. Such nucleophiles are ambident nucleophiles.
![]()
Question 17.
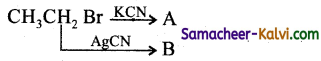
The products A and B are:
(a) A = CH3CH2CN; B = CH3CH2NC
(b) A = CH3CH2NC ; B = CH3CH2CN
(c) 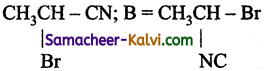
(d) A = CH3CH2CN; B = C3CH2CN
Answer:
A = CH3CH2CN; B = CH3CH2NC
Hint:
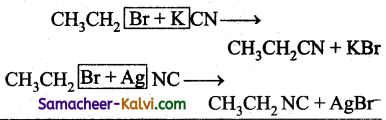
Question 18.
The major product in the following reaction:
 is:
is:
(a) CH2 = CH CH2CH3
(b) CH3CH CH2 CH3
(c) CH3CH = CH CH3
(d) CH3CH = CH2
Answer:
(c) CH3CH = CH CH3
Hint:
The product follows Saytzeff’s rule: In a dehydrohalogenation reaction, the preferred product is that alkene which has more number of alkyl groups attached to the doubly bonded carbon (more substituted double bond is formed).
![]()
Question 19.
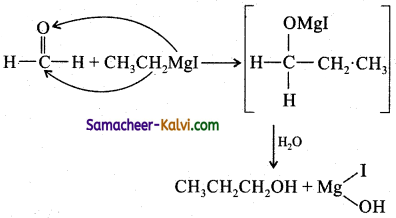
Which of the following is used to prepare propanol – 1 from formaldehyde?
(a) CH3MgI
(b) CH3CH2MgI
(c) 
(d) CH3MgBr
Answer:
(b) CH3CH2MgI
Hint:
Propanol – 1 contains three carbon atoms. Alkyl magnesium halides teact with HCHO, to give primary alcohol. Thus RMgX, should contain two carbon atoms, i.e., CH3CH2MgI.
Question 20.
The correct sequence of reactions involved -for the formation of isopropyl alcohol is/are:
(a) CH3CHO, CH3MgI, H2O/H+
(b) HCHO, CH3MgI, H2O/H+
(c) CH3Mg I, CH3CHO, H+/ H2O
(d) CH3COCH3, CH3CHO, H+/ H2O
Answer:
(a) CH3CHO, CH3MgI, H2O/H+
Hint:
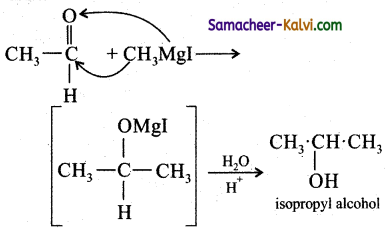
![]()
Question 21.
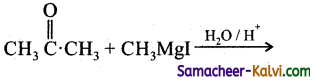
The product is:
(a) 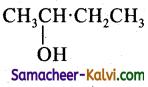
(b) 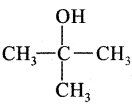
(c) CH3CH2CH2CH2OH
(d) 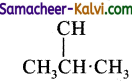
Answer:
(b) 
Hint:
Question 22.
Which of the following reaction is known as Sandmeyer reaotion?
(a) Benzene diazonium chloride ![]() Chloro benzene
Chloro benzene
(b) Benzene diazonium chloride + HBF4 → Fluoro benzene
(c) Benzene + Chlorine ![]() Chloro benzene
Chloro benzene
(d) Benzene + Nitric acid ![]() Nitro benzene
Nitro benzene
Answer:
(a) Benzene diazonium chloride ![]() Chloro benzene
Chloro benzene
Hint:
![]()
Question 23.
Which one of the following is/are ‘gem’ dihalide?
(a) 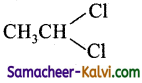
(b) 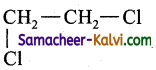
(c) 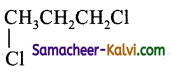
(d) 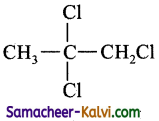
Answer:
(a) 
Question 24.
Chloropicrin is:
(a) trichloro nitro ethane
(b) trichloro nitro methane
(c) nitro methane
(d) nitro ethane
Answer:
(b) trichloro nitro methane
![]()
Question 25.
The reagents used to test primary amines are:
(a) chloroform and KOH
(b) mixture of conc H2SO4 and HNO3
(c) aqueous KOH
(d) bromine water
Answer:
(a) chloroform and KOH
Hint:
Chloroform reacts with aliphatic or aromatic primary amine and alcoholic caustic potash, to give foul smelling alkyl isocyanide (carbylamines).
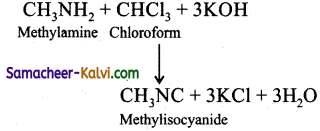
This reaction is used to test primary amine.
Question 26.
SWARTZ reaction is used to prepare:
(a) Chloro benzene
(b) Toluene
(c) DDT
(d) Freon 12
Answer:
(d) Freon 12
Hint:
Freon – 12 is prepared by the action of hydrogen fluoride on carbon tetrachloride in the presence of catalytic amount of antimony pentachloride. This is called swartz reaction.
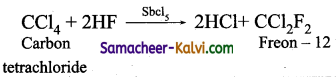
![]()
Question 27.
Assertion:
Chlorination of methane gives different products having differences in boiling points.
Reason:
Chlorination of methane gives a mixture of chloromethane, dichloromethane, tri-chloro methane and carbon tetra chloride.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
Question 28.
Assertion:
Bromo ethane reacts with ammonia in the molar ratio 1 : 1 gives ethyl amine.
Reason:
The reaction follows SN1 mechanism.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
Being a primary alkyl halide bromo ethane undergoes nucleophilic substitution by SN2 mechanism.
![]()
Question 29.
Assertion:
SN2 reaction of an optically active haloalkane is always accompanied -by inversion of configuration at the asymmetric carbon atom.
Reason:
In the transition state, the nucleophile and the leaving group are attached to the asymmetric carbon atom opposite to each other.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
Question 30.
Assertion:
E1 (elimination, unimolecular) follows first order kinetics.
Reason:
Primary alkyl halides undergo elimination reaction by E1 mechanism.
(a) Both assertion and reason are true and reason is the correct explanation of assertion.
(b) Both assertion and reason are true but reason is not the correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(c) Assertion is true but reason is false.
Hint:
Primary alkyl halides undergo elimination reaction by E2 mechanism.
![]()
Question 31.
Identify a tertiary alkyl halide from the following.
(a) CH3CH2Br ‘
(b) 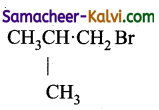
(c) 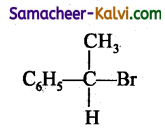
(d) 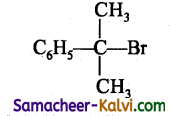
Answer:
(d) 
Question 32.
Which among the following will have the least boiling point?
(a) CH3Cl
(b) CH3 CH2 Cl
(c) CH3(CH2)2Cl
(d) CH3 (CH2)3 Cl
Answer:
(a) CH3Cl
Hint:
For the same halogen atom, the boiling points will increase with the size of the alkyl group.
![]()
Question 33.
Which of the following pairs, will undergo nucleophilic substitution reaction by SN2 mechanism only?
(a) CH3 Br and CH3 CH2 Br
(b) (CH3)2 CH Br and CH3 CH2 Br
(c) 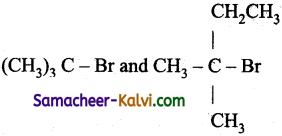
(d) 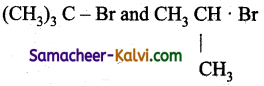
Answer:
(a) CH3 Br and CH3 CH2 Br
Hint:
Only primary alkyl halides undergo nucleophilic substitution reaction by SN2 mechanism.
Question 34.
Identify the pair of compounds which contain a chiral carbon atoms.
(a) 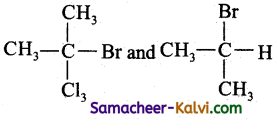
(b) 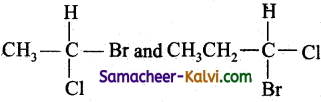
(c) (CH3)3 C – Br and (CH3)2 CH2 Br
(d) CH3CH2Br and CH3CH2I
Answer:
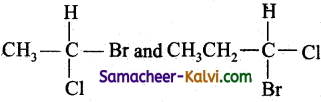
Hint:
Chiral carbon atoms are those to which form different atoms or groups attached to Cl.
![]()
Question 35.
Assertion:
Benzyl chloride when kept in acetone-water, produces, benzyl alcohol.
Reason:
The reaction follows SN2 mechanism.
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
Answer:
(c) If assertion is true, but reason is false.
Hint:
Correct reason: Benzyl halides undergo hydrolysis by SN1 mechanism.
Question 36.
Assertion:
The presence of nitro group facilitates nucleophilic substitution reactions in aryl halides.
Reason:
The intermediate carbanion is- stabilised due to the presence of the nitro group.
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
Answer:
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
![]()
Question 37.
Assertion:
Nucleophilic substitution reactions on an optically active alkyl halide gives a mixture of enantiomer.
Reason:
The reaction occurs through SN2 mechanism.
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
Hint:
The reaction occurs through SN1 mechanism.
Question 38.
Assertion:
1 – Butene on reaction with HBr in the presence of peroxide produces 1-bromobutane.
Reason:
It involves the formation of primary free radical.
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
Answer:
(c) If assertion is true, but reason is false.
Hint:
The reaction occurs through the formation of more stable 2° free radical.
Question 39.
Assertion:
Chloral reacts with phenyl chloride to form DDT.
Reason:
It is an electrophilic substitution reaction.
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
(b) If both assertion and reason are true but reason is not the correct explanation of the assertion.
(c) If assertion is true, but reason is false.
(d) If both assertion and reason are false.
Answer:
(a) If both assertion and reason are true and reason is the correct explanation of the assertion.
![]()
Question 40.
Consider the following reaction:
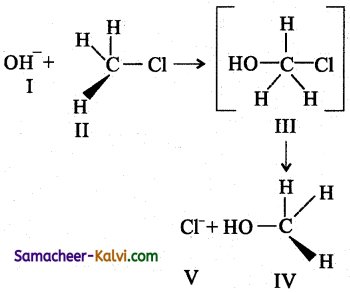
Which statements are correct about the above reaction?
(a) I and V both are nucleophile.
(b) In III carbon atom is sp3 hybridised.
(c) In IV carbon atom is sp2 hybridised.
(d) I and V both are electrophile.
Answer:
(a) I and V both are nucleophile.
Question 41.
Choose the incorrect statement with regard to SN1 reactions:
(a) The order of reactivity depends on the stability of intermediate carbocations.
(b) Among the isomeric butanes, 1 – bromo butane undergo SN1 reactions.
(c) Among the isomeric butanes, (CH3)3 C — Br undergo SN1 reactions.
(d) The carbocation (CH3)3 C+ is most reactive.
Answer:
(b) Among the isomeric butanes, 1 – bromo butane undergo SN1 reactions.
Hint.
The increasing reactivity of the four isomeric butanes towards SN1 reaction is
CH3 CH2 CH2 CH2 Br < (CH3)2 CH CH2 Br < (CH3)3 C – Br < 
![]()
Question 42.
The incorrect statement is the carbon atom is:
(a) The C – Cl bond is chlorobenzene is shorter than C – Cl bond in methyl chloride.
(b) The C – Cl bond in both chloro benzene and methyl chloride is sp3 hybridised.
(c) In chloro benzene, the lone pair of electrons on chlorine enters into conjugation with tho benzene ring.
(d) There is no resonance in methyl chloride, whereas, chloro benzene exhibits resonance.
Answer:
(b) The C – Cl bond in both chloro benzene and methyl chloride is sp3 hybridised.
Hint:
The carbon atom in C – Cl bond in aryl halides is sp2 hybridised whereas in methyl chloride it is sp3 hybridised.
Question 43.
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(a) Both compounds form same products on treatment with alcoholic KOH.
(b) Both compounds form same products on treatment with aqueous KOH.
(c) Both compounds form the same product on reduction.
(d) Both compounds are optically active.
(a) I, II
(b) I, III
(c) II, IV
(d) I, IV
Answer:
(b) I, III
Question 44.
Choose the incorrect statement:
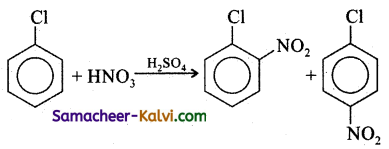
(a) The electrophile in this reaction is NO2+
(b) Chlorine deactivates the benzene ring, by -I effect while activates the benzene ring by + M effect.
(c) Chlorine is an ortho paradirecting group. Nitration is a nucleophilic substitution reaction.
(d) Nitration is a nucleophilic subctitution reaction.
Answer:
(d) Nitration is a nucleophilic subctitution reaction.
![]()
Question 45.
Match the entities of column I with appropriate entities of column II.
| Column I | Column II |
| (i) DDT | (A) pesticide |
| (ii) chloroform | (B) refrigerant |
| (iii) BHC | (C) insecticide |
| (iv) Freon – 12 | (D) anaesthetic |
(a) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)
(b) (i) – (D), (ii) – (A), (iii) – (B), (iv) – (C)
(c) (i) – (A), (ii) – (D), (iii) – (B), (iv) – (C)
(d) (i) – (B), (ii) – (C), (iii) – (A), (iv) – (D)
Answer:
(a) (i) – (C), (ii) – (D), (iii) – (A), (iv) – (B)
![]()
Question 46.
Match the entities of column I with appropriate entities of column II.
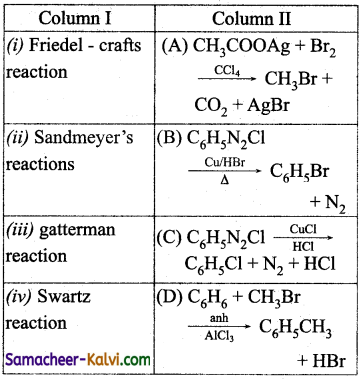
(a) (i)- (B), (ii) – (C), (iii) – (A), (iv) – (D)
(b) (i) – (D), (ii) – (C), (iii) – (A), (iv) – (B)
(c) (i) – (C), (ii) – (A), (iii) – (D), (iv) – (B)
(d) (i) – (A), (ii) – (C), (iii) – (B), (iv) – (D)
Answer:
(b) (i) – (D), (ii) – (C), (iii) – (A), (iv) – (B)