Students get through the TN Board 12th Chemistry Important Questions Chapter 14 Biomolecules which is useful for their exam preparation.
TN State Board 12th Chemistry Important Questions Chapter 14 Biomolecules
Question 1.
What are monosaccharides?
Answer:
Monosaccharides are carbohydrates which cannot be hydrolysed to simple molecules. Their general molecular formula is Cn(H2O)n where n = 3 to 7. There are two types. Those which contain an aldehyde group are called aldoses and those which contain a ketonic group are called ketoses. They are further classified as trioses, tetroses, pentose, hexoses etc according to as they contain 3, 4, 5, 6 carbon atom respectively.
Question 2.
Classify the following as monosaccharides and disaccharides:
Ribose: 2 – deoxy ribose; maltose, galactose, fructose, and lactose.
Answer:
Monosaccharides: Ribose, 2-Deoxyribose, galactose and fructose.
Disaccharides: Maltose and lactose.
![]()
Question 3.
What are the products of hydrolysis of (i) sucrose (ii) lactose.
Answer:
Both sucrose and lactoses are disaccharides. Sucrose on hydrolysis gives one molecule of each of glucose and fructose.
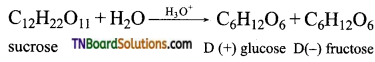
Lactose on hydrolysis gives one molecule of glucose and galactose.
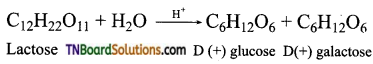
Question 4.
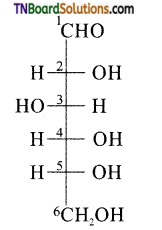
How do you explain the presence of all six carbon atoms in glucose in a straight chain?
Answer:
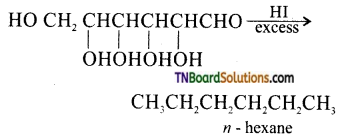
Since, n – hexane has six carbon atoms connected in a straight chain, therefore glucose also has six carbon atoms connected in a straight chain.
Question 5.
The letter ‘D’ or ‘L’ before the name of a stereo isomer indicates the correlation of configuration of that particular stereo isomers. This refers to their relationship with one of the isomers of glyceraldehyde. Predict whether the compound is has D or L configuration.
Answer:
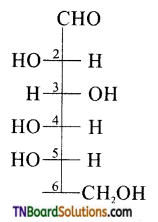
Since the configuration of the ‘OH’ group at the penultimate chiral carbon (last but one or C5) is towards left, therefore the given compound has ‘L’ configuration.
![]()
Question 6.
How do you explain the presence of five – OH groups in a glucose molecule?
Answer:
On acetylation with acetic anhydride in presence of pyridine, or a few drops of cone. H2SO4, it is converted into glucose penta acetate.
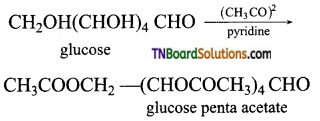
This confirms the presence of five OH groups.
Question 7.
Why does compound (A) given below does not form an oxime?
Answer:
Glucose penta acetate does not have a free OH group at carbon atom 1(C1) and hence cannot be converted into the open chain form having a free CHO group therefore, glucose penta acetate does not form an oxime.
Question 8.
How do you explain the presence of an aldehyde group in glucose molecule?
Answer:
Glucose reacts with hydroxyl amine, (NH2OH) to form an oxime, and adds one molecule of hydrogen cyanide (HCN) to give a cyano hydrin. Therefore, glucose contains a carbonyl group which can be either an aldehyde or ketone. On mild oxidation with bromine water, it gives a carboxylic acid i.e., gluconic acid containing same six carbon atoms present in glucose. This indicates that the carbonyl group present in glucose is an aldehyde group.
Question 9.
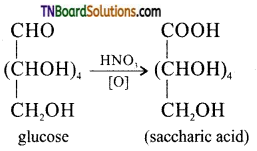
How do you distinguish 1° and 2° alcoholic groups present in glucose? Explains with reactions.
Answer:
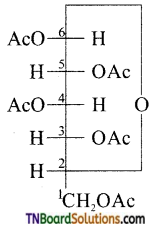
Consider the structure of glucose
The ‘OH’ group present on the terminal carbon (C6) is called the primary alcoholic group while all other four ‘OH’ groups present in C2, C3, C4 and C5 are called secondary alcoholic groups. 1° alcoholic groups are readily oxidised to carboxylic acids but 2° alcoholic groups undergo oxidation under drastic conditions. For example glucose on oxidation cone. HNO3 gives a dicarboxylic acid, saccharic acid, having the same number of carbon atoms as in glucose. This indicates that glucose contains one 1° alcoholic group while the remaining four are 2° alcoholic groups.
Question 10.
Write the reactions of D glucose which can’t be explained by its open chain structure. Reactions of D- glucose which can’t be explained by its open chain structure.
Answer:
(i) Glucose does not form NaHSO3 additions product, aldehyde-ammonia, 2-4 DNP derivative and does not respond to schiff reagent test. These are the characteristic reactions of aldehyde.
(ii) Glucose reacts with NH2OH to form an oxime but glucose penta acetate does not. This shows that the aldehyde group is absent in glucose penta acetate.
(iii) D(+) glucose exists in two isomeric forms i.e., α glucose and β glucose. Both these undergo mutarotation.
(iv) D(+) glucose forms two isomeric methyl glycosides. (Aldehydes normally react with two moles of methanol per mole of aldehyde where as one mole of glucose reacts with one mole of methanol to form a mixture of two methyl D – glucosides)
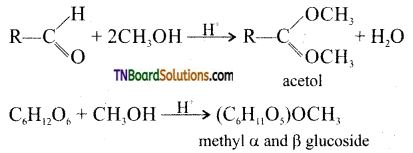
The two methyl glucosides behave as acetates.
![]()
Question 11.
Write the cyclic structure of glucose.
Answer:
The cyclic structures of a -D glucose and β -D glucose are as follows:
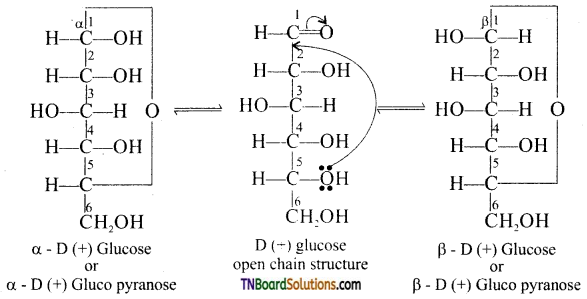
Question 12.
What is the structure feature characterising reducing sugars?
Answer:
The main structural feature of reducing sugars is the presence of an aldehyde group (— CHO) such as in glucose, mannose, galactose or a ketol group (—CO—CH2OH) as present in fructose.
Question 13.
Fructose contains a keto group but it still reduces Tollen’s reagent. Explain.
Answer:
Under basic conditions of Tollen’s reagent fructose undergoes a rearrangement as shown below:
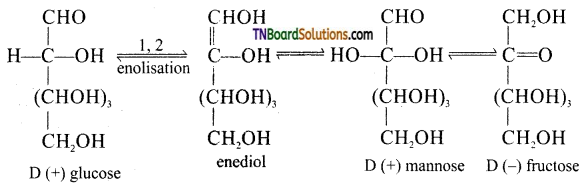
The same equilibrium is obtained even of one strands with D(+) mannose or D(-) fructose, i.e., Fructose gives an equilibrium mixture of fructose, glucose and mannose. Since both glucose and mannose contain —CHO group, they reduce Tollen’s reagent.
Question 14.
Explain the term glycosidic linkage with an example.
Answer:
The ethereal or oxygen linkage through two monosaccharides are joined together by the loss of a molecule of water to form a molecule of a disaccharide is called a glycosidic linkage. The glycosidic linkage of two α – D glucose in maltose is given below:
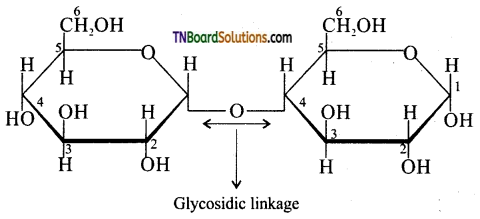
![]()
Question 15.
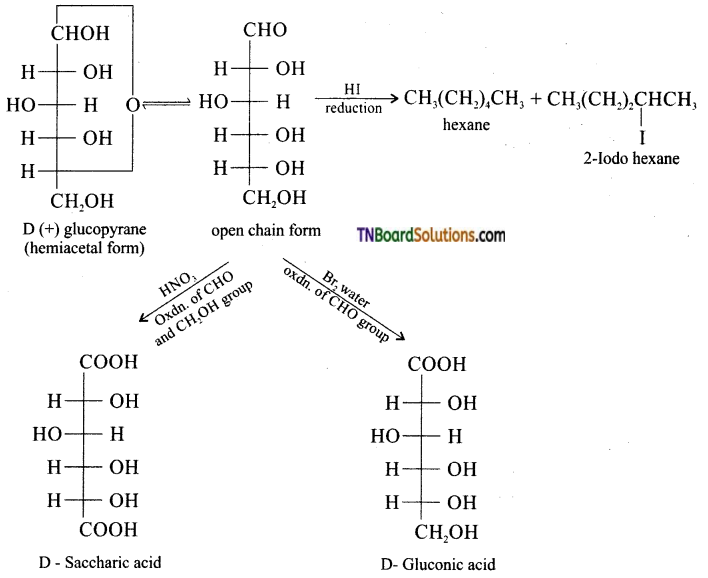
What happens when D – glucose is treated with the following reagents (i) HI (ii) Bromine water (iii) HNO3.
Answer:
Question 16.
Glucose and fructose give the same osazone. Give reason.
Answer:
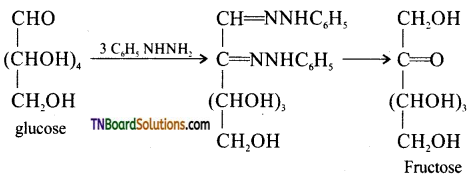
During osazone formation the reaction occurs only at C1 and C2. As glucose and fructose differ from each other only in the arrangement of atoms C1 and C2 they give the same osazones.
Question 17.
Name two components of starch. How do they differ from each other structurally?
Answer:
Starch is a polymer of a – glucose and consists of two components amylose and amylopectin. Amylose is a long unbranched chain with 200 – 1000 a D(+) glucose units held by C1 – C4 glycosidic linkage.
Amylopectin is a branched chain polymer of a D glucose units in which chain is formed by C1 – C4 glycosidic linkage branching occurs by C1 – C6 glycosidic linkage.
Question 18.
Name the reaction which proves the presence of carbonyl group in fructose.
Answer:
Fructose reacts with hydroxylamine (NH2OH) and hydrogen cyanide (HCN). These reactions . prove the presence of carbonyl group in fructose.
![]()
Question 19.
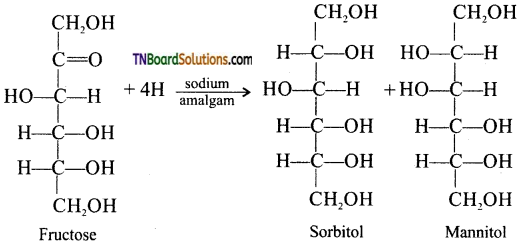
Explain the reaction which indicates the presence of a carbonyl group in fructose.
Answer:
Partial reduction of fructose with sodium amalgam and water produces mixtures of sorbitol and mannitol which are epimers at second carbon. New asymmetric carbon is formed at C2. This confirms the presence of keto group.
Question 20.
Oxidation of fructose with nitric acid gives glycolic acid and tartaric acid. What information that this reaction gives to establish the structure of fructose.
Answer:
On oxidation with nitric acid, it gives glycolic acid and tartaric acids which contain smaller number of carbon atoms than in fructose.
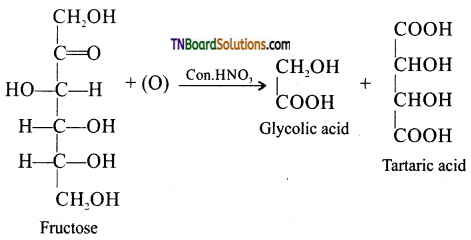
This shows that a keto group is present in C2. It also shows the presence of 1° alcoholic groups at C1 and C6.
Question 21.
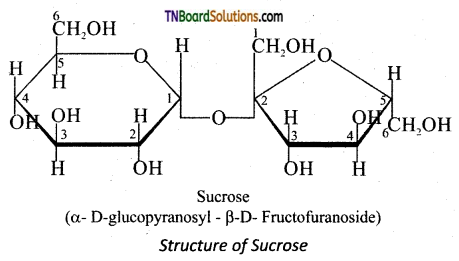
Explain why sucrose is called ‘invert’ sugar.
Answer:
Sucrose (+ 66.6°) and glucose (+ 52.5°) are dextrorotatory compounds while fructose is levo rotatory (- 92.4°). During hydrolysis of sucrose the optical rotation of the reaction,mixture changes from dextro to levo. Hence, sucrose is also called as invert sugar.
![]()
Question 22.
Write the open chain structure of D(+) fructose and indicate the asymmetric carbon atoms present in it.
Answer:
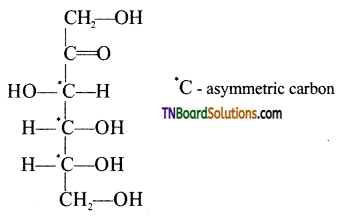
Question 23.
Briefly discuss the cyclic structure of fructose.
Answer:
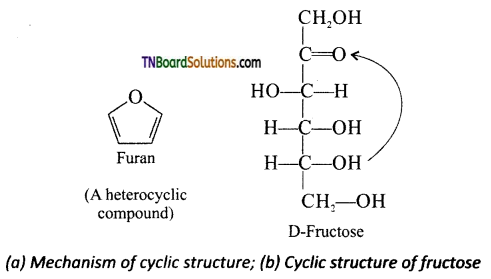
Like glucose, fructose also forms cyclic form. Unlike glucose it forms a five membered ring similar to furan. Hence it is called furanose form. When fructose is a component of a saccharide as in sucrose, it usually occurs in furanose form.
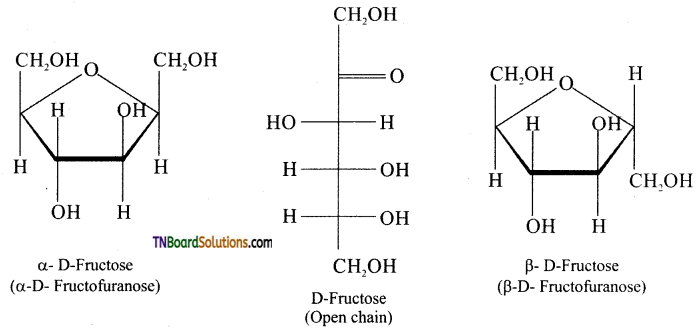
Question 24.
Explain why sucrose is called a non reducing sugar.
Answer:
In sucrose, C1 of α-D-glucose is joined to C2 of -D-fructose. The glycosidic bond thus formed is called α-1,2 glycosidic bond. Since, the both the carbonyl carbons (reducing groups) are involved in the glycosidic bonding, sucrose is a non-reducing sugar.
![]()
Question 25.
Based on its cyclic structure, explain why lactose is a reducing sugar.
Answer:
On hydrolysis, it yields galactose and glucose. Here, the β-D-galactose and β-D-glucose are linked by β -1,4 glycosidic bond as shown in the figure. The aldehyde carbon is not involved in the glycosidic bondbence; it retains its reducing property and is called a reducing sugar.
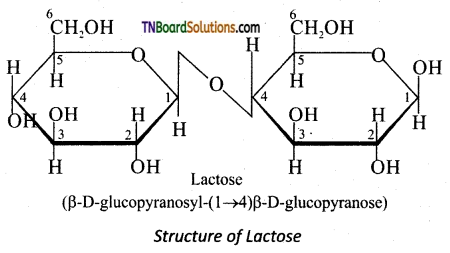
Question 26.
Maltose is a reducing sugar. Explain.
Answer:
Maltose consists two molecules of α-D-glucose units linked by an α -1,4 glycosidic bond between anomeric carbon of one unit and C-4 of the other unit. Since one of the glucose has the carbonyl group intact it is also acts as a reducing sugar.
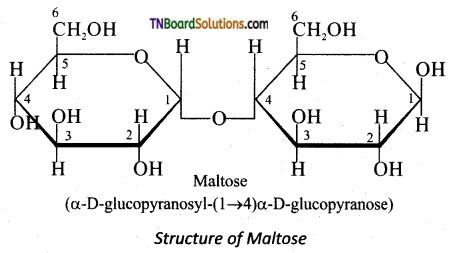
Question 27.
Mention the uses of cellulose.
Answer:
Cellulose is used extensively in manufacturing paper, cellulose fibres, rayon, explosive, (Gun cotton – Nitrated ester of cellulose) and so on. Humans cannot use cellulose as food because our digestive systems do not contain the necessary enzymes (glycosidases or cellulases) that can hydrolyse the cellulose.
Question 28.
Briefly explain the structure of cellulose.
Answer:
Cellulose is a straight chain polysaccharide.
The glucose molecules are linked by β(1, 4) glycosidic bond.
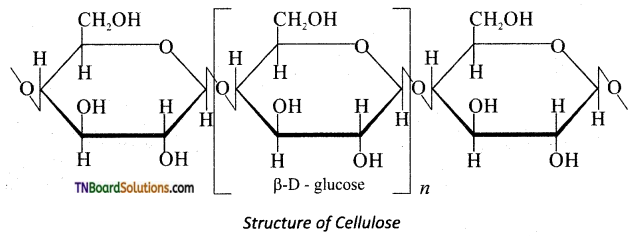
![]()
Question 29.
Following two amino acids lysine and glutamine dipeptide linkage. What are the two possible dipeptides?
Answer:
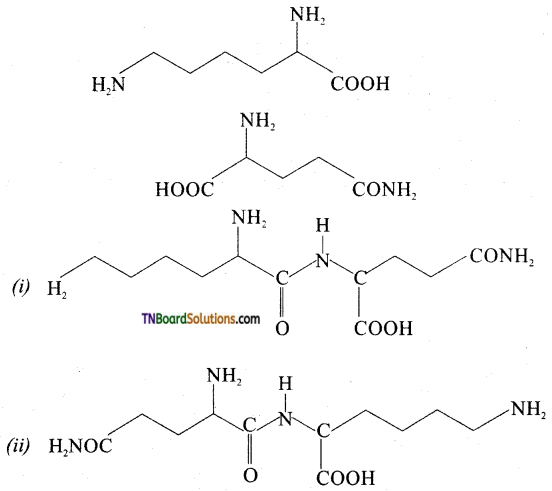
Question 30.
Give the. important uses of carbohydrates.
Answer:
- Carbohydrates, widely distributed in plants and animals, acts mainly as energy sources and structural polymers.
- Carbohydrate is stored in the body as glycogen and in plant as starch.
- Carbohydrates such as cellulose which is the primary component of the plant cell wall is used to make paper, furniture (wood) and cloths (cotton).
- Simple sugar glucose serves as an instant source of energy.
- Ribose sugars are one of the components of nucleic acids.
- Modified carbohydrates such as hyaluronate (glycosaminoglycans) act as shock absorber and lubricant.
Question 31.
What is glycogen? How is it different from starch?
Answer:
Glycogen is a carbohydrates stored in animal body. Starch is a mixture of two components a water soluble compounds called amylose (15 – 20%) and water insoluble amylopectin
(80 – 85%) chemically, amylose is a long unbranched chain with 200 – 1000 a – D (+) glucose units held by C1 – C4 glycosidic linkage. But both glycogen and amylopectin are branched polymers of α – D glucose rather glucogen is more highly branched than amylopectin. Whereas amylopectin chain consist of 20 – 25 glucose units glucose chain consists of 10 – 14 glucose units.
![]()
Question 32.
What is the basic structural difference between starch and cellulose?
Answer:
Starch contains amylose and amylopectin. Amylose is a linear polymer of α D glucose whereas cellulose is a linear polymer of β – D – glucose. In amylose, C-1 of one glucose unit is connected to C-4 of the other through glycosidic linkage.
Cellulose is a straight chain polysaccharides composed of only β -D glucose units which are joined by glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit.
Question 33.
What are essential and non essential amino acids? Give two examples of each type.
Answer:
α – amino acids which are required for health and growth of human beings but are not synthesised by the human body are known as essential amino acids, eg: valine, leucine, phenylalanine etc.,
On the other hand, α amino acids which are needed for health and growth of human being and are synthesised by the body are called non essential amino acids, eg: glycine, alanine, aspartic acid etc.
Question 34.
Give examples for fibrous and globular proteins.
Answer:
Fibrous protein: Keratin in skin, hair, nails, and wood, collagen in tendous. Fibroin in silk, myosin in muscles.
Globular protein: All enzymes, hormones like insulin, from pancreas, thyroglobulin from thyroid gland antibodies, haemoglobin.
Question 35.
What is isoelectic point? Explain with a suitable examples.
Answer:
The pH at which there is no net migration of the amino acid under the influence of an applied electrified is called isoelectric point.
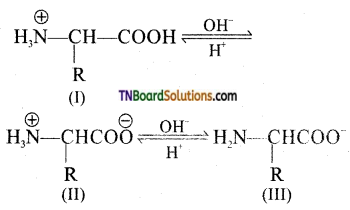
Question 36.
What is peptide bond?
Answer:
The covalent bond —NH—CO— formed between —NH2 group of one amino acid and —COOH group of the other with the elimination of a molecule of water is called a peptide bond.
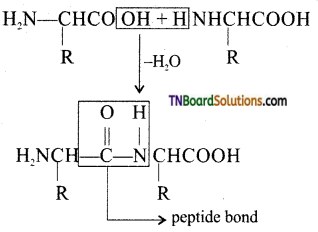
![]()
Question 37.
How are epimers differ from anomers?
Answer:
Carbohydrates which differ in configuration at the glycosidic carbon (i.e., C1 in aldoses and C2 in ketoses) are called anomers while those differ in configuration at any carbon other than glycosidic carbon are called epimers.
For example α – D glucose and β-D glucose are anomers since they differ in configuration at C1 while glucose and mannose are called epimers since they differ in configuration at C2 (other than glycosidic carbon C1). In other words glucose and mannose are C2 epimers. Similarly glucose and galactose are C4 epimers.
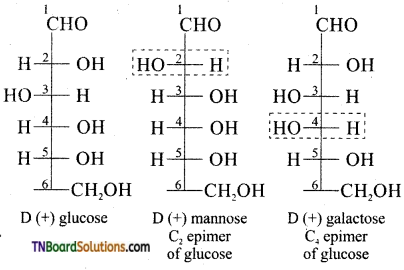
Question 38.
Differentiate between globular and fibrous proteins.
Answer:
| Globular protein | Fibrous protein |
| Have almost spherical shape due to folding of polypeptide chains. | Polypeptide chains consists of thread like molecule which tend to lie side by side to form fibres. |
| Soluble in water. | Insoluble in water. |
| Sensitive to small changes of temperature and pH. Therefore they undergo denatura- tion on heating or an treatment with acid or base. | Stable to moderate change of temperature and pH. |
| The interaction are (i) disulphide bridging (ii) intermolecular hydrogen (iii) vanderwaals attraction (iv) dipolar attraction |
Held together by disulphide bridges and weak intermolecular hydrogen bonds. |
Question 39.
Describe (i) primary structure (ii) secondary structure (iii) tertiary structure (iv) quaternary structure of proteins.
Answer:
(i) Primary structure: Proteins may contain one or more polypeptide chains. Each polypeptide chain has a large number of α – amino acids which are linked to one another in a specific sequence. The specific sequence in which the various α – amino acids present in a protein are linked to one another is called its primary structure. Any change in the sequence of α – amino acids creates a different protein.
(ii) Secondary structure: It refers to shape in which a long polypeptide chain exists. A protein may assume a – helix structure or β – pleated structure. The α – helix structure results due to regular coiling of polypeptide chain which is stabilized by intramolecular hydrogen bonding. In β pleated sheet structure, all peptide chains are stretched to a nearly maximum extention and then arranged side by side and held together by intramolecular hydrogen bonding.
(iii) Tertiary structure: The tertiary structure of proteins represent overall folding of the polypeptide chains, i.e., further folding of the secondary structure. The main force which stabilises 2° and 3° of proteins are hydrogen bonds, disulphide linkages, Vanderwaals forces of attraction and electrostatic force of attraction.
(iv) Quaternary structure: Same of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these sub units with respect to each other is called quaternary structure.
![]()
Question 40.
What are the common types of secondary structure of protein?
Answer:
The conformations which the polypepetide chains assume as a result of hydrogen bonding is called the secondary structure of protein. The two types are
- α helix and
- β pleated structure
Question 41.
What types of bonding helps in stabilising α – helix structure of proteins?
Answer:
The α helix structure of proteins is stabilised by intramolecular hydrogen bonding between
C = O of one amino acid residue and —N—H of the fourth amino acid residue in the chain.
Question 42.
What is the effect of denaturation on the structure of proteins?
Answer:
During denaturation, 2° and 3° structures of proteins are destroyed but 1° structure remains intact. As a result of denaturation, the globular protein (soluble in H2O) are converted to fibrous proteins (insoluble in H2O) and their biological activity is lost.
Question 43.
Mention the causes for the denaturation of proteins.
Answer:
The denaturation of proteins occurs when the protein is exposed to the higher temperature the presence of certain such as urea, alteration of pH, ionic strength etc.
Question 44.
Mention the importance of protein.
Answer:
- All biochemical reactions that occur in the living systems are catalysed by the catalytic proteins called enzymes.
- Proteins such as keratin, collagen acts as structural backbones.
- Proteins are used for transporting molecules (Haemoglobin), organelles (Kinesins) in the cell and control the movement of molecules in and out of the cells (Transporters).
- Antibodies help the body to fight various diseases.
- Proteins are used as messengers to coordinate many functions. Insulin and glucagon control the glucose level in the blood.
- Proteins act as receptors that detect the presence of certain signal molecules and activate the proper response.
- Proteins are also used to store metals such as iron (Ferritin) etc.
![]()
Question 45.
What are enzymes? What is the most important reason for their specific action?
Answer:
Enzymes are biomolecules & which catalyst biological reactions. Chemically they are globular proteins (water-soluble) that have very high molecular near ranging from 15,000 to 1,000,000 mol-1.
Enzymes are highly specific in their actions. The specificity is due to the presence of active sites of definite size and shape on their surfaces so that only specific substrates can fit into them. This specific binding leads to the formation of enzyme substrates complex which accounts for high specificity.
Question 46.
Name the enzyme that converts (i) carbonic acid to CO2 and H2O (ii) hydrolysis of sucrose to fructose and glucose. (iii) hydrolysis of lactose to glucose and galactose.
Answer:
(i) Carbonic anhydrase enzyme catalyses the interconversion of carbonic acid to water and carbon dioxide.
(ii) Sucrose enzyme catalyses the hydrolysis of sucrose to fructose and glucose.
(iii) Lactase enzyme hydrolysis the lactose to glucose and galactose.
Question 47.
Explain the mechanism of enzyme action.
Answer:
Mechanism of Enzymatic action: The general scheme is represented as
Step 1: Binding of the enzyme (E) to the substrate (S) to form a complex (ES) is Enzyme – substrate complex.
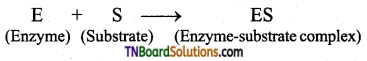
Step 2: Product formation in the complex

Step 3: Release of enzyme product complex and leaving the enzyme as unchanged.
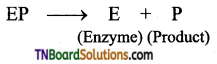
Question 48.
What are lipids? How are they classified?
Answer:
Lipids are the principal components of cell membranes including cell walls. They act as an energy source for living systems. Classification of lipids: Based on their structures Lipids can be classified as simple lipids, compounds lipids and derived lipids. Simple lipids can be further classified into fats, which are esters of long-chain fatty acids with glycerol (triglycerides) and waxes which are the esters of fatty acids with long-chain monohydric alcohols (Beeswax). Compounds lipids are the esters of simple fatty acid with glycerol which contain additional groups. Based on the groups attached, they are further classified into phospholipids, glycolipids and lipoproteins. Phospholipids contain a phospho-ester linkage while the glycolipids contain a sugar molecule attached. The lipoproteins are complexes of lipid with proteins.
![]()
Question 49.
How are vitamins classified?
Answer:
Vitamins are classified depending on their solubility in water.
- Water-soluble Vitamins: These include Vitamin B – complex (B1, B2, B5, i.e., nicotinic acid, B5, B12 and vitamin C)
- Water-insoluble Vitamins (or fat-soluble Vitamins): These include Vitamins D, A, E and K. They are stored in the liver and in fat-storing tissues.
Question 50.
What are coenzymes and prosthetic groups?
Answer:
coenzymes are loosely held to the protein and can be easily separated by dialysis.
Prothetic group: These are tightly held to the protein molecule by covalent bonds. They can be separated only by hydrolysis.
Question 51.
What are nucleic acids? Mention their two important functions?
Answer:
Nucleic acids are polynucleotides i.e., they are formed by the condensation of thousand molecules of nucleotides with the elimination of water molecules.
Nucleic acids are of two types: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The two main functions of nucleic acids are:
- DNA is responsible for the transmission of heredity effects from one generation to another. This is due to the unique property of replication during cell division as a result of which two identical DNA strands are transmitted to daughter cells.
- DNA and RNA are responsible for protein synthesis needed for the growth and maintenance of our body. Actually, proteins are synthesised by various RNA molecules (rRNA, mRNA and tRNA) in the cell but the message for the synthesis of a particular protein is given by DNA molecules.
Question 52.
Write the structure of nucleic acids.
Answer:
Primary structure: The primary structure of nucleic acid refers to the sequence in which the four nitrogen bases (A, G, C and T in DNA or A, G, C and U in RNA) are attached to the sugar-phosphate backbone of a nucleotide chain.
Secondary structure: DNA consists of two strands of polynucleotides coiled around each other in form of a double helix. The backbone of each strand consists of sugar-phosphate units and the base unit of each strand are pointed into the interior of the helix and are linked together through hydrogen bonds. Whereas G and C are held by three H – bonds, A and T are held by H – bonds.
![]()
Question 53.
Write down the structure of sugar present in DNA.
Answer:
DNA contains β D – 2 deoxyribose as sugar. In structure in the furanose form is
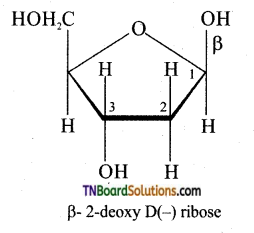
Question 54.
What purine and pyrimidine bases are present in DNA and RNA?
Answer:
Purines: adenine and guanine Pyrimidine: Cytosine and thymine are present in DNA.
Cytosine and uracil is present in RNA.
Question 55.
What is a nucleoside?
Answer:
A nucleoside consists of two components, i.e., a nitrogenous base (purine or pyramidine)’ and a five-carbon sugar (ribose or deoxyribose). It is obtained when the nitrogenous base is attached, to C1 of sugar by a β – linkage. Thus, in general, a nucleoside may be represented as a base – super. Examples of nucleosides are adenosine, cytidine etc.,
Question 56.
What is a nucleotide?
Answer:
A nucleotide consists of all the three basic components of nucleic acids, i.e., a nitrogenous purine or a pyrimidine base, a five-carbon ribose or deoxyribose and phosphoric acid. A nucleotide is obtained when a nitrogenous base is attached to C1 of the sugar by a β linkage and a nucleotide is thus obtained when C5—OH of the sugar is esterified with phosphoric acid. Thus, in general, nucleotides may be represented as a base – sugar-phosphate.
![]()
Question 57.
What type of linkage holds together the monomers of DNA?
Answer:
The monomers of DNA are polydeoxy ribo nucleotides. These are held together by H bonds. There are three H bonds between guanine and cytosine (G ≡ C) and two between adenine and thymine (A = T).
Question 58.
The two strands in DNA are not identical but complimentary explain.
Answer:
The two strands in the DNA molecule are held together through H — bonds between the purine base of one strand and pyrimidine base of the other and vice versa. Because of different sizes and geometries of the base, the only
possible pairing in DNA is G(guanine) and C(cytosine) through two hydrogen bonds i.e., bases (C ≡ G) and between A(adenine) and thymine (T) through two hydrogen bonds (i.e., A = T) due to this base – pairing principle the sequences of bases in one strand automatically fixes the sequence of bases in the other strand. Thus the two strands are not identical but complementary.
Question 59.
Mention the functional difference DNA and RNA.
Answer:
| DNA | RNA |
| DNA has unique property of replication. | RNA does not replicate. |
| DNA controls transition of hereditary effects. | RNA controls the synthesis of proteins. |
Question 60.
What is DNA fingerprinting? Explain.
Answer:
The DNA fingerprint is unique for every person. By using this method, individual-specific variation in human DNA can be detected.
![]()
Question 61.
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does it suggest about the structure of RNA?
Answer:
A DNA molecule has two strands in which four complementary bases pair each other i.e., cytosine (C) always pairs with guanine (G) while Thymine (T) always pair with adenine(A). Therefore when a DNA molecule is hydrolysed, the molar amount of cytosine is equal to that of guanine and that of adenine is always equal to that of thymine. Since RNA has no such relationship between the quantities of the four bases (C, G, A and U) obtained, therefore, based on the base-pairing principle, i.e., A pairs with U and C pairs with G is not followed. Therefore, unlike DNA, RNA has a single strand.
Choose the correct answer:
1. Glucose does not react with:
(a) Br2 / H2O
(b) NH2 OH
(c) (CH3 CO)2O
(d) NaHSO3
Answer:
(d)
2. The letter ‘D’ in D – glucose signifies:
(a) Configuration at all chiral carbons
(b) Dextro rotatory
(c) That it is a monosaccharide
(d) Configuration at the penultimate chiral carbon.
Answer:
(d)
3. The term anomer of glucose refers to:
(a) isomers of glucose that differ in configuration at carbon one and four (C-1 and C-4).
(b) a mixture of D – glucose and L – glucose
(c) enantiomers of glucose
(d) isomers of glucose that differ in configuration at C-1.
Answer:
(d)
![]()
4. The helical structure of proteins is stabilised by:
(a) dipeptide bonds
(b) hydrogen bonds
(c) ether bonds
(d) peptide bonds
Answer:
(b)
5. The vitamins absorbed from intestine along with fats are:
(a) A, D
(b) A, B
(c) A, C
(d) D, B
Answer:
(a)
6. The human body does not produce:
(a) enzymes
(b) DNA
(c) vitamins
(d) hormones
Answer:
(c)
7. Adenosine is an example of:
(a) nucleotide
(b) nucleoside
(c) purine base
(d) pyramidine base
Answer:
(b)
8. Which of the following statements is not true about glucose?
(a) It is aldohexose.
(b) On heating with HI it forms n-hexane
(c) It is present in furanose form
(d) It does not give 2-4 DNP test.
Answer:
(c)
9. Which of the following reactions of glucose can be explained only by its cyclic structure?
(a) Glucose forms Penta acetate
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Answer:
(c)
Hint: Due to the absence of free ‘OH’ group at C1 cyclic structure of glucose Penta acetate cannot revert to open-chain aldehyde form and hence cannot form Penta acetate.
![]()
10. Three cyclic structures of monosaccharides are given below, which are anomers?
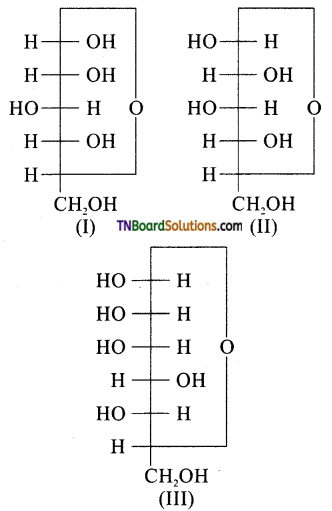
(a) I and II
(b) II and III
(c) I and III
(d) III is anomer of I and II
Answer:
(a)
Hint: Structure (I and II) differ only in the position of OH at C1 and hence anomers.
11. Each polypeptide in a protein has amino acids linked with each other in a specific sequence. This sequence of amino acids is said to be :
(a) primary structure of proteins
(b) secondary structure of proteins
(c) tertiary structure of proteins
(d) quaternary structure of proteins
Answer:
(a)
12. RNA and DNA contains four bases each which of the following bases is not present in RNA.
(a) Adenine
(b) Uracil
(c) Thymine
(d) Cytosine
Answer:
(c)
![]()
13. In fibrous proteins, polypeptide chains are held together by:
(I) Vander waals forces
(II) disulphide linkages
(III) electrostatic forces of attraction
(IV) hydrogen bonds
(a) I and II
(b) II and IV
(c) III and IV
(d) IV only
Answer:
(b)
14. Which of the following does not exhibit the phenomenon of muta rotation?
(a) (-) Fructose
(b) (+) sucrose
(c) (+) Lactose
(d) (+) Maltose
Answer:
(b)
Hint: Only monosaccharides i.e., (-) fructose and reducing disaccharides i.e., (+) lactose and (+) maltose show mutarotation. Since (+) sucrose is a non reducing sugar, it does not show mutarotation.
15. Which of the following statements is not true regarding (+) lactose?
(a) (+) lactose, C12H22O11 contains 8 (—OH) groups.
(b) On hydrolysis, (+) lactose gives equal amounts of D(+) glucose and D(+) galactose.
(c) (+) Lactose is a β – glycoside formed by the union of a molecule of D(+) glucose and a molecule of D(+) galactose.
(d) (+) Lactose is a reducing sugar and does not exhibit mutarotation.
Answer:
(d)
16. The vitamin which is neither soluble in water nor in fat is:
(a) biotin
(b) phylloquinone
(c) thiamine
(d) ergocalciferol
Answer:
(a)
17. Which of the following is not a fat soluble vitamins?
(a) Vitamin B complex
(b) Vitamin D
(c) Vitamin E
(d) Vitamin A
Answer:
(a)
![]()
18. Which of the following structure represent the peptide chain?
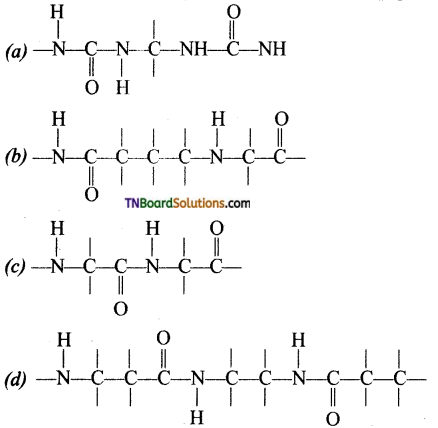
Answer:
(c)
19. The tripeptide is written as glycine – alanine – glycine. The correct structure of the tripeptide is:
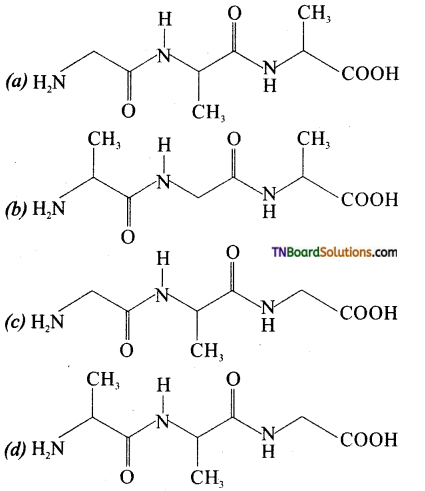
Answer:
(c)
![]()
20. The hormone which controls the processes of the burning of fats, proteins and carbohydrates and liberates energy in the body is:
(a) thyroxine
(b) adrenaline
(c) insulin
(d) cortisone
Answer:
(c)
21. The secondary structure of a protein refers to:
(a) fixed configuration of polypeptide backbone.
(b) a- helical backbone
(c) hydrophobic interactions
(d) sequence of amino acids
Answer:
(b)
22. Which of the following statements about “denaturation” given below are correct?
(i) denaturation of proteins causes loss of secondary and tertiary structure of proteins.
(ii) denaturation leads to the conversion of double strand DNA to a single strand.
(iii) denaturation affects the primary structure which gets distorted.
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (i), (ii) and (iii)
Answer:
(c)
23. The pyramidine bases present in DNA are :
(a) Cytosine and adenine
(b) Cytosine and guanine
(c) Cytosine and thymine
(d) Cytosine and uracil
Answer:
(c)
![]()
24. Nitrogen base that is found in RNA but not in DNA is:
(a) uracil
(b) thymine
(cl cytosine
(d) adenine
Answer:
(a)
25. RNA and DNA are chiral molecules, then chirality is due to:
(a) D – sugar component
(b) L – sugar component
(c) Chiral bases
(d) Chiral phosphate ester unit
Answer:
(a)
26. In DNA, the complimentary bases are:
(a) Adenine and guanine; thymine and cytosine
(b) Uracil and adenine; cytosine and guanine
(c) Adenine and thymine; guanine and cytosine
(d) adenine and thymine; guanine and uracil
Answer:
(c)
27. Assertion: Fructose does not contain an aldehyde group but still reduces Tollen’s reagent.
Reason: In presence of base, fructose undergoes rearrangement to form glucose and mannose.
(a) Both assertion and reason are correct and the reason is a correct explanation of assertion.
(b) Both assertion and reason are correct but reason is not a correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a)
![]()
28. Assertion: Glycosides are hydrolysed in acidic conditions.
Reason: Glycosides are acetals.
(a) Both assertion and reason are correct and the reason is a correct explanation of assertion.
(b) Both assertion and reason are correct but reason is not a correct explanation of assertion.
(c) Assertion is true but reason is false.
(d) Both assertion and reason are false.
Answer:
(a)
29. Match the entries in column I with appropriate entries in column II and choose the correct option.
| Column I | Column II |
| (A) Glycosidic linkage | (1) Monomeric units of nucleic acids |
| (B) peptide bond | (2) Sugar heterocyclic base combination |
| (C) Nucleoside | (3) linkage between two amino acid units |
| (D) Nucleotide | (4) Linkage between two monosaccharide units. |
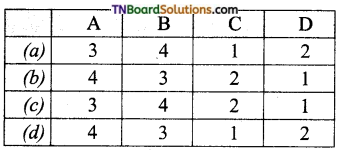
Answer:
(b)
![]()
30. The two forms of D – glucopyranose are called:
(a) Enantiomers
(b) Anomers
(c) Epimers
(d) Diastereomers
Answer:
(b)