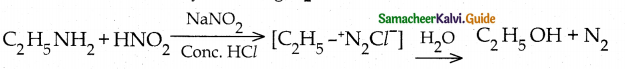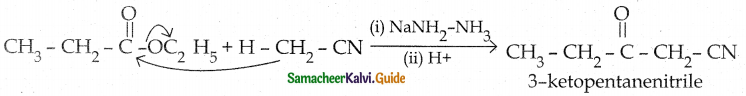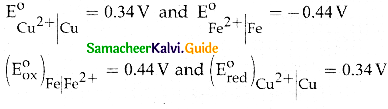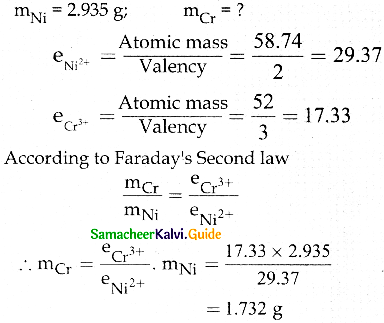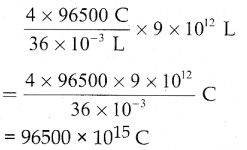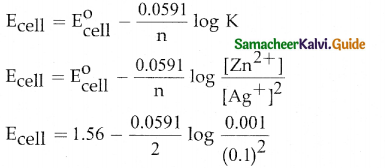Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Commerce Guide Pdf Chapter 17 Rights, Duties & Responsibilities of Consumers Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Commerce Solutions Chapter 17 Rights, Duties & Responsibilities of Consumers
12th Commerce Guide Rights, Duties & Responsibilities of Consumers Text Book Back Questions and Answers
I. Choose the correct answers.
Question 1.
The final aim of modern marketing is …………….
a. Maximum profit
b. Minimum profit
c. Consumer satisfaction
d. Service to the society
Answer:
c. Consumer satisfaction
Question 2.
…………………. is the king of modern marketing.
a. Consumer
b. Wholesaler
c. Producer
d. Retailer
Answer:
a. Consumer
![]()
Question 3.
As the consumer is having the rights, they are also having …………….
a. Measures
b. Promotion
c. Responsibilities
d. Duties
Answer:
c. Responsibilities
Question 4.
Which of the following is not a consumer right summed up by John F. Kennedy
a. Right to safety
b. Right to choose
c. Right to consume
d. Right to informed
Answer:
c. Right to consume
![]()
II. Very Short Answer Questions
Question 1.
Write short notes on “Right to be informed”
Answer:
Consumers should be given all the relevant facts about the product. This implies that the manufacturer and the dealer are expected to disclose all the material facts relevant and relating to the product.
Question 2.
What do you understand about the “Right to safety”?
Answer:
“Right to safety” or “right to protection” against the marketing of goods which are hazardous and dangerous to consumer health, lives, and property.
Question 3.
What are the rights of consumers according to John F. Kennedy?
Answer:
The former president of U.S.A Mr. John F. Kennedy defined the basic consumer rights as “The Right of Safety“ the Right to be informed, the Right to choose and the Right to be heard.”
Question 4.
Which is the supreme objective of a business?
Answer:
The prime and supreme objective of the business is to satisfy the consumer’s wants.
Question 5.
What are the important aspects to be kept in mind by consumers while purchasing goods related to the quality of goods?
Answer:
The consumer has to have the knowledge about the quality from his own experiences or from the experiences of other persons who used the product or by browsing the website.
![]()
III. Short Answer Questions
Question 1.
What do you understand by “Right to redressal”?
Answer:
- Right to seek redressal against unfair trade practices or unscrupulous exploitation of consumers.
- The complaints and protests of the aggrieved consumer is to be settled and compensated within a reasonable time.
- There should be fair and just settlement of deserving claims in a definite time frame.
Question 2.
What do you understand about “Right to protection of health and safety”?
Answer:
- Right to safety or right to protection against marketing of goods which are hazardous and dangerous to consumers health, lives and property.
- The health hazards which are likely to arise have to be eradicated.
- Both life saving and life sustaining safety is to be guaranteed in case of food items drugs.
![]()
IV. Long Answer Questions
Question 1.
What are the rights of consumers? [HIS] [CR|
Answer:
Right to be heard:
Consumers have right to show and register their dissatisfaction.
Right to be heard and to be assured that the consumer’s interest will receive due consideration b\ the sellers
Right to be informed:
The manufacturers and the sellers are expected to disclose (inform ) all the material facts regarding to the products [Quality, Quantity, potency, purity, price etc.|
Right to safety:[Right to protection of health and safety]
Right to safety or right to protection against marketing of goods which are hazardous and dangerous to consumer health, lies and properly.
Right to choose:
Right to access [choose] variety of goods [quality and brands] at reasonable price.
Right to redressal:
- Right to seek redress against unfair trade practices or unscrupulous exploitations or
- The complaints and protests of the aggrieved consumer is to be settled and compensated
- There should be fair and just settlement of deserving claims in a definite lime frame.
Right to consumer education:
The Consumer has to acquire knowledge and stay well informed although his life
He should be aware about his rights and the reliefs granted to him where a product or service falls short or his expectations.
![]()
Question 2.
Explain the duties of consumers. [BEEBI] [N]
Answer:
Apart from rights, there are certain duties imposed on the consumer. The following are the duties of consumers
- Buying Quality Products at Reasonable Price: It is the duty of a consumer to purchase a product after gaining a thorough knowledge of its price, quality, and other terms and conditions.
- Ensure the Weights and Measurement before Purchase: The consumer should ensure that he/she is getting the product of exact weight and measure.
- Reading the Label Carefully: It is the duty of the consumer to read the label of the product thoroughly.
- Beware of False and Attractive Advertisements: It is the prime duty of the consumer about the genuineness of the advertisement, before purchasing the product.
- Ensuring the Receipt of Cash Bill: It is a legitimate duty of consumers to get the cash receipt and warranty card supplied along with the bill.
![]()
Question 3.
What are the responsibilities of consumers?
Answer:
- The consumer must pay the price of the goods according to the terms and conditions of the sale contract.
- The duty of the consumer to apply to the seller for the delivery of goods. Otherwise, goods will not be delivered
- He is responsible to take delivery of goods on the due date and time.
- The consumer has to bear any loss which may arise to the seller when the consumer refuses to take delivery or delays in taking delivery in time.
- The consumer is bound to pay any interest or special damages caused to the seller in case if there is a delay in the payment.
- The consumer must ask and collect the invoice, cash receipt, delivery note, guarantee card.
- The consumer has to follow and observe strictly the instruction and precautions while using the products.
- He has to follow the prescriptions and directions of Doctors and such other professionals.
- He has a responsibility to tell the seller of his requirements and expectations from the product.
- He must file a complaint with the seller concerned about defects or shortcoming noticed in their products and services.
- The consumer should never compromise on the quality of goods. He must watch for ISO, Agmark, FPO, the standard quality certification marks on the label.
12th Commerce Guide Rights, Duties & Responsibilities of Consumers Additional Important Questions and Answers
I. Choose the Correct Answers
Question 1.
The consumer is to be protected against any __________
(a) unfair practices of trade
(b) family functions
(c) profit-making firm
(d) loss in business
Answer:
(a) unfair practices of trade
Question 2.
…………………………. Should be conscious of his duties.
a. Producer
b. Consumer
c. Seller
d. NOTA
Answer:
b. Consumer
![]()
Question 2.
The consumer is the __________ of the modem marketing.
(a) Manager
(b) Director
(c) King
(d) None of these
Answer:
(c) King
II. Match the following.
Question 1.
|
List – I |
List -II |
| i. Right to choose | 1. Prompt settlement |
| ii. Right to heard | 2. Offering the widest range |
| iii. Right to be informed | 3. Register their satisfaction |
| iv. Right to seek redressal | 4. Relevant facts |
a. i-2, ii-3, iii-4, iv-1
b. i-1, ii-2, iii-3, iv-4
c. i-3, ii-4, iii-1, iv-2
d. i-4, ii-1, iii-2, iv-3
Answer:
a. i-2, ii-3, iii-4, iv-1
![]()
III. Assertion and Reason
Question 1.
Assertion (A) : Every consumer has a right to get basic necessities of life such as food, clothing, water and a pure and healthy environment.
Reason (R) : Community life should be free from various modes of pollution
a. Both (A) and (R) are true
b. Both (A) and (R) false
c. (A) is true (R) is false
d. (A) is false (R) is true
Answer:
a. Both (A) and (R) are true
IV. Short Answer Questions
Question 1.
What do you mean by Rights to quality of life?
Answer:
- Quality of life refers to the perceived well-being of people in groups and individually and well being of the environment in which these people live.
- Consumerism has been defined as an improved quality of life.
![]()
Question 2.
How the consumer got the basic needs, as per the right of the consumer?
Answer:
Every consumer has a right to get basic necessities of life such as food, clothing, and water, and the right to pine and a healthy environment. It is the latest addition to the consumer bill of rights.
Question 3.
Define “consumer rights”.
Answer:
- The Right to have information about the Quality, Quantity, potency, purity, price and standard of goods or services.
- It is mandatory for the consumer to know these rights.
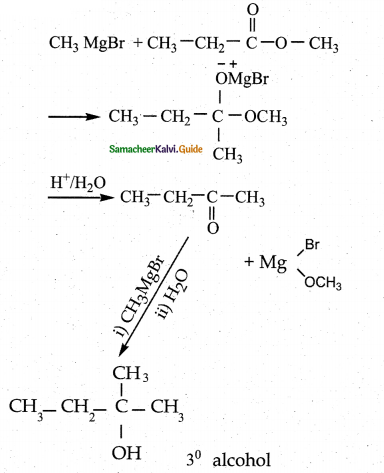
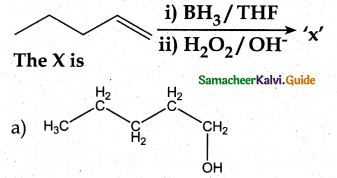
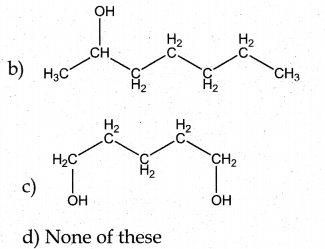

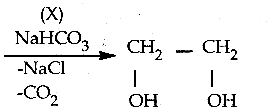
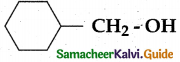
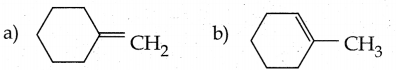
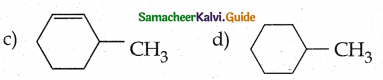
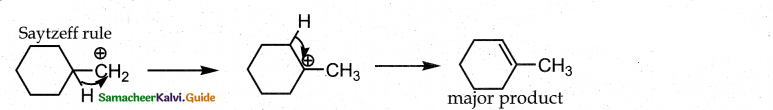
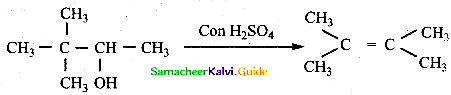
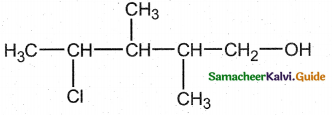
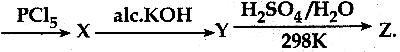
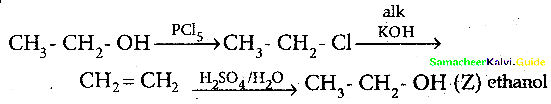
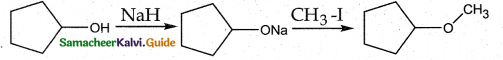
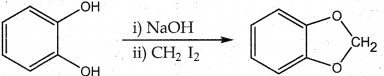
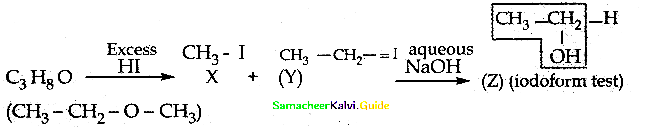
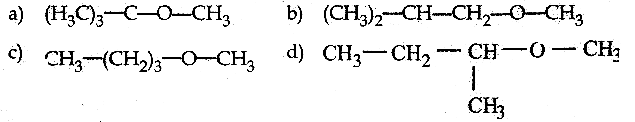
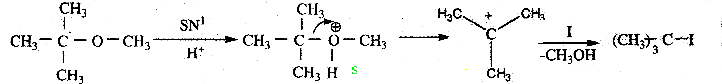
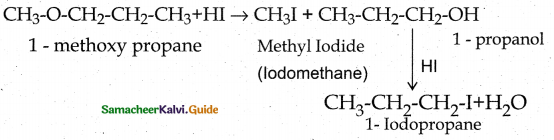
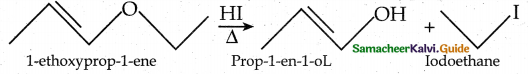
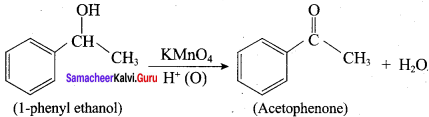
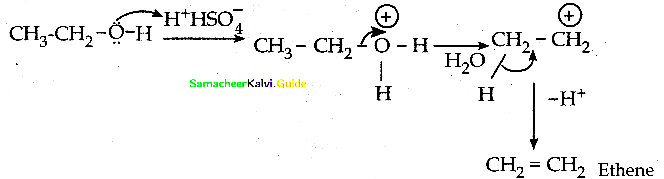
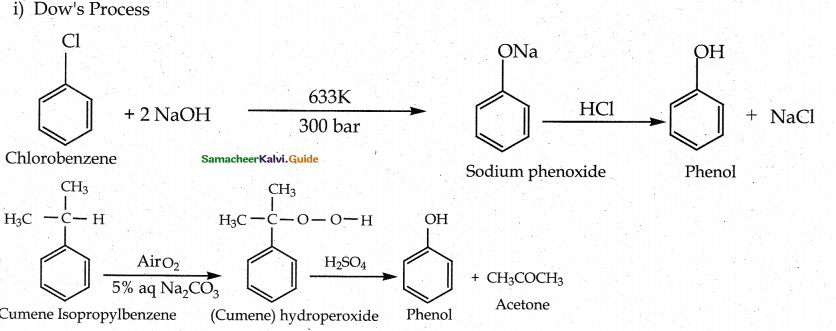
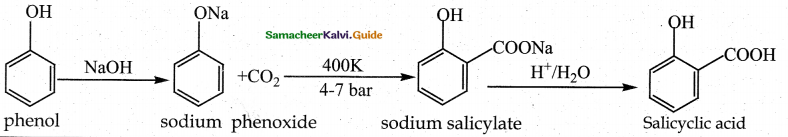
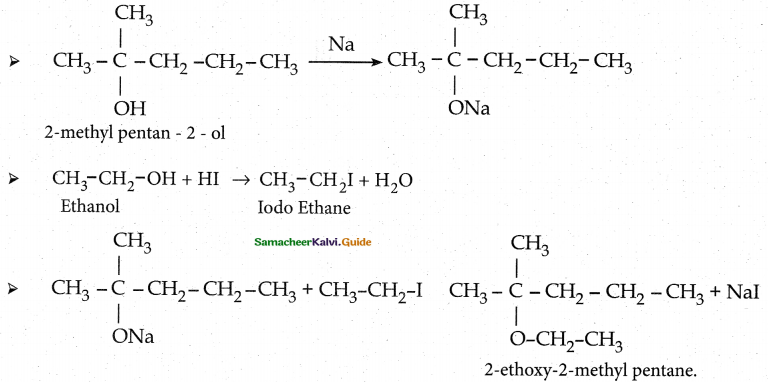
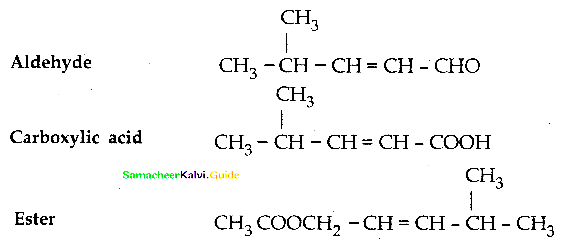
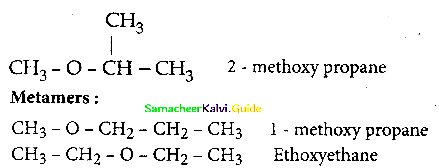
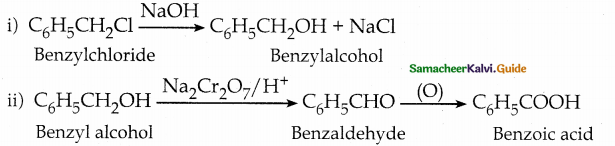
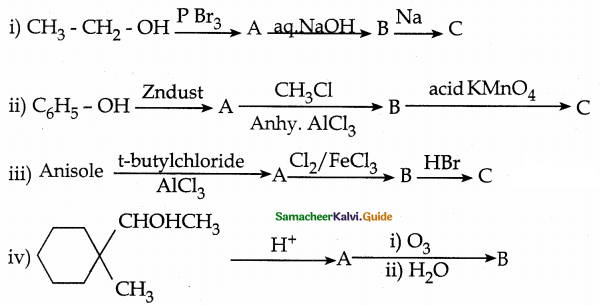
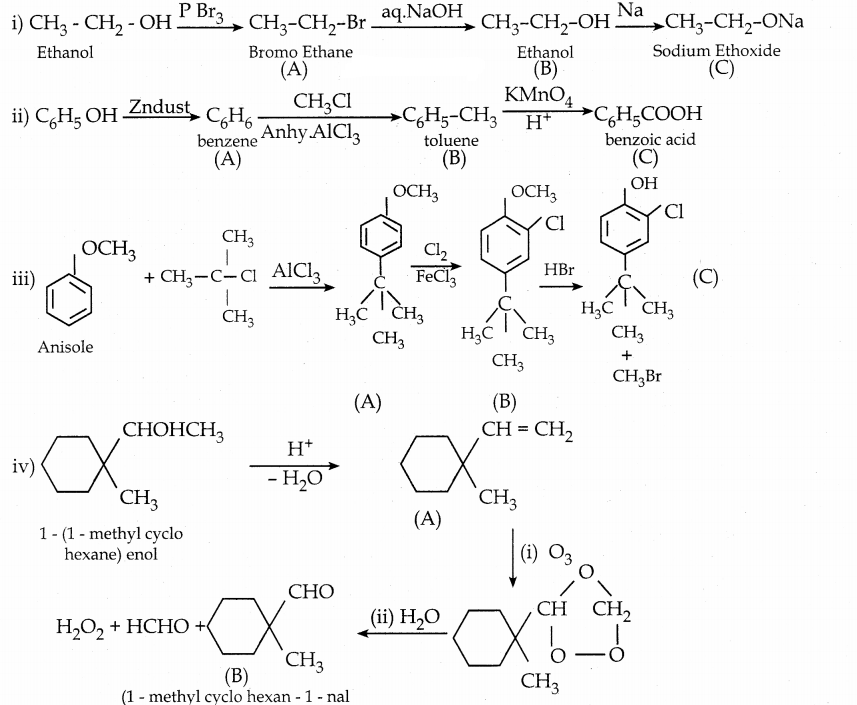
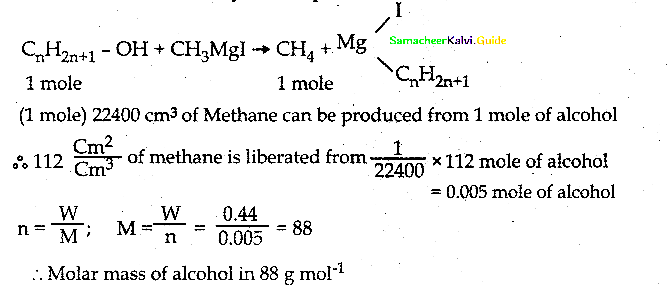
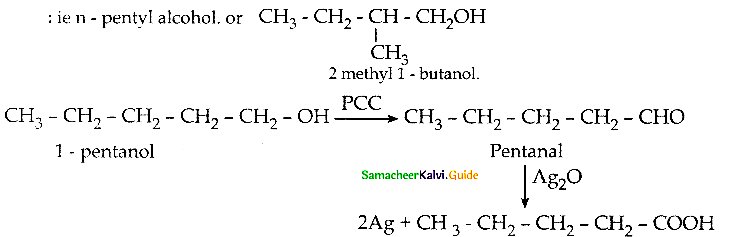
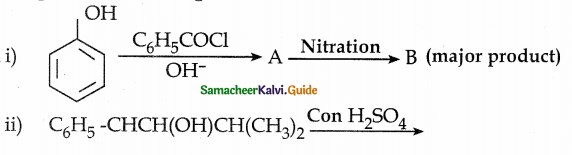
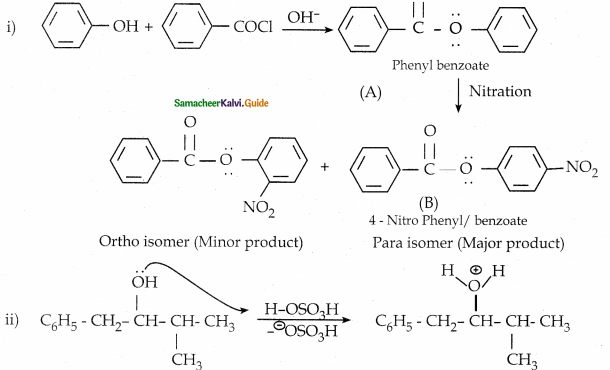
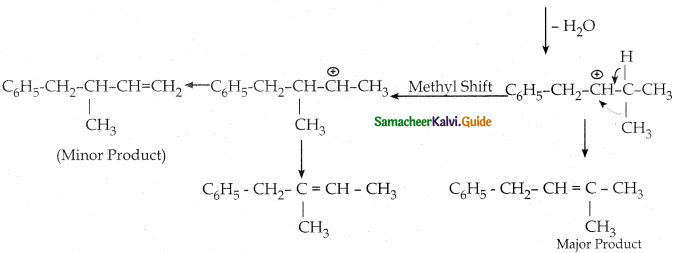
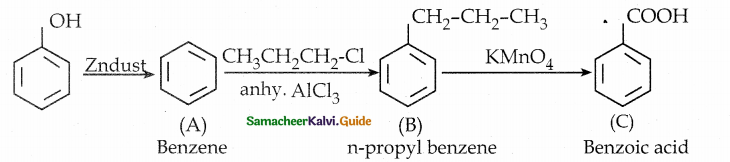
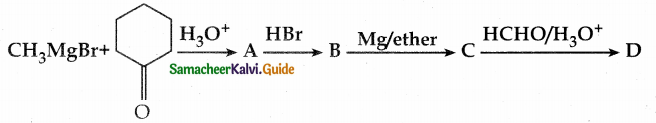
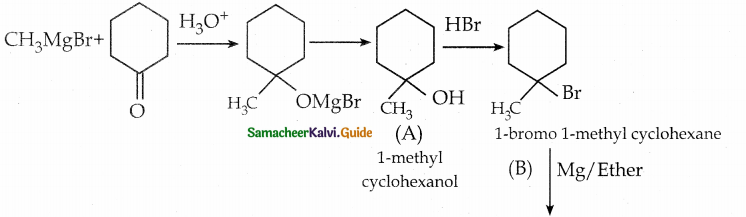
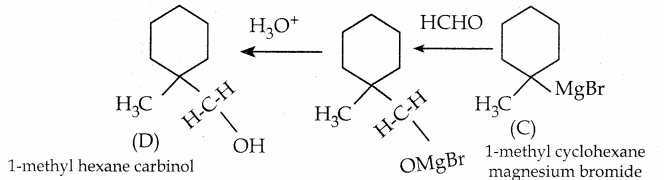
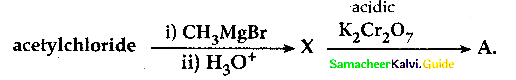
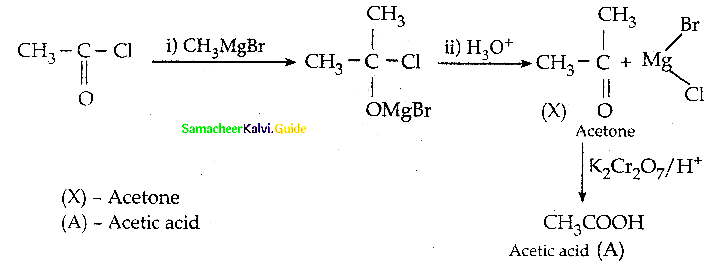
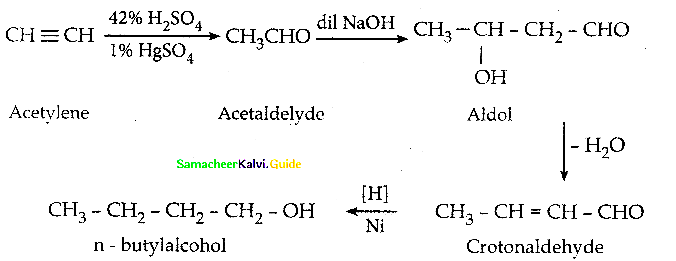
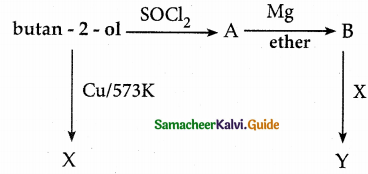
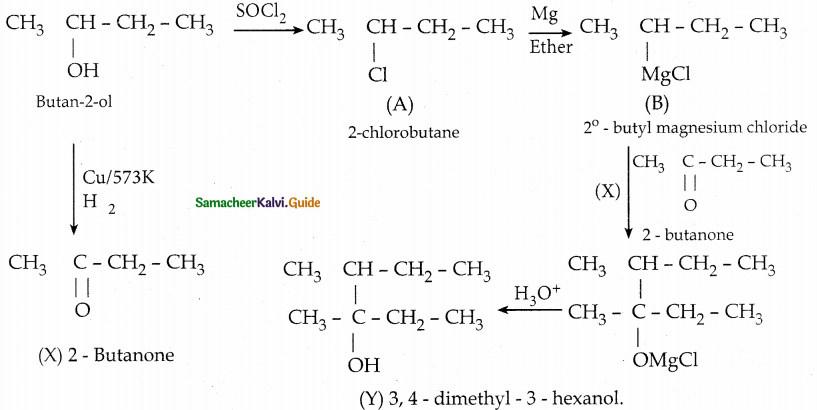
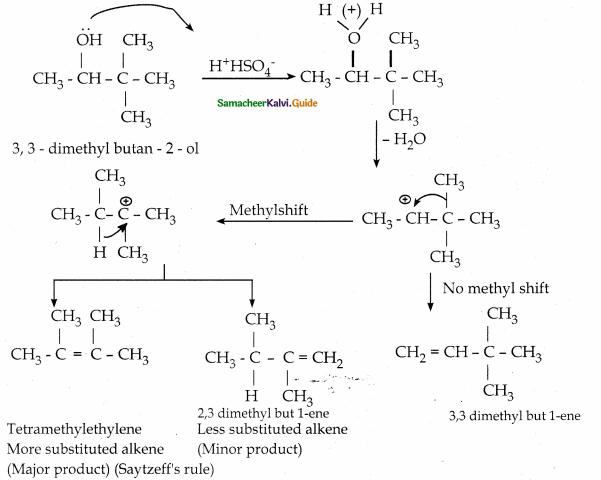
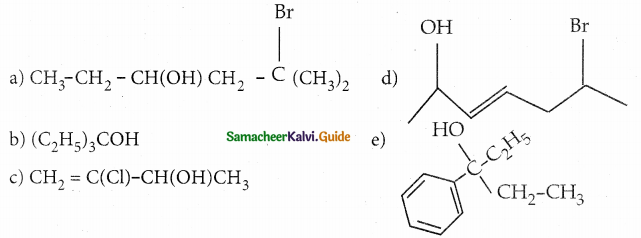
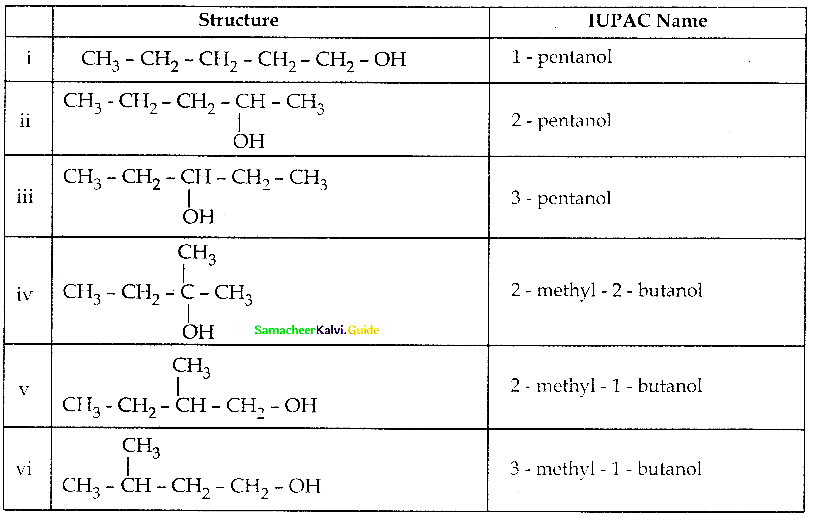
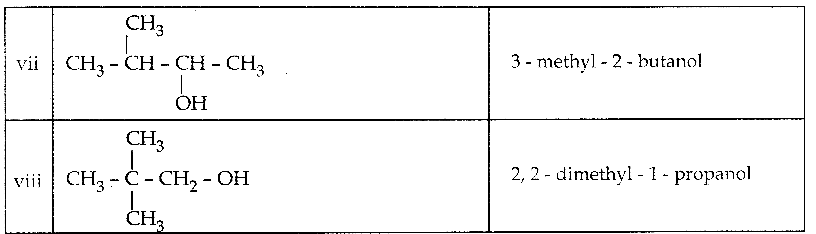

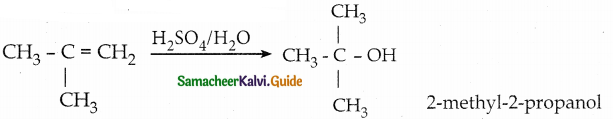
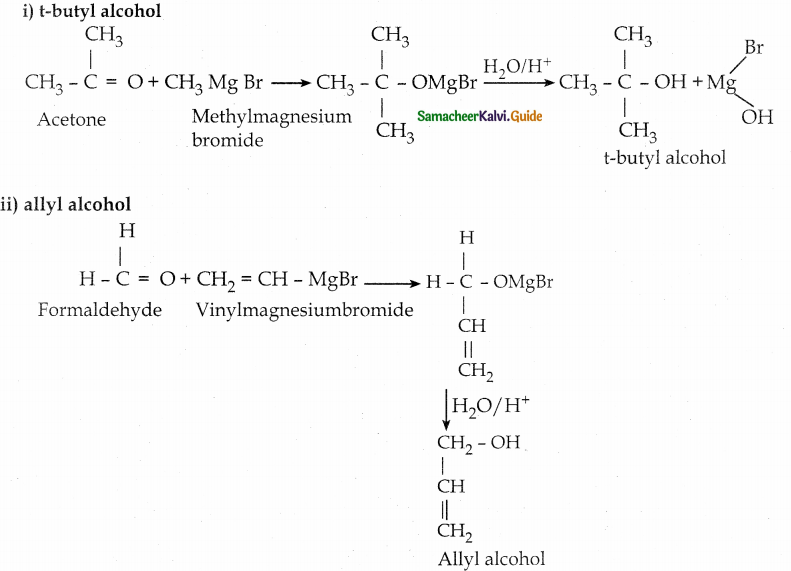

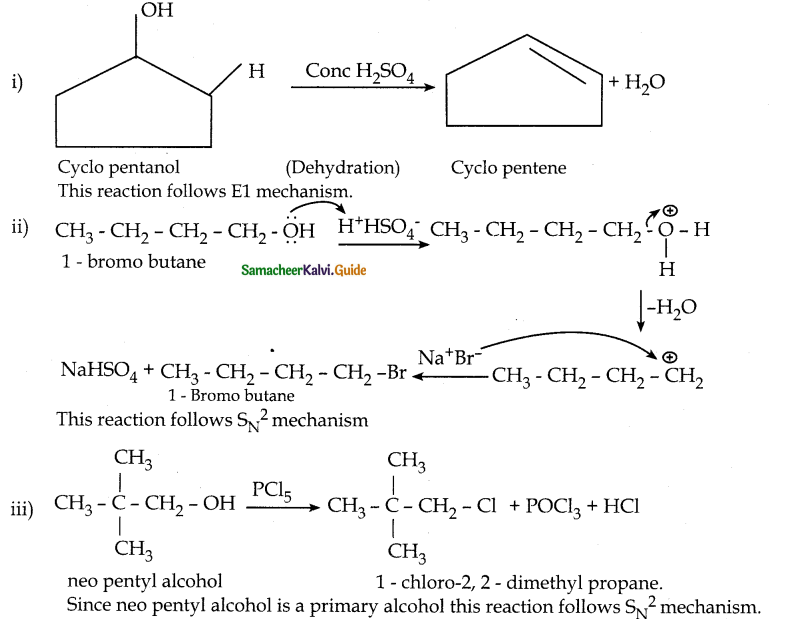
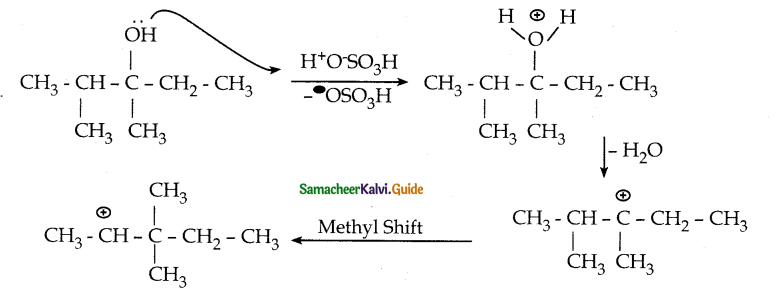
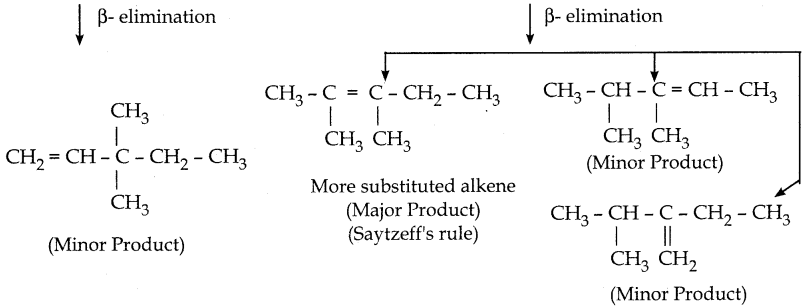
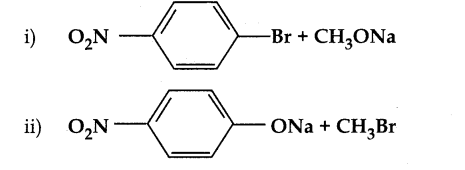
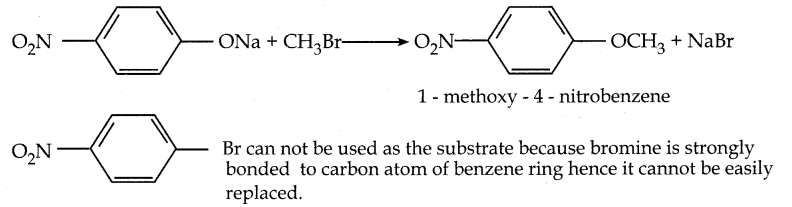
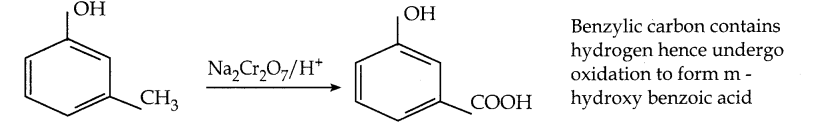
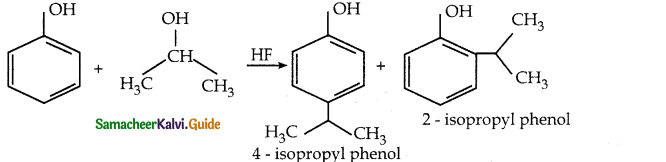
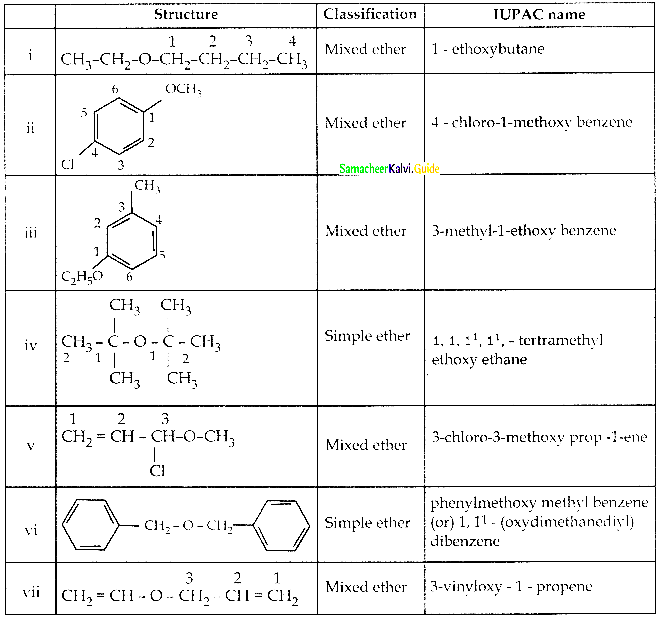
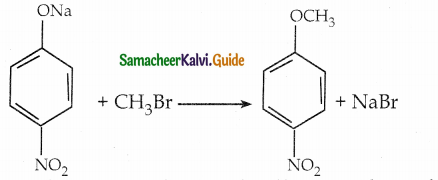
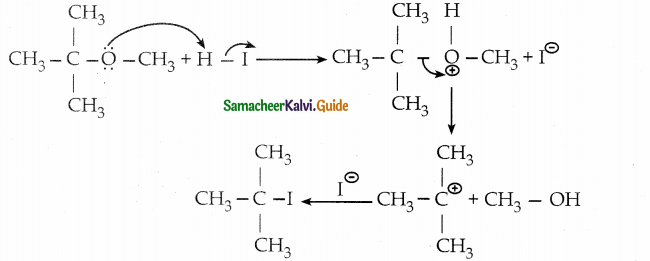
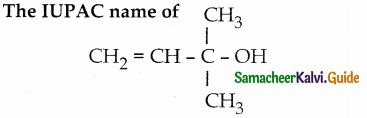

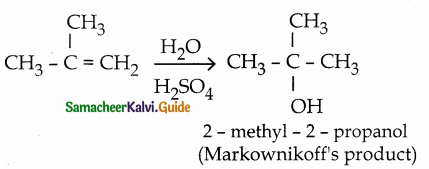
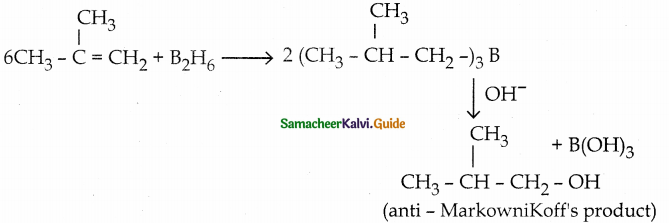

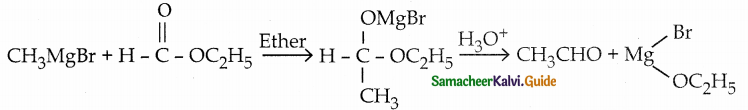
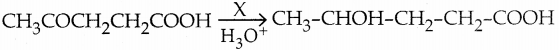
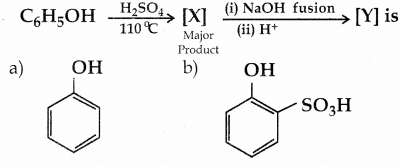
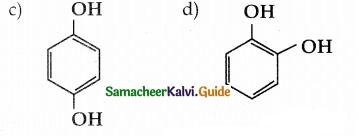
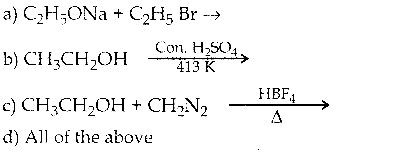
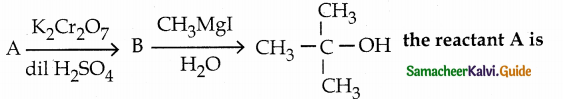
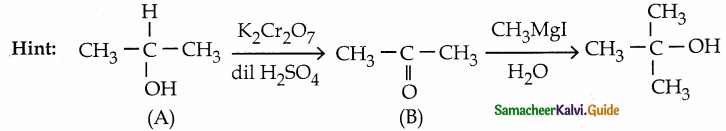
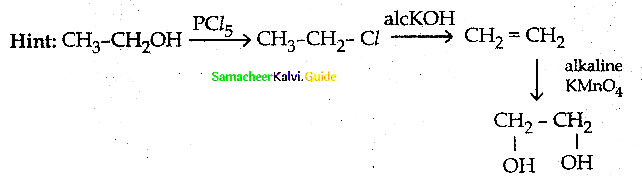
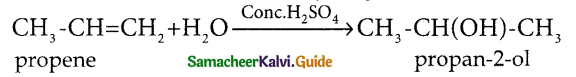
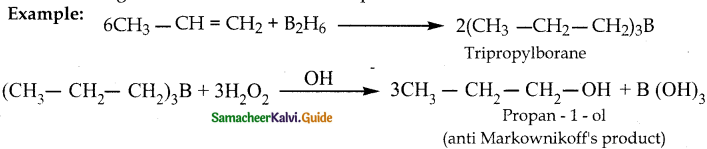
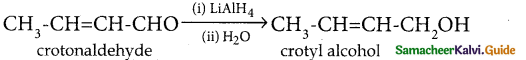
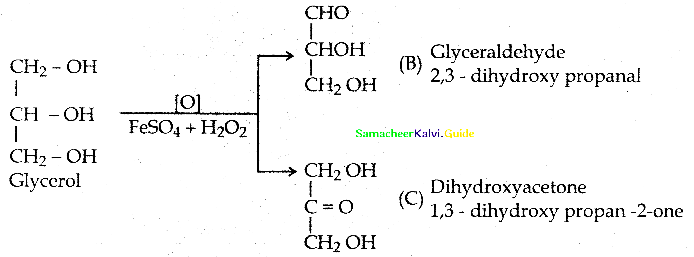
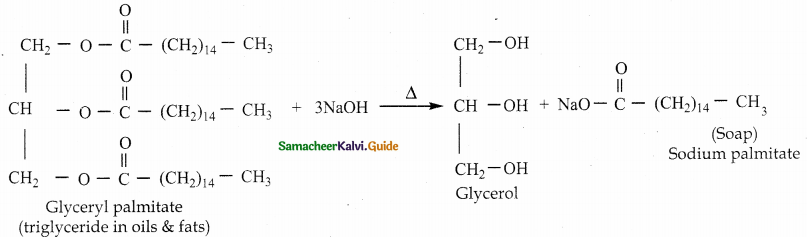

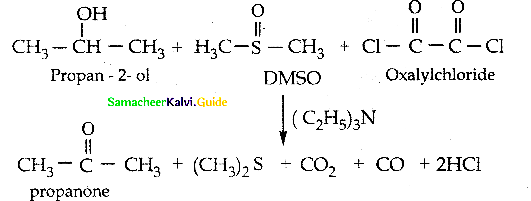
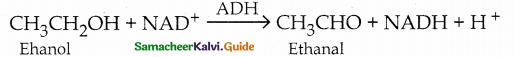
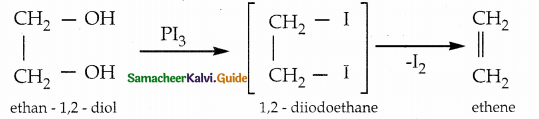
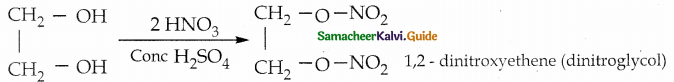
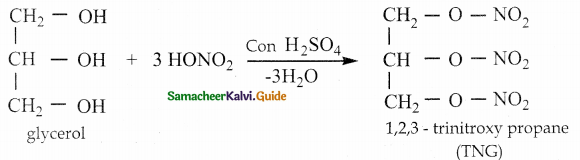
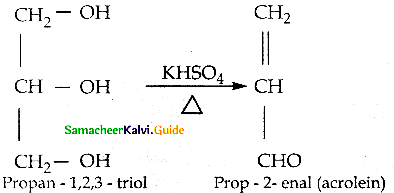
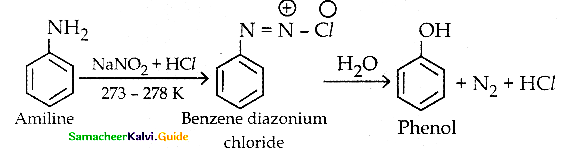
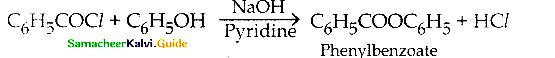
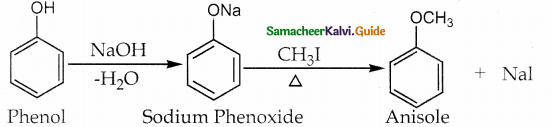
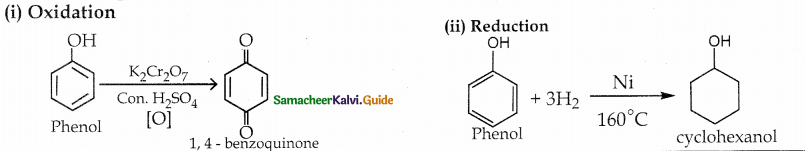
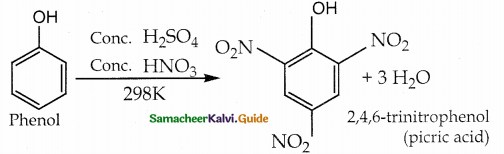
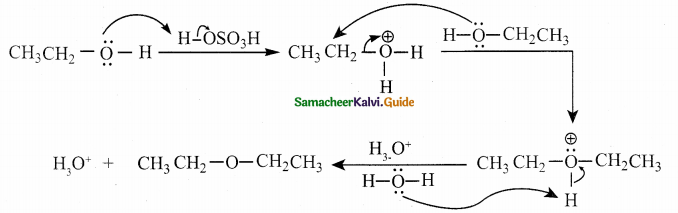
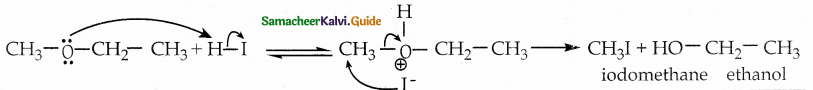
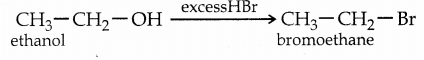
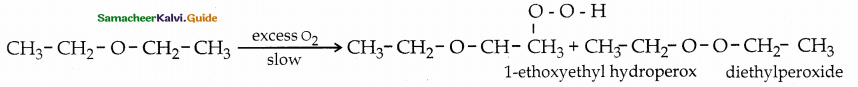
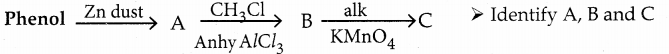
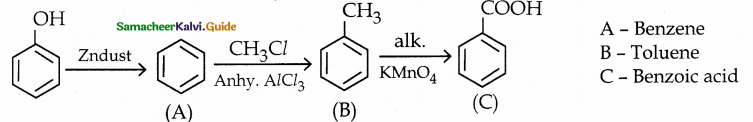
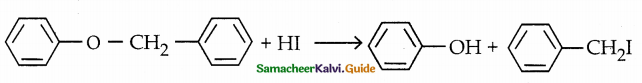
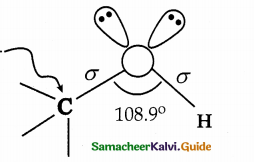
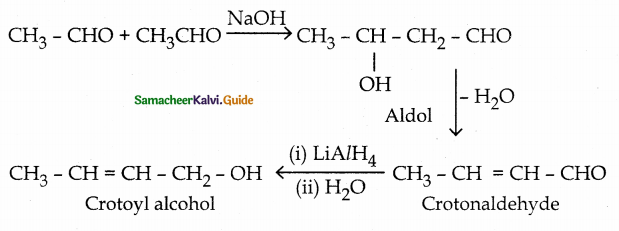
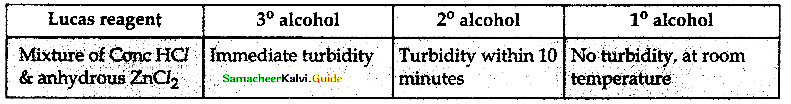
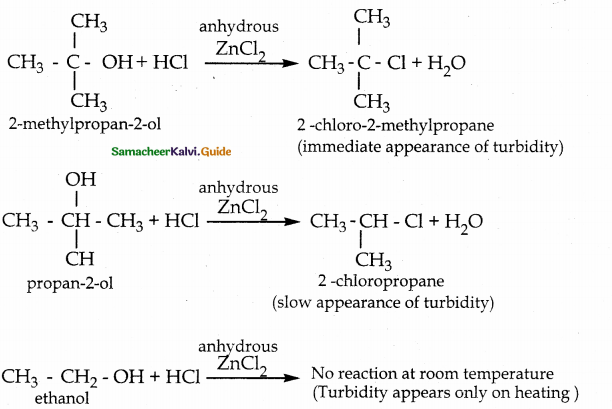
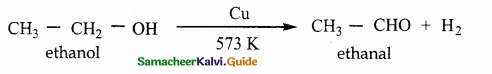
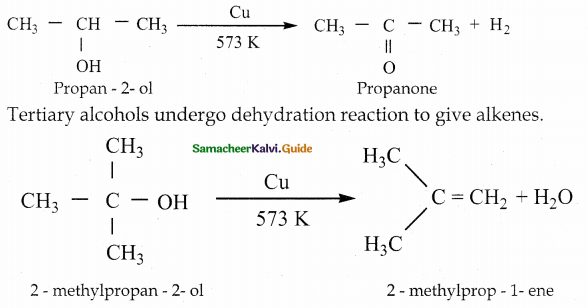
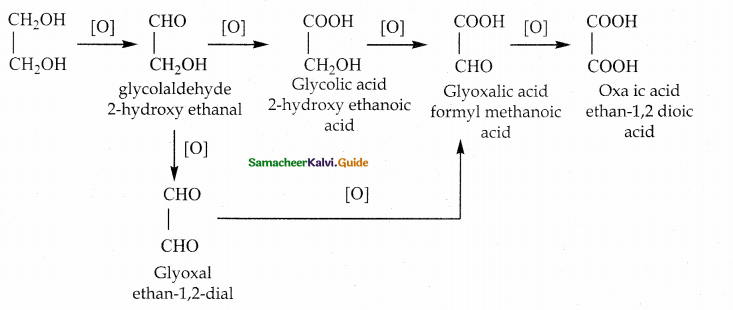
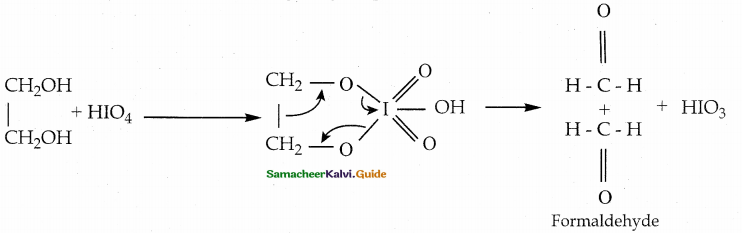
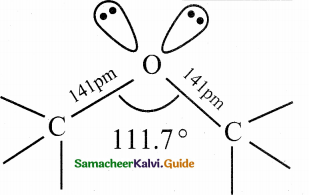
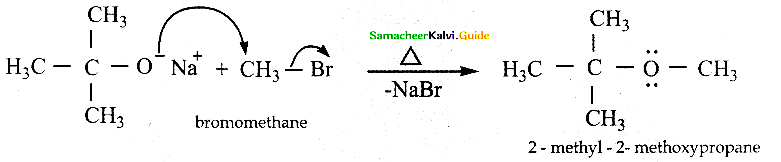

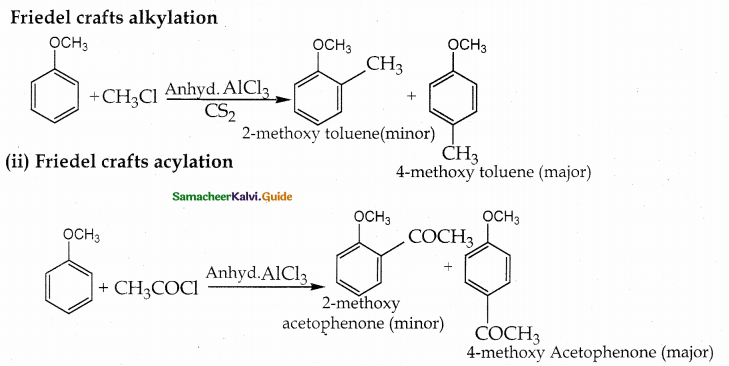
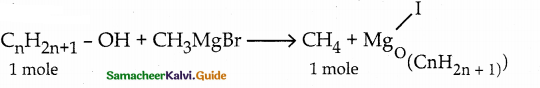
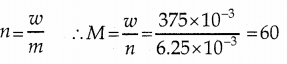
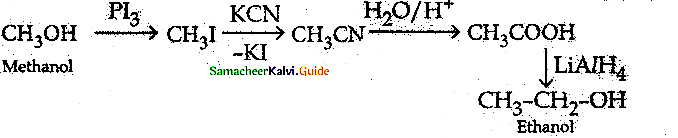
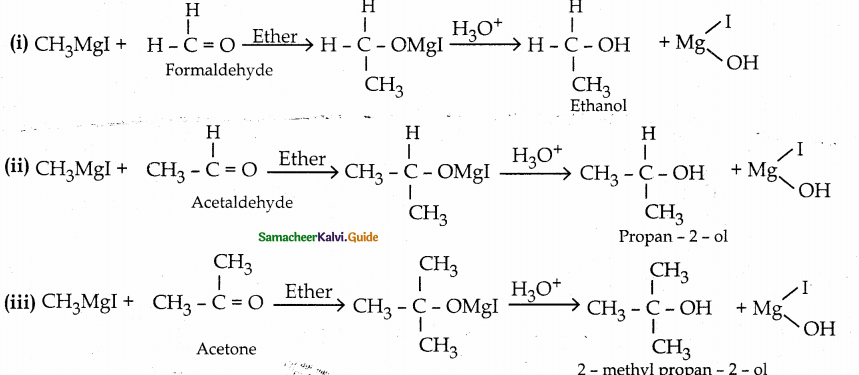
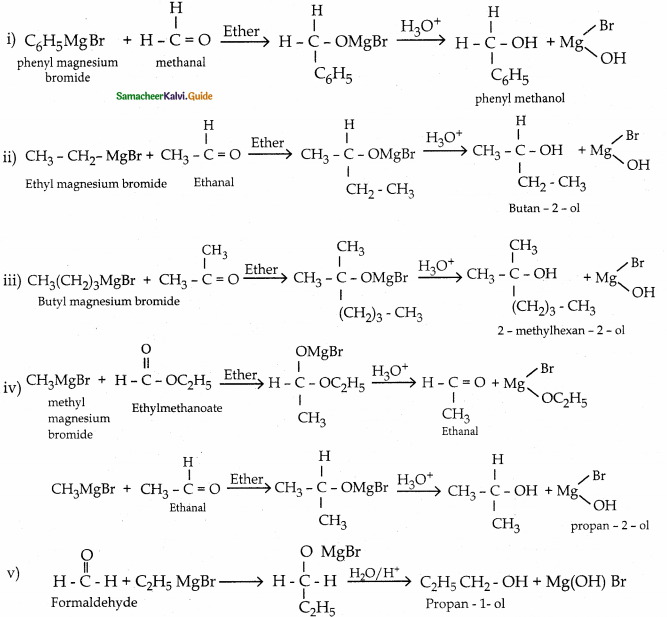
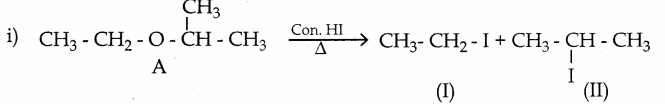
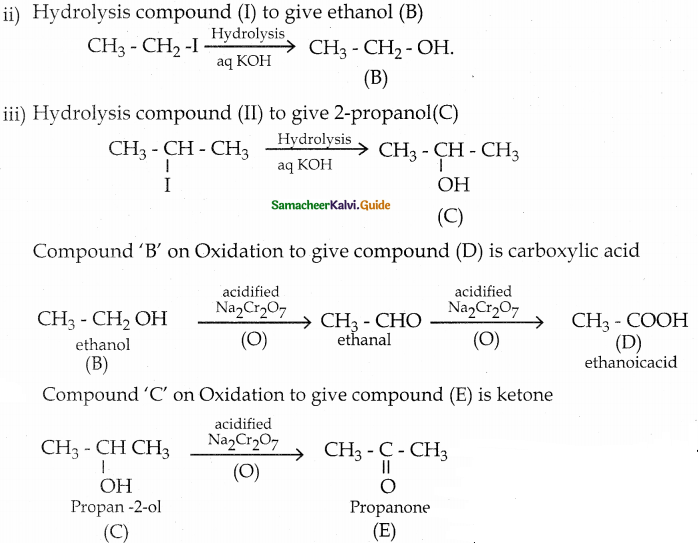
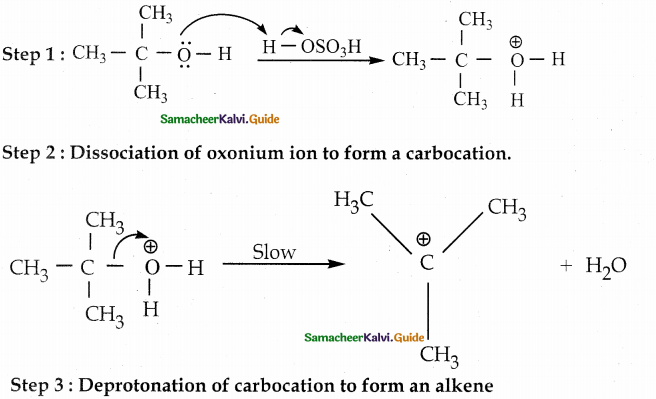
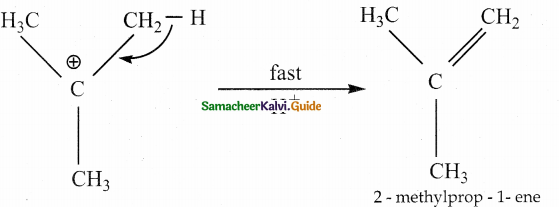

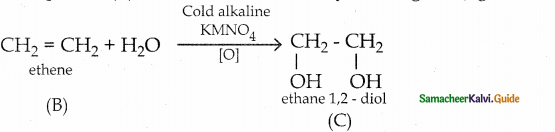
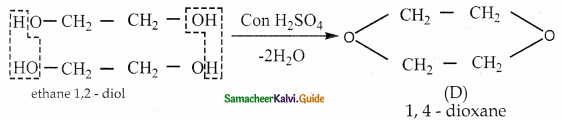
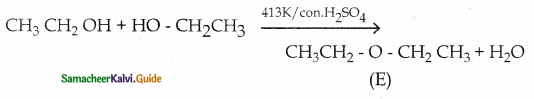
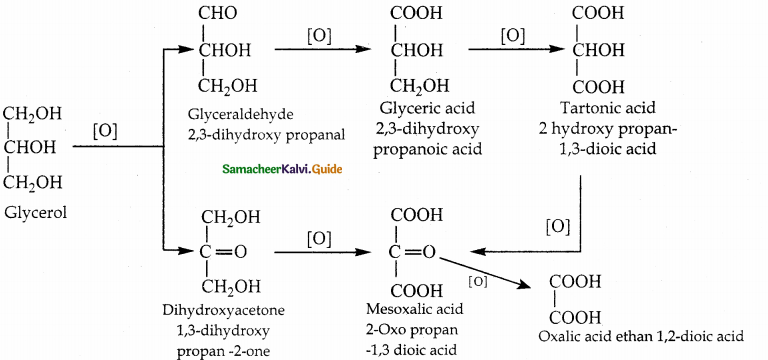
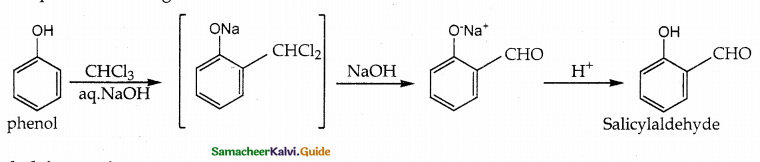
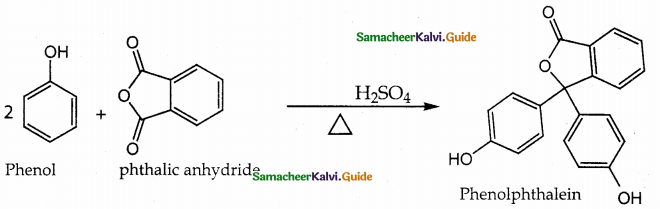
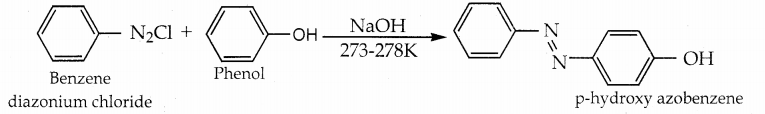
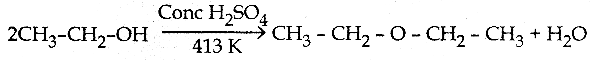
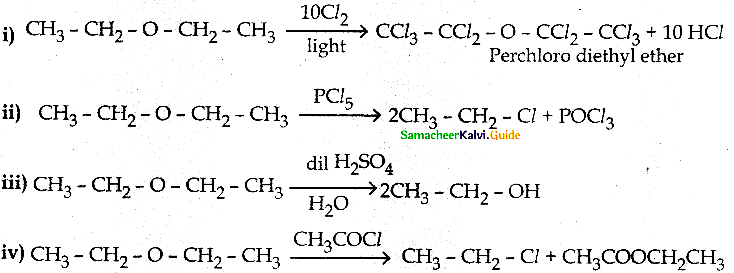
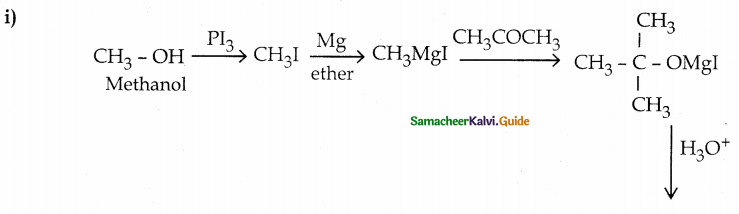
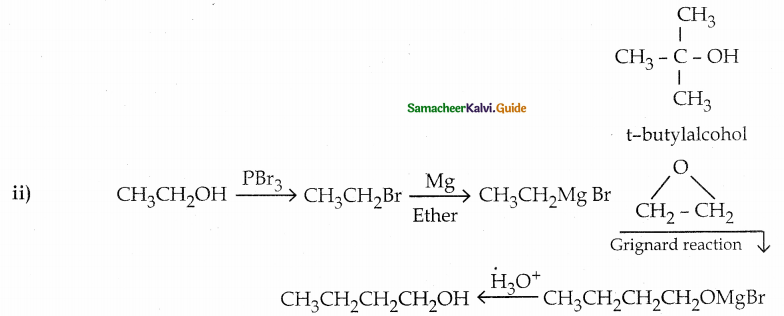
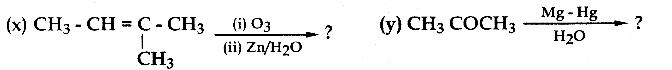
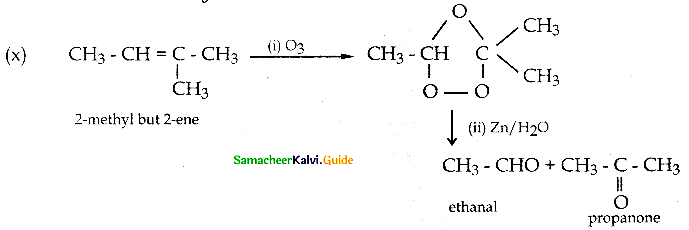
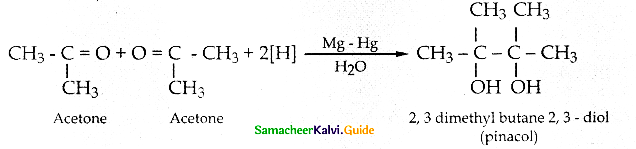





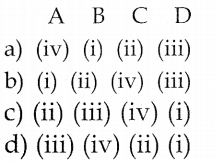

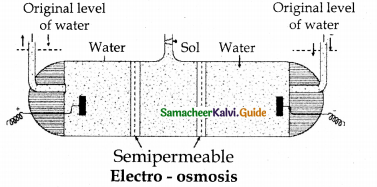
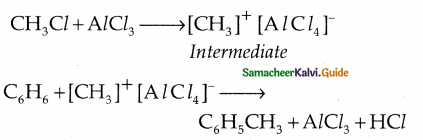
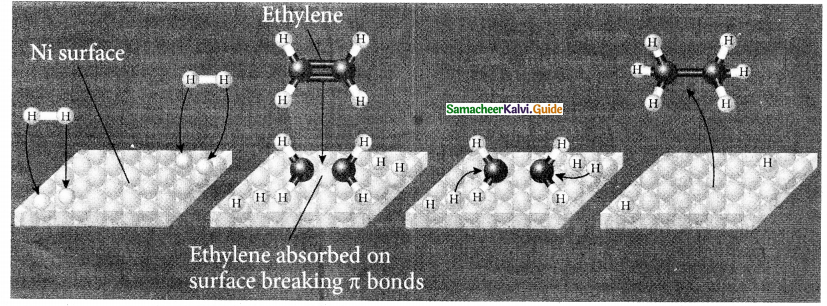
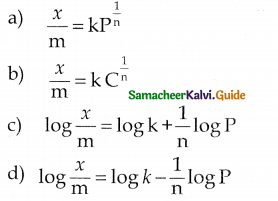

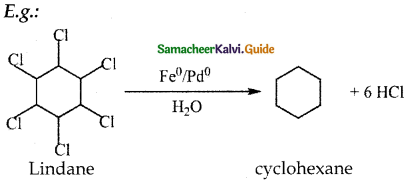
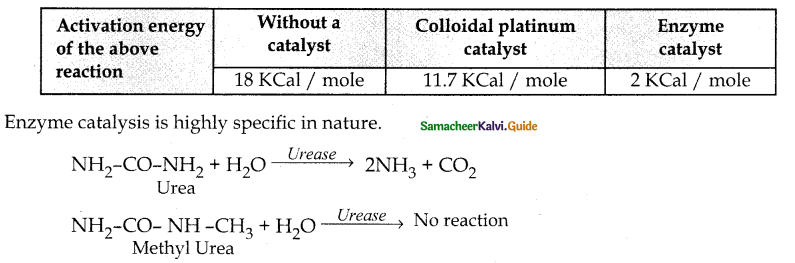
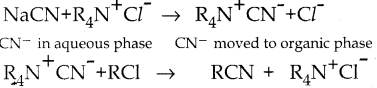
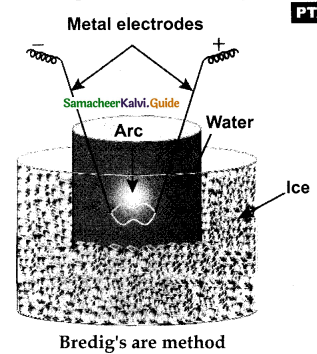
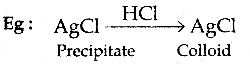
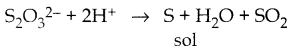
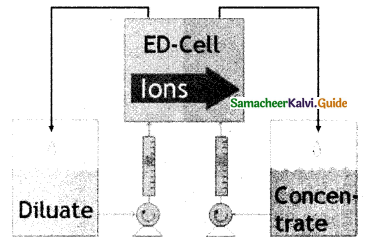
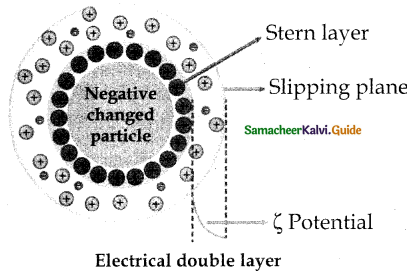
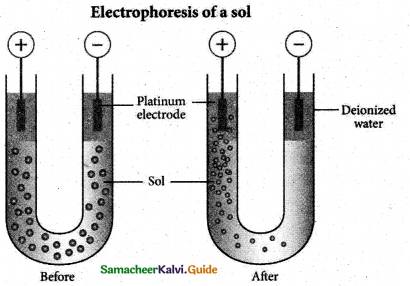
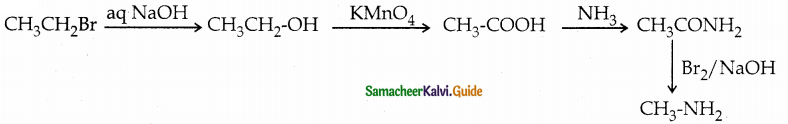
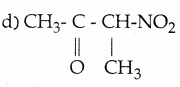

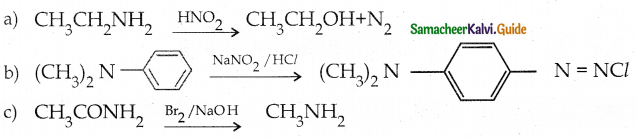
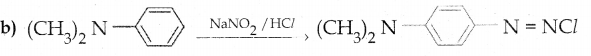
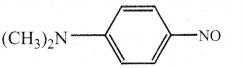
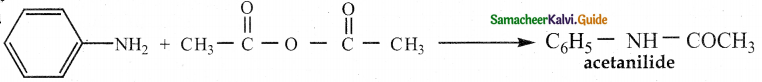

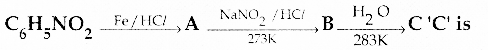
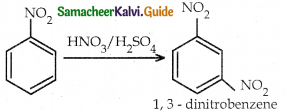
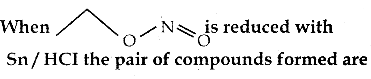
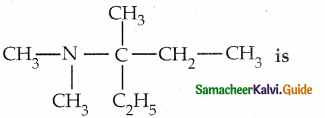
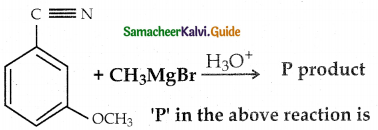
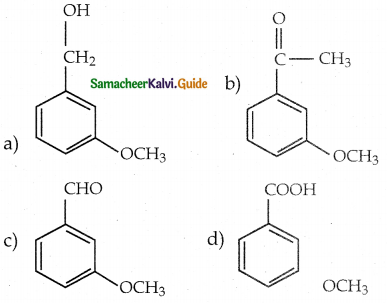
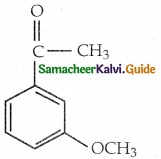
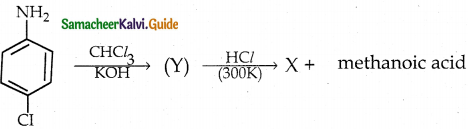
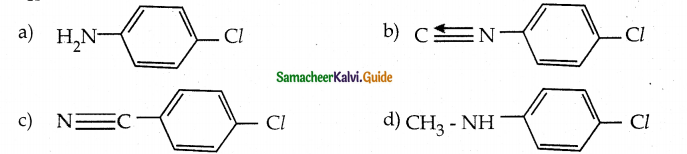
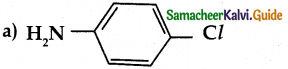
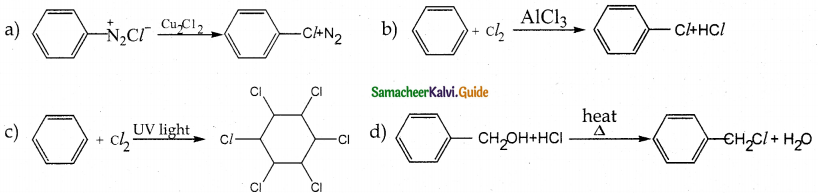
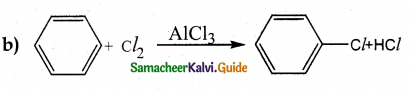
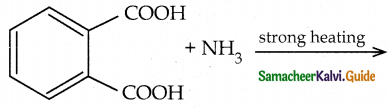
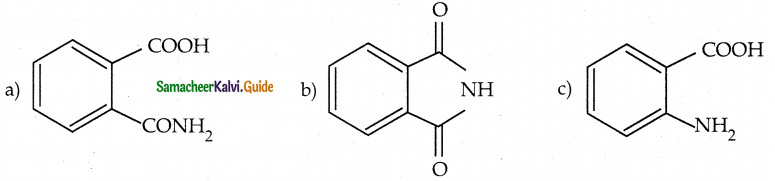
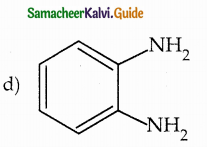
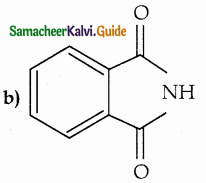
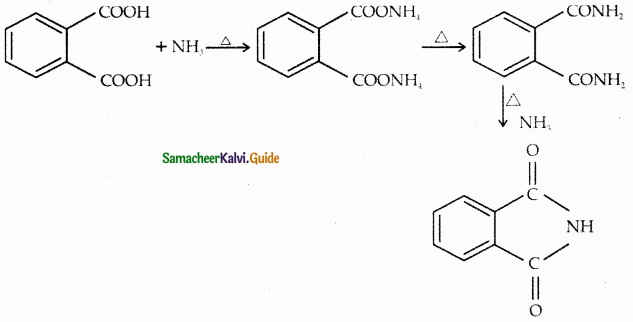
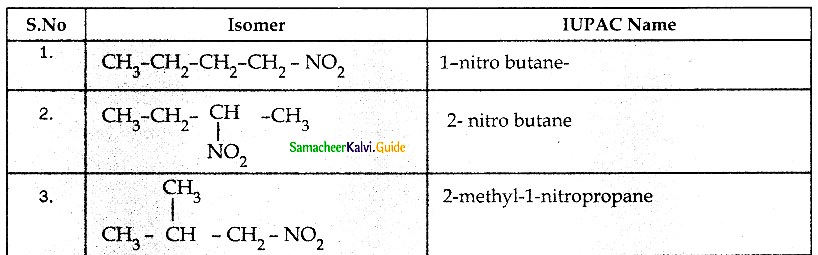
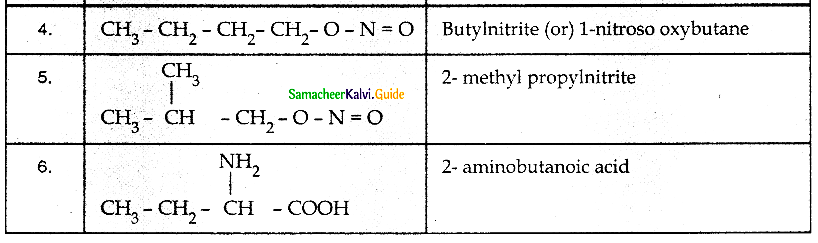
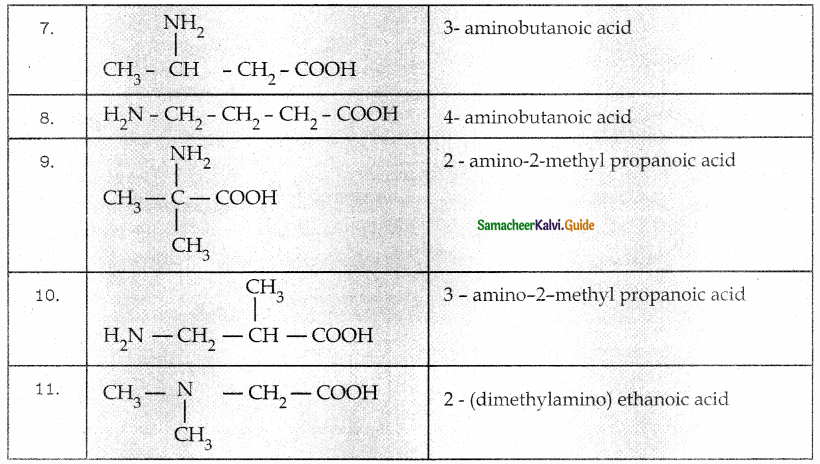
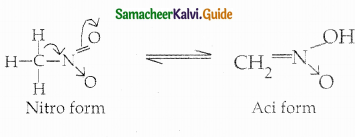
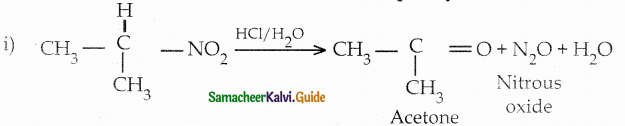
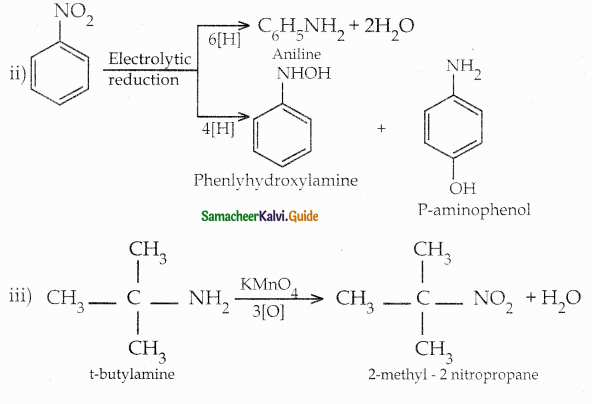

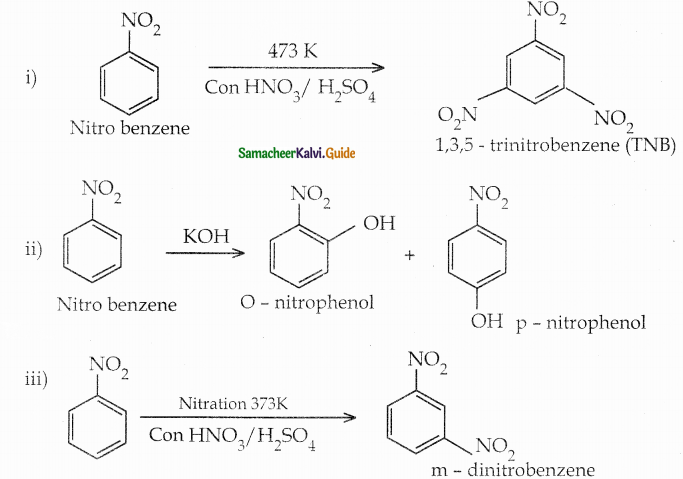
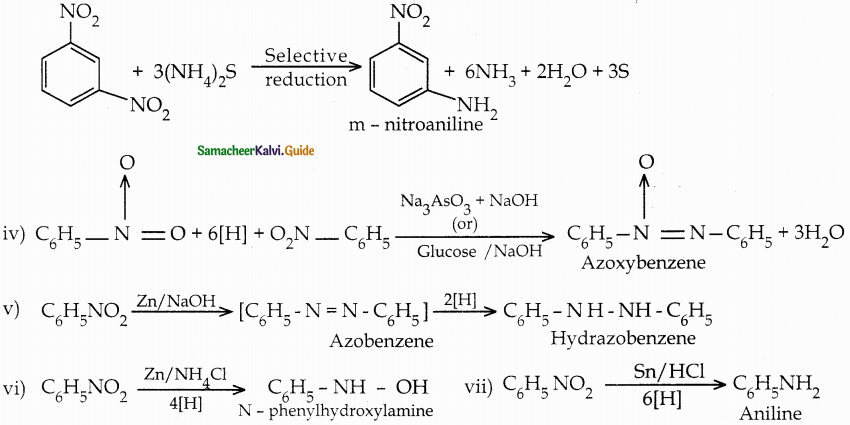
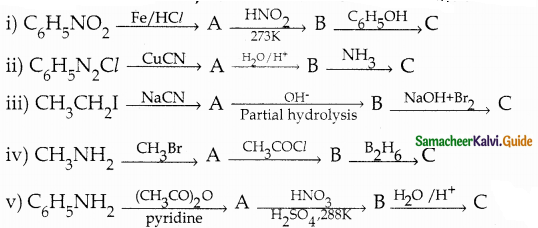
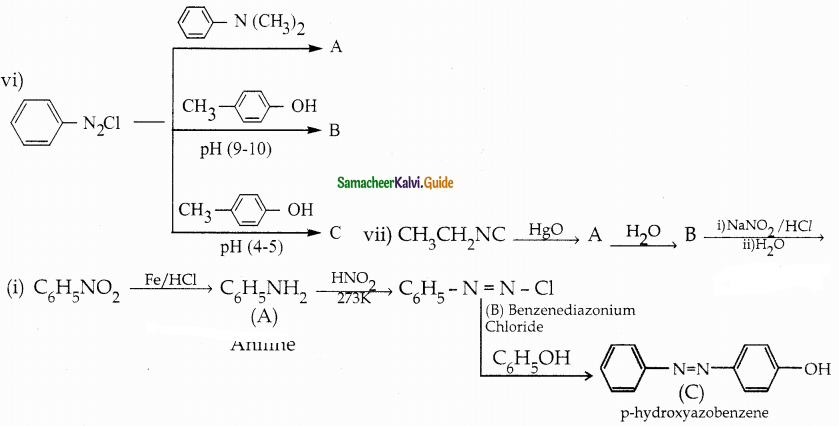
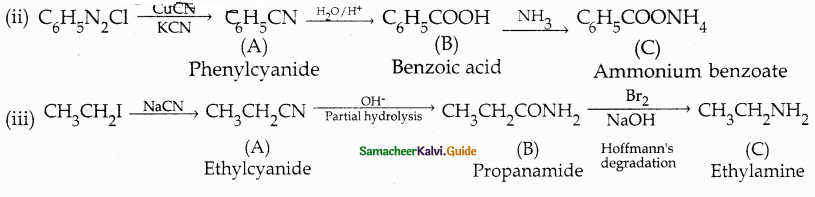
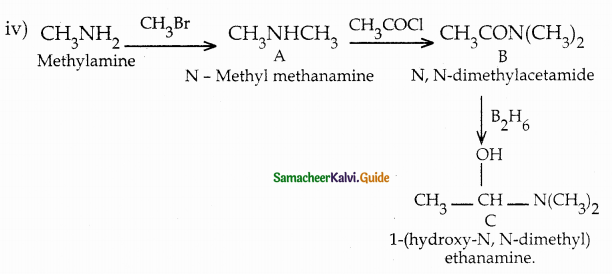
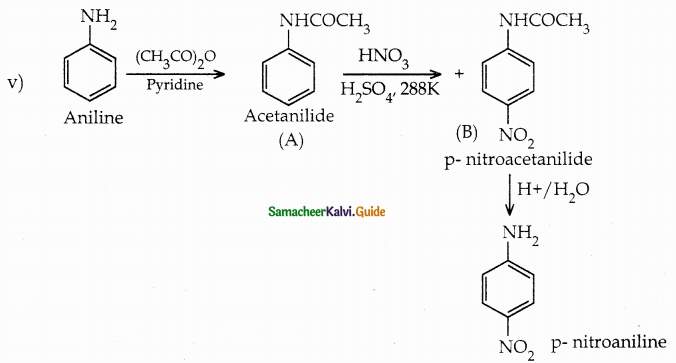
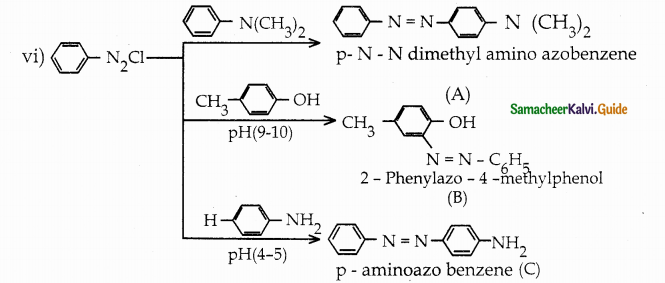
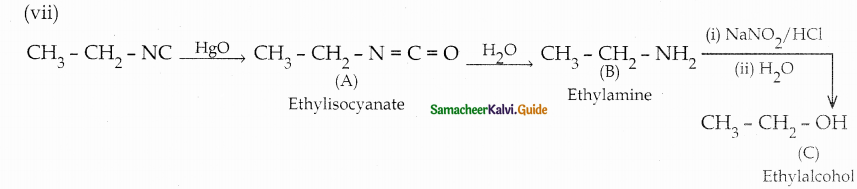

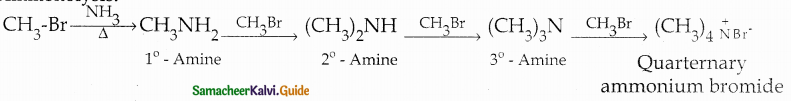
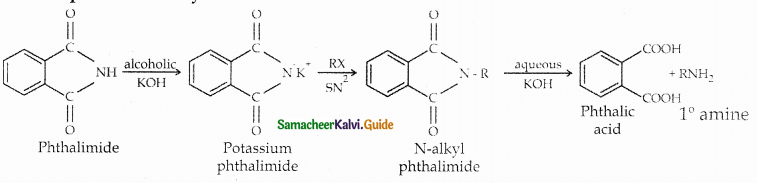
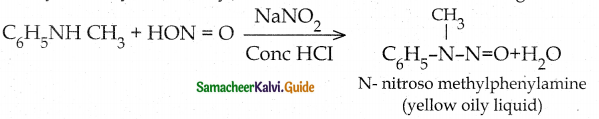
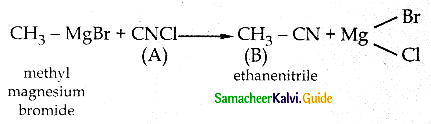
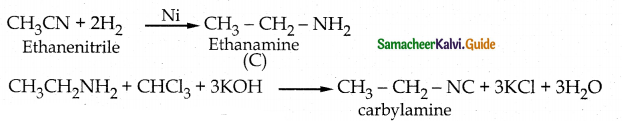
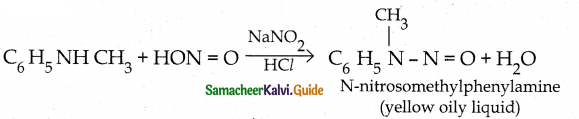
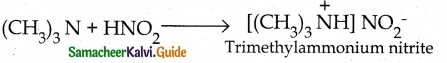
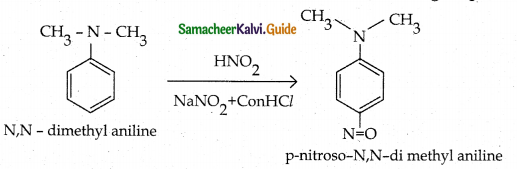
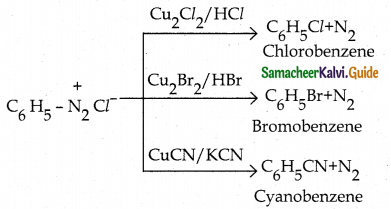
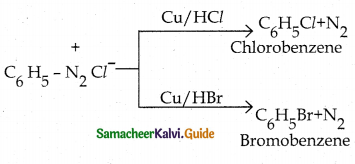
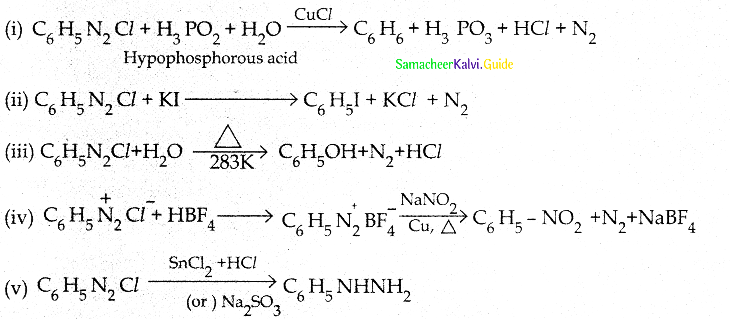
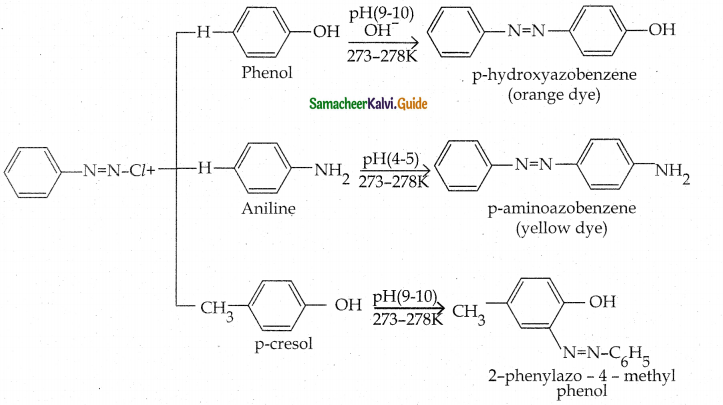
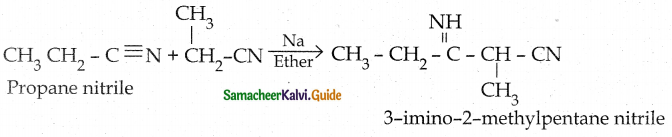
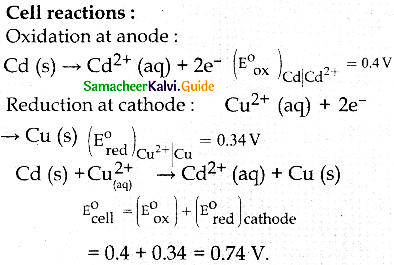
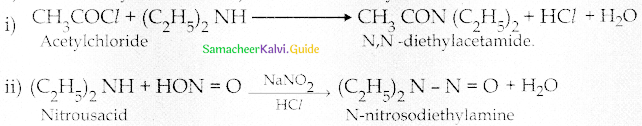
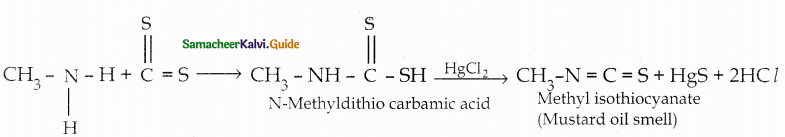 When primary amines are treated with carbon disulphide, N-alkyl dithiocarbamic acid is formed which on treatment with HgCl2 gives an alkyl isothiocyanate.
When primary amines are treated with carbon disulphide, N-alkyl dithiocarbamic acid is formed which on treatment with HgCl2 gives an alkyl isothiocyanate.

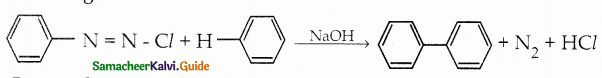
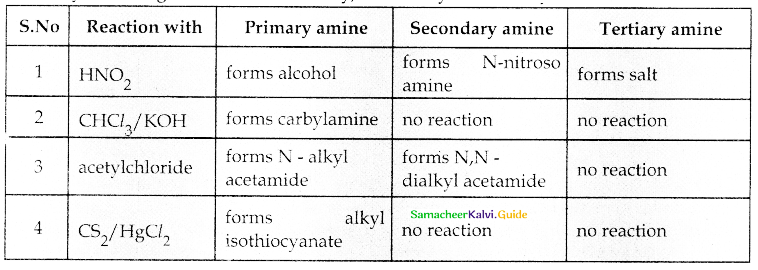
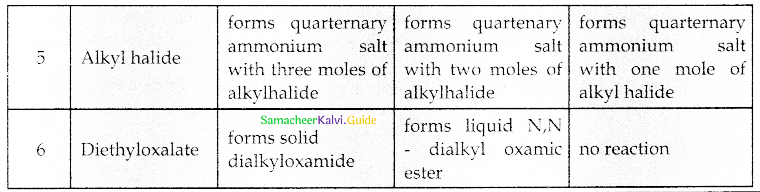
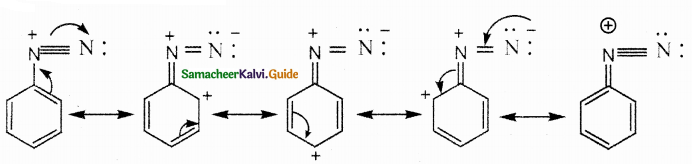
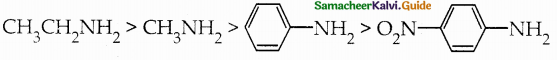
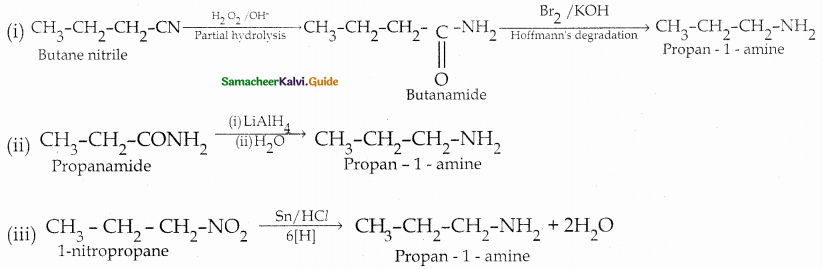
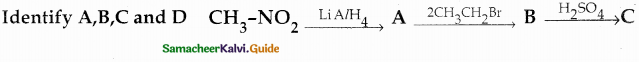
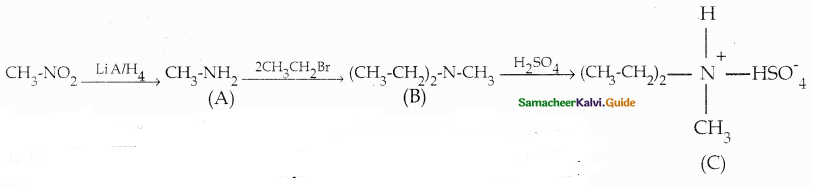
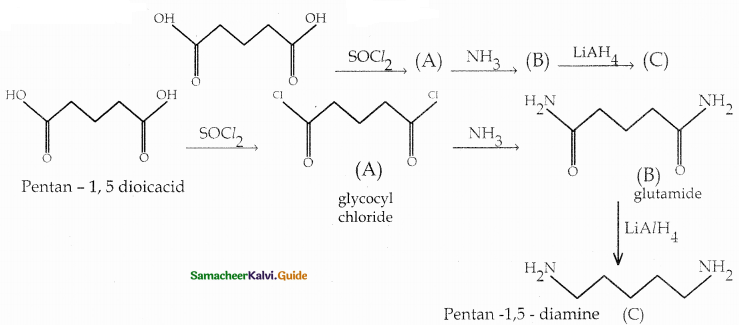
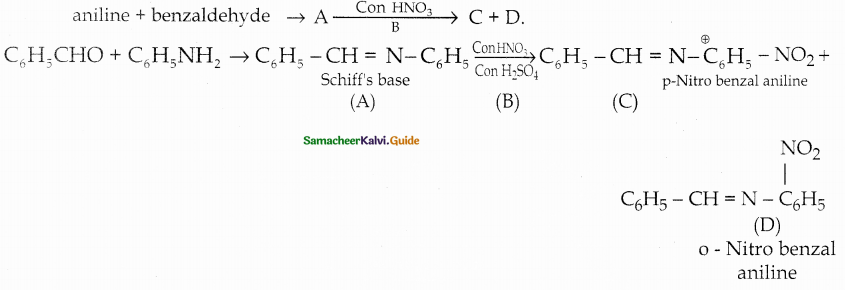
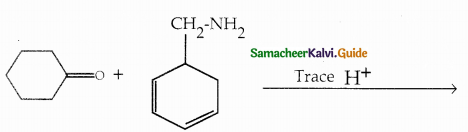
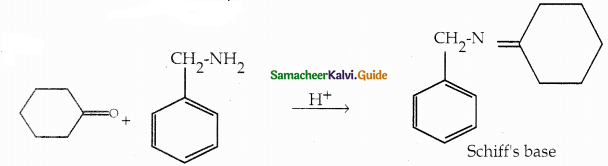
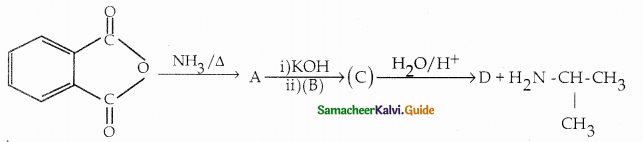
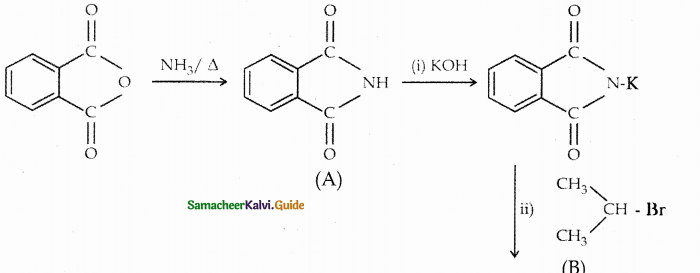
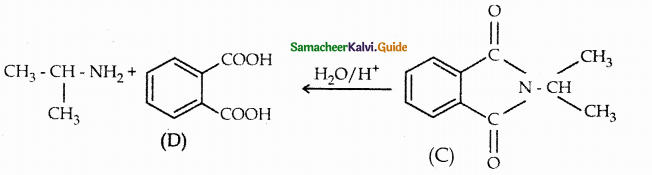
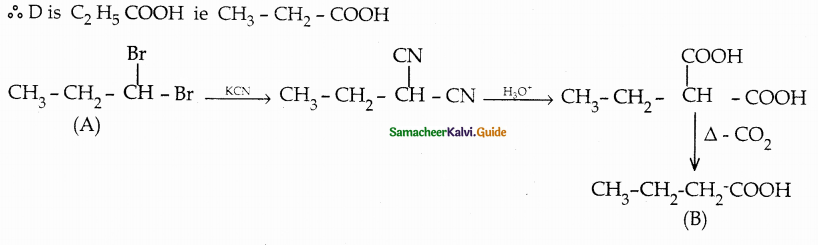
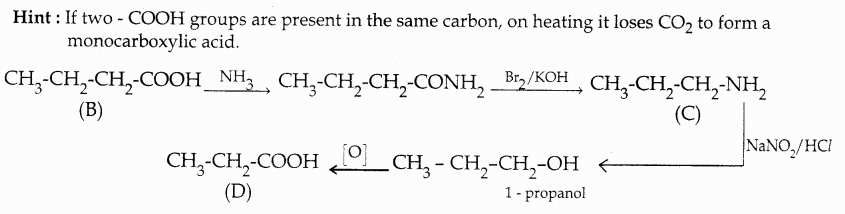
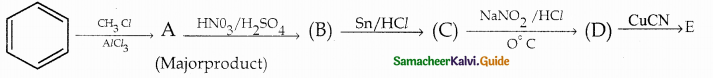
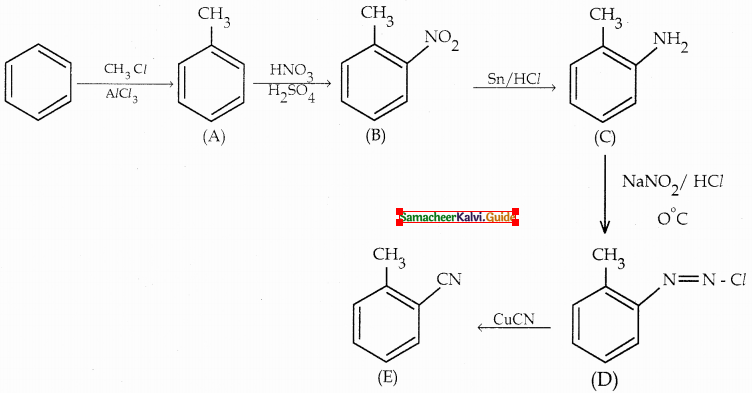
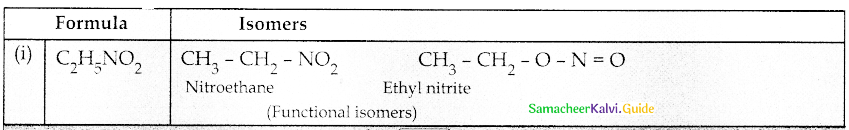
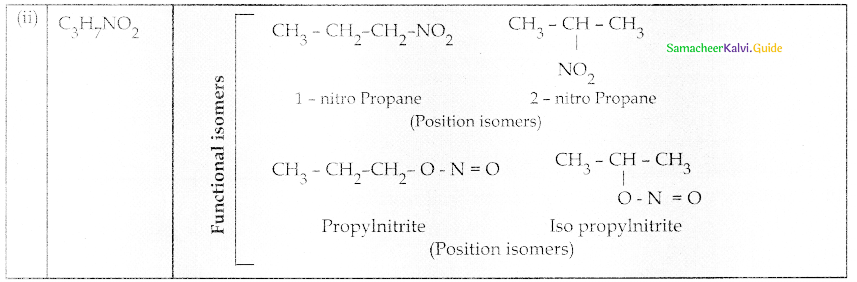
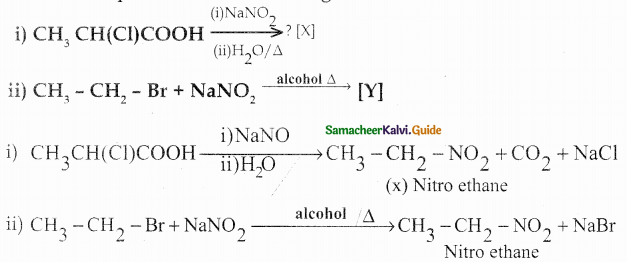
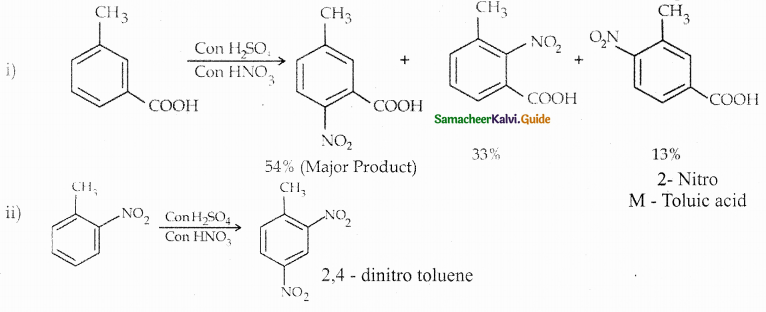
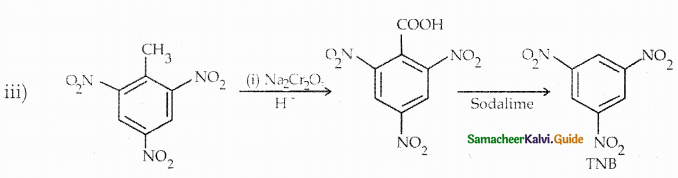
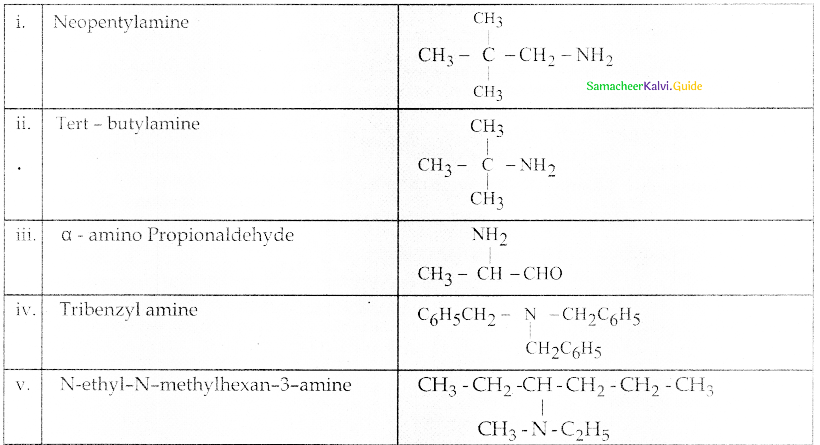
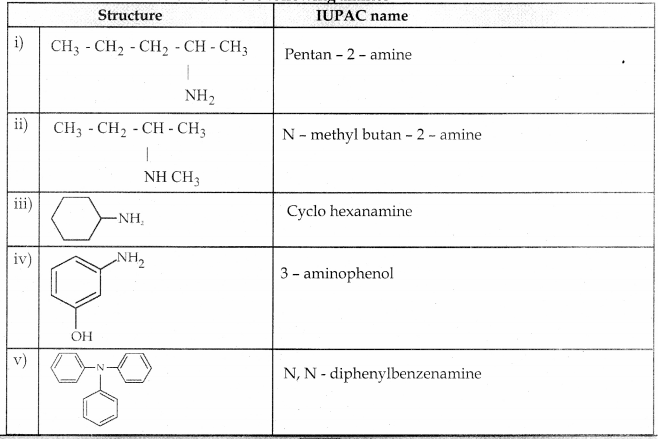

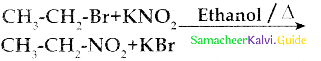
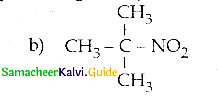
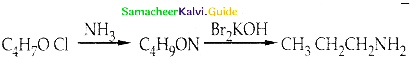
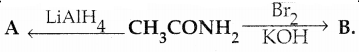
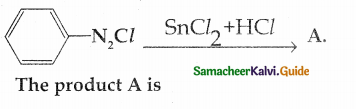
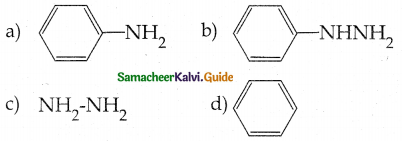
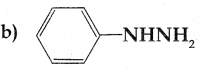
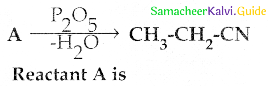
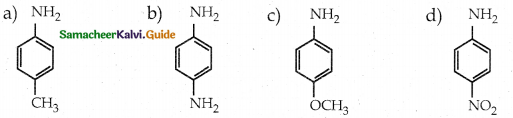
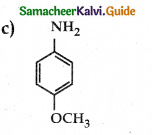

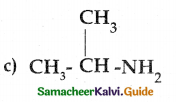
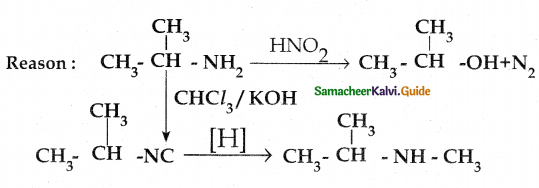

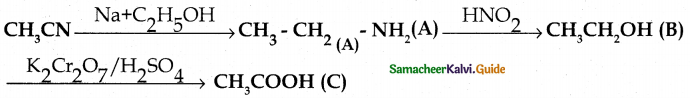
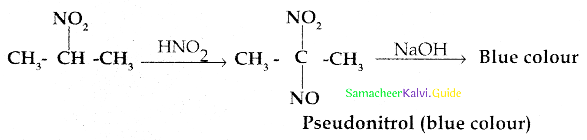
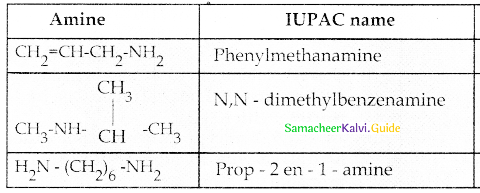
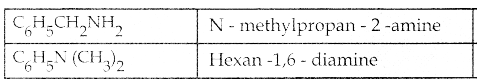
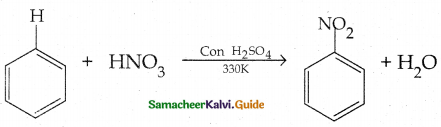
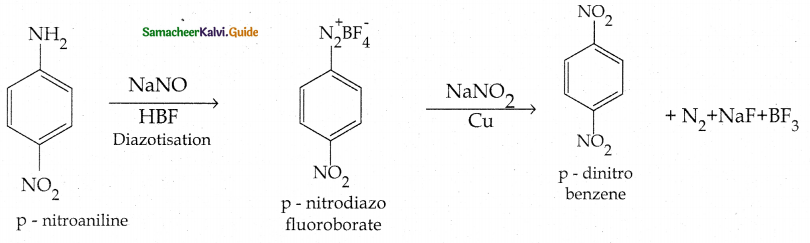
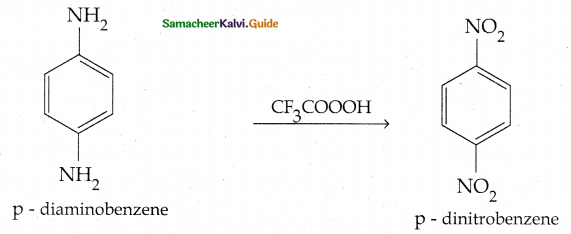
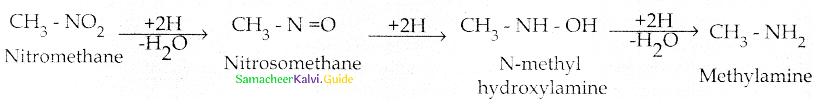
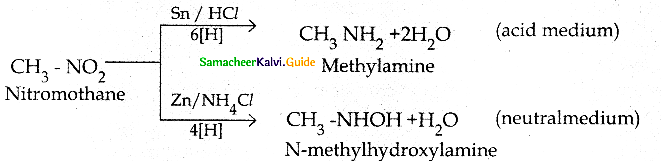
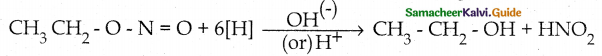
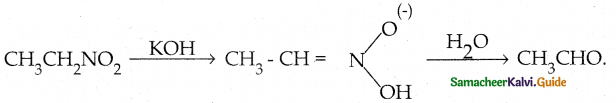
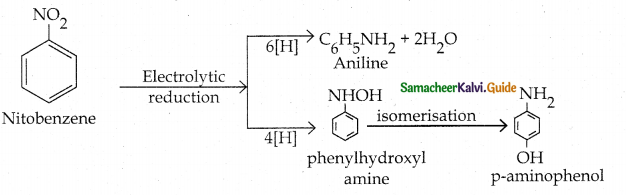
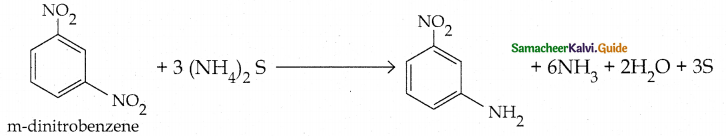
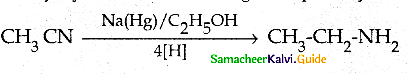
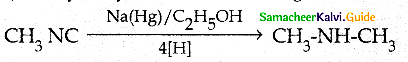
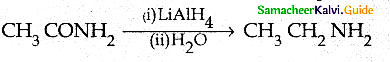
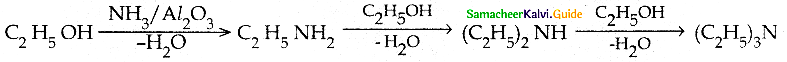
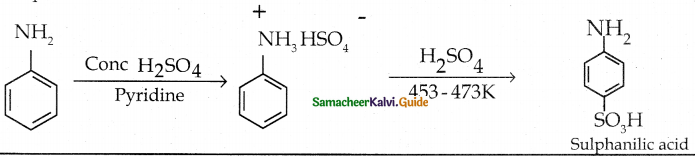
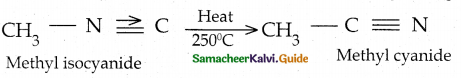
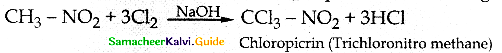
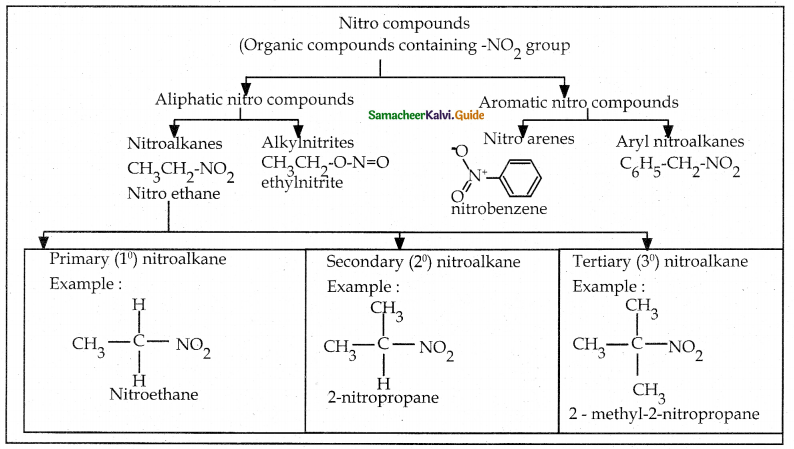
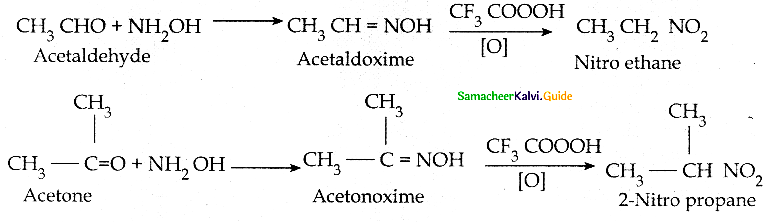
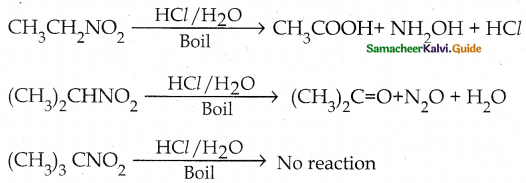
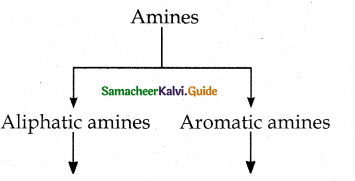
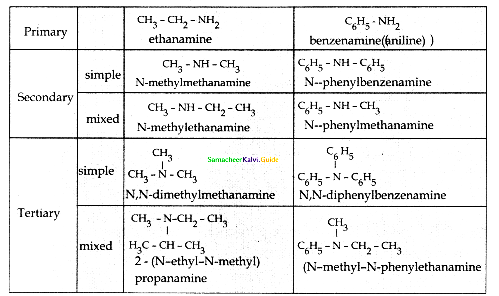
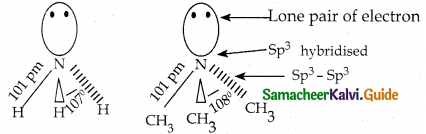
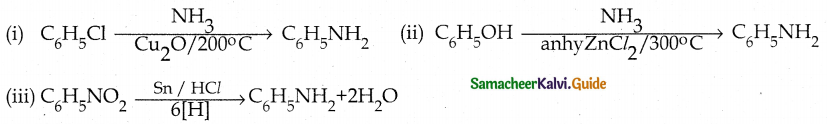
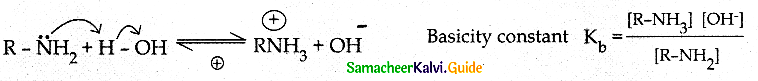
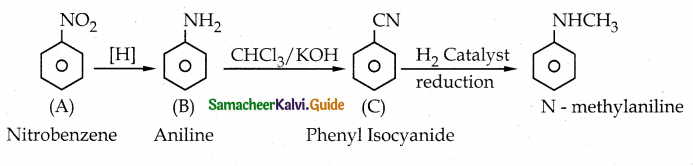
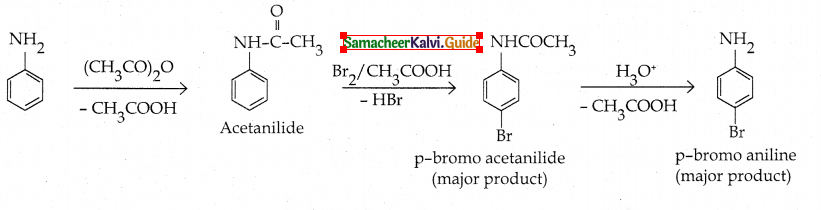
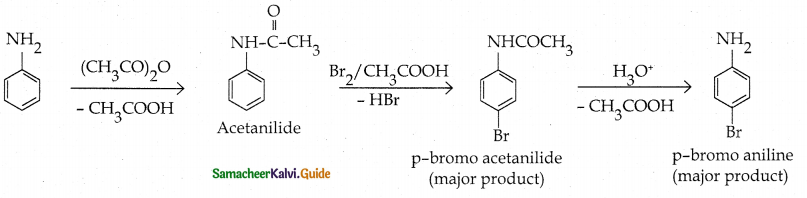
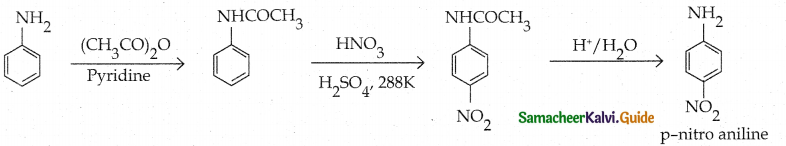
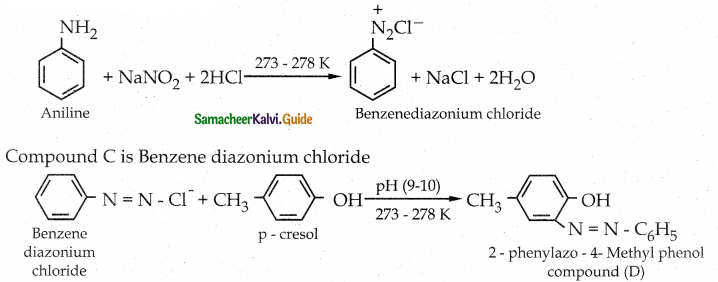
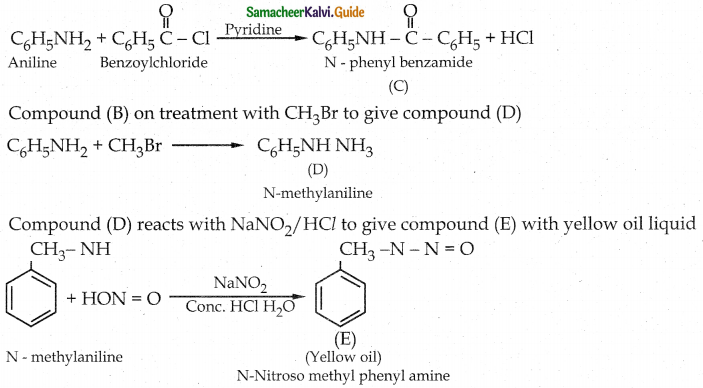
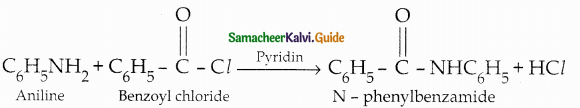
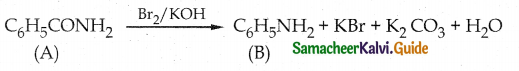 Compound B is Aniline (primary amine)
Compound B is Aniline (primary amine)