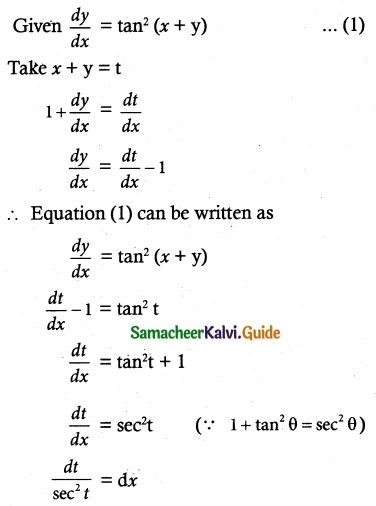Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Commerce Guide Pdf Chapter 7 Stock Exchange Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Commerce Solutions Chapter 7 Stock Exchange
12th Commerce Guide Stock Exchange Text Book Back Questions and Answers
![]()
I. Choose The Correct Answer.
Question 1.
…………. is the oldest stock exchange in the world.
a) London Stock Exchange
b) Bombay Stock Exchange
c) National Stock Exchange
d) Amsterdam Stock Exchange
Answer :
d) Amsterdam Stock Exchange
Question 2.
There are ………………. stock exchanges in the country.
a) 21
b) 24
c) 20
d) 25
Answer :
a) 21
![]()
Question 3.
Stock exchanges deal in
a) Goods
b) Serves
c) Financial Securities
d) Country’s Currency
Answer:
c) Financial Securities
Question 4.
Stock exchange allow trading in
a) All types of Shares of any Company
b) Bonds issued by the Govt.
c) Listed Securities
d) Unlisted Securities
Answer:
c) Listed Securities
![]()
Question 5.
Jobbers transact in a stock exchange
a) For their Clients
b) For their Own Transactions
c) For other Brokers
d) For other Members
Answer:
b) For their Own Transaction
Question 6.
A pessimistic speculator is
a) Stag
b) Bear
c) Bull
d) Lame Duck
Answer:
b) Bear
![]()
Question 7.
An optimistic speculator is
a) Bull
b) Bear
c) Stag
d) Lame Duck
Answer:
a) Bull
Question 8.
A bull operator believes in
a) Increase in Prices
b) Decrease in Prices
c) Stability in Prices
d) No change in Prices
Answer:
a) Increase in Prices
![]()
Question 9.
…………………… means the price at which securities are bought and sold are recorded and made public.
a) Market Quotations
b) Trade Quotations
c) Business Quotations
d) Buyers Quotations
Answer:
a) Market Quotations
Question 10.
The rules and regulations of Stock exchange is framed by ……………………. guide lines.
a) RBI
b) Central Government
c) SEBI
d) BSE
Answer:
c) SEBI
![]()
II. Very Short Answer Questions.
Question 1.
What is meant by Stock Exchange?
Answer:
Stock Exchange is an organized market for the purchase and sale of industrial and financial security. It is also called the stock market or share market.
Question 2.
Define Stock Exchange.
Answer:
Stock Exchange:
- “An association, organization or a body of individuals, whether incorporated or not, established for the purpose of assisting, regulating and controlling business in buying, selling and dealing in securities.
- Indian securities contracts [Regulation] Act of 1956.
![]()
Question 3.
Write any 5 Stock Exchanges in India.
Answer:
- The Bombay Stock Exchange
- Bangalore Stock Exchange Ltd
- The Madras Stock Exchange Ltd
- The Hyderabad Stock Exchange Ltd
- The Cochin Stock Exchange Ltd
Question 4.
What is meant by Remisier?
Answer:
- He acts as an Agent of a member of a Stock Exchange.
- He obtains business for his principal.
- For that service, he gets a commission.
![]()
Question 5.
Who is called a Broker?
Answer:
The broker is a commission agent. He acts as an intermediary between buyers and sellers of securities. He charges a commission for his services from both parties.
Question 6.
What are the types of Speculator?
Answer:
- Bull – Tejiwala
- Bear – Mandiwala
- Stag – Premium hunter
- Lame Duck
![]()
Question 7.
Mention the Recent Development in Stock Exchange?
Answer:
A commodity exchange is an exchange where commodities are purchased and sold. Commodities are listed here: Metals, agricultural products.
III. Short Answer Questions.
Question 1.
What are the limitations of Stock exchange? AL FAN
Answer:
- Absence of restriction on the membership of Stock Exchange.
- Lack of uniformity and control of Stock Exchanges.
- Failure to control unhealthy speculation.
- Allowing more than one exchange in a place.
- No proper regulation on the listing of securities.
Question 2.
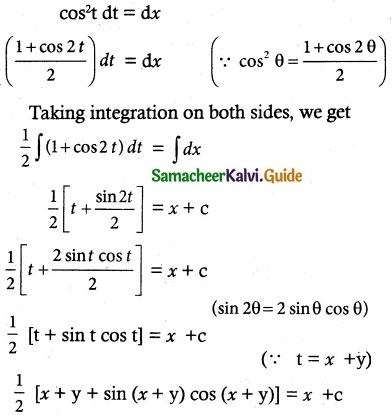
Explain Bull and Bear.
Answer:
Speculators in a stock market are of different types based on animal behaviour.
They are:
- Bull: A Bull or Tejiwala is an operator who expects a rise in prices of securities in the future. He purchases the securities expecting the price of rise in future.
- Bear: A bear or Mandiwala speculator expects prices to fall in future and sells securities at present with a view to purchase them at lower price.
![]()
Question 3.
Explain Stag and Lame Duck.
Answer:
Stag: [Premium Hunter]
- He is a Cautious Speculator.
- He applies for shares in new companies and expects to sell them at a premium.
- If he gets an allotment, he sells the shares before being called to pay the allotment money.
Lame Duck:
- When a Bear finds it difficult to fulfill his commitment, he is said to be struggling like a lame duck.
- Bear contracts to sell securities at a later date.
- At the appointed time he is not able to get the securities as the holders are not willing to part with them.
- In such situations, he feels concerned.
Question 4.
Explain National Stock Market System. (NSMS)
Answer:
The national stock market system was advocated by the High Powered Group. It is headed by Shri. Pherwani (popularly known as Pherwani Committee). At present the National Stock Market comprises the following:
- National Stock Exchange of India Limited (NSE)
- Stock Holding Corporation of India Limited (SHCIL)
- Securities Trading Corporation of India (STCI)
![]()
Question 5.
Explain National Stock Exchange (NSE)
Answer:
National Stock Exchanges:
- NSE was incorporated in November 1992.
- It is a Countrywide, Screen-based, Online, and order-driven trading system.
- It uses a satellite link to spread trading throughout the country thereby connecting members scattered all over India.
- NSE has two segments Debt and Capital Segments.
- It has revolutionized stock trading in India.
- Through a computer network, member’s orders for buying and selling within prescribed prices are matched by the central computers with each other and instantly communicate to the trading member.
IV. Long Answer Questions.
Question 1.
Explain the functions of the stock Exchange.
Answer:
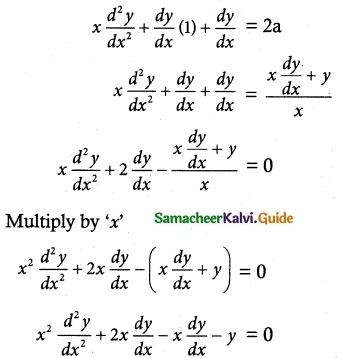
- Ready and Continuous Market: Stock exchange helps investors to sell their securities easily. And also he can convert his cash into securities.
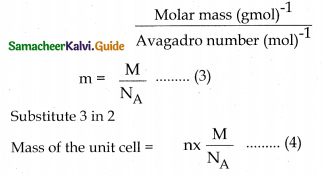
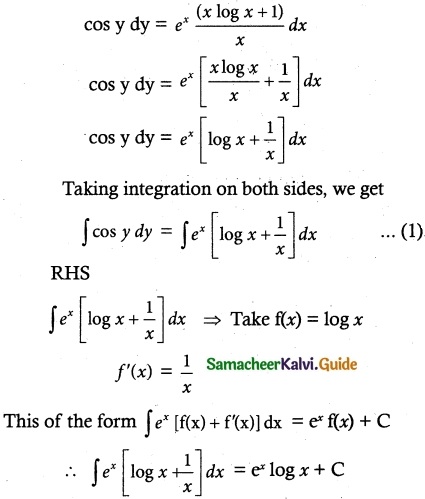
- Correct Evaluation of Securities: The prices at which securities are bought and sold are recorded and informed to the public. These prices are called “market quotations”
- Protection to Investors: All dealings in stock exchange are in accordance with well- defined rules and regulations. Any malpractice will be severely punished.
- Proper Channelisation of Capital: People like to invest in the shares of companies which yield good profits. Also, people invest in the companies which are giving good dividends.
- Facilities for Speculation: Speculation is an integral part of stock exchange operations. As a result of speculation, demand for and supply of securities are equalized.
![]()
Question 2.
Explain the features of the Stock Exchange.
Answer:
Features:
Market for Securities:
Stock Exchange is a market, where securities of corporate, bodies Government and Semi-Government bodies are bought and sold.
Association of Persons:
It is an association of persons or body of individuals whether incorporated or not.
Deals in second-hand securities:
In the secondary market (Stock Exchange) Shares, Debentures, Bonds already issued by the companies are (re-sale) traded.
Regulates Trade in Securities :
It regulates the trade activities so as to ensure free and fair trade.
Allow dealings only in Listed Securities:
- It maintains the list of companies (securities) that could be bought and sold on its floor.
- Unlisted Securities can’t be traded in the stock exchange.
Specific location:
- Stock exchange is a particular place where authorized brokers come together daily (on working days – working hours) on the floor of market.
![]()
Question 3.
Explain the Benefits of Stock Exchange.
Answer:
The benefits of the Stock Exchange are classified into benefits to the Community, Company, and Investors.
Benefits to the Community:
- Economic Development: It increases economic development by ensuring a steady flow of savings for production.
- Fund Raising Platform: It enables well-managed companies to raise funds by issue of shares.
Benefits to the Company:
- Enhances Goodwill or Reputation: Companies whose shares are quoted on a stock exchange enjoy greater goodwill and credit standing.
- Raises huge funds: Stock exchange helps the companies to raise huge funds by issue of shares and debentures.
Benefits to Investors:
- Liquidity: Stock exchange helps to convert his shares into cash quickly.
- Investor protection: The stock exchange safeguards, investor’s interests by following strict rules and regulations.
Question 3.
Benefits to the Investors [MLA].
Answer:
- The mechanism to trade security – It provides a mechanism by which listed securities can be bought and sold within few minutes.
- Liquidity – An investor can convert his. securities into cash and cash into securities quickly and easily.
Adding collateral value -It is good collateral security for obtaining loans from banks.
Question 4.
Distinguish between Stock Exchange and Commodity Exchange. MP SOFA
Answer:
|
Basis of Difference |
Money Market |
Capital Market |
| 1. Regulator | Central Bank is the Regulator | Central Bank and SEBI are the Regulators. |
| 2. Underwriting | Underwriting is not a primary function. | It is a primary function |
| 3. Risk | Low credit and market risk. | High credit and market risk. |
| 4. Availability of Instruments | Money Market instruments generally do not have a secondary market. |
Capital Market instruments generally have secondary market. |
| 5. Liquidity | High liquidity | Low liquidity |
| 6. Duration | Short-term loanable Funds not exceeding one year. |
Long-term loanable Funds exceeding one year. |
![]()
12th Commerce Guide Stock Exchange Additional Important Questions and Answers
I. Choose the correct answer.
Question 1.
A cautious speculator is……….
a) Bull
b) Bear
c) Stag
d) Lame Duck
Answer:
c) Stag
Question 2.
A Bear operator believes in ………….
a) Increase in Price
b) Decrease in Price
c) Stability in Price
d) No Change in Price
Answer:
b) Decrease in Price
![]()
Question 3.
Which is not a foreign Stock exchange?
(a) London Stock Exchange
(b) Bombay Stock Exchange
(c) Tokyo Stock Exchange
(d) New York Stock Exchange
Answer:
(b) Bombay Stock Exchange
Question 4.
Which one of the following is not correctly matched?
a) Jobbers – India pendent operators
b) Brokers – Intermediaries
c) Authorised clerks – Employee
d) Bull – Mandiwala
Answer:
d) Bull – Mandiwala
![]()
Question 5.
Pick the odd one out:
a) Bull
b) Bear
c) Stag
d) Swan
Answer:
d) Swan
Question 6.
Pick the odd one out:
a) Khazimar Street
b) Dalai Street
c) Wall Street
d) Lombard Street
Answer:
a) Khazimar Street
![]()
Question 7.
Choose the correct statement.
i) Future market is an Auction Market
ii) Right to sell a security is called “Put Option”
iii) Right to buy a security is called “Call Option”
a) (i) is correct
b) (ii) is correct
c) (iii) is correct
d) All (i) (ii) and (iii) are correct
Answer:
d) All (i) (ii) and (iii) are correct
![]()
II. Match the following
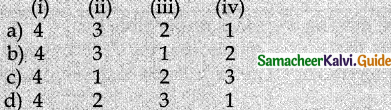
1. Match List I with List – II
| List -I | List – II |
| (i) Treasure Bill Market | 1. Short term Funds |
| (ii) CD Market | 2. Long term Funds |
| (iii) Money Market | 3. Higher degree of liquidity |
| (iv) Capital Market | 4. Issued by Commercial Banks |

Answer:
a) (i) 4, (ii) 3, (iii) 2, (iv) 1
Question 2.
|
List-I |
List-II |
| i. NASDAQ | 1. China |
| ii. Euronext | 2. New York City |
| iii. TMX Group | 3. France, Portugal, Netherlands |
| iv. Shenzhen Stock Exchange | 4. Toronto, Canada |

Answer:
a) (i) 2, (ii) 3. (iii) 4, (iv) 1
III. Assertion and Reason
Question 1.
Assertion (A): Bear or Mandiwala speculator expects prices to fall in future and sells the securities at present with a view to purchase them at lower prices.
Reason (R): A Bear usually presses its viction down to down to the ground. Similarly, the Bear speculator tends to force down prices of securities,
a) (A) is True (R) is False
b) (A) is False (R) is True
c) Both (A) and (R) are False
d) Both (A) and (R) are True
Answer:
c) Both (A) and (R) are True
IV. Very Short Answer Questions.
Question 1.
What is Future Market?
Answer:
- A “Future Market” is an auction market in which participants Buy and Sell commodities and future contracts for delivery on a Specified Future Date.
- (e.g) York Mercantile Exchange.
Question 2.
What is Options Market?
Answer:
An “Option” is a type of Security that can be Bought and Sold as a specified price within a specified period of time in exchange for a non-refundable upfront deposit.
![]()
Question 3.
What is leverage?
Answer:
- Options help you profit from changes in share prices without puffing down the full price of the share.
- You get control over the shares without buying them outright.
Question 4.
What is hedging?
Answer:
- They can also be used to protect yourself from fluctuations in the price of a share.
- Letting you buy or sell the shares at a pre-determined price for a specified period of time.
Question 5.
What is Sensex?
Answer:
- Sensex in an Index of the Stocks in BSE.
- It has a list of 30 stocks.
- BSE decides the stocks that are to be listed on sensex.
- The criteria for picking a stock to be listed on Sensex is volume of the trade of that stock and the total volume of stock in BSE.
Question 6.
What is NIFTY?
Answer:
- NIFTY derived from two words. “National” and “Fifty”.
- It means the index of the 50 most actively traded stocks from across all sectors.
Question 7.
What is meant by Commodity Exchange?
Answer:
Commodity Exchange is an Exchange where Commodities are traded.
Question 8.
What is the stock trading time in India?
Answer:
Equity Market:
- Normal Trading Time – 9.15 a.m to 3.30 p.m [Monday to Friday]
- Closed on – All Saturday Sunday and National Holidays.
Commodity Market:
- Normal Trading Time – 10.00 a.m to 11.30 a.m [Monday to Friday]
- Closed on – All Saturdays and Sundays and National Holidays.
Question 9.
Explain Dalai Street.
Answer:
- DalaI Street is an area in down town Mumbai, India that houses the Bombay Stock Exchange (BSE).
- The largest Stock Exchange of India.
- It received the name Dalai Street after the BSE moved to the area in 1874.
![]()
V. Long Answer Questions.
Question 1.
Explain Lombard street and Wall street.
Answer:
Lombard Street:
- Lombard street in London is notable for its connections with the city of London’s Merchant, Banking, Insurance Industries stretching back to medieval times.
- From Bank Junction,where NINE streets coverage by the Bank of England.
- It runs south east for a short distance before bearing left into a most easterly direction and terminates at a junction with Grace church Street and Fenchruch Street.
Wall Street:
- Wall Street in New York is a street in Lower Manhattan that is the original home of New York Stock Exchange.
- The Historic Head Quarters of the largest US brokerages and investment Banks.
- It is the collective name for the financial and Investment community which includes stock exchanges and – banks, Brokerages, Securities and underwriting firms and big Businesses.