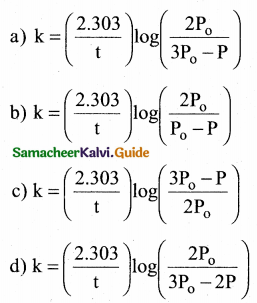
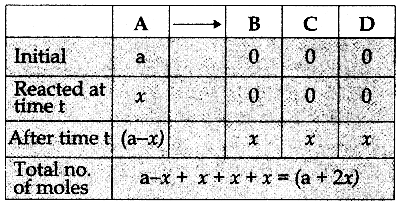
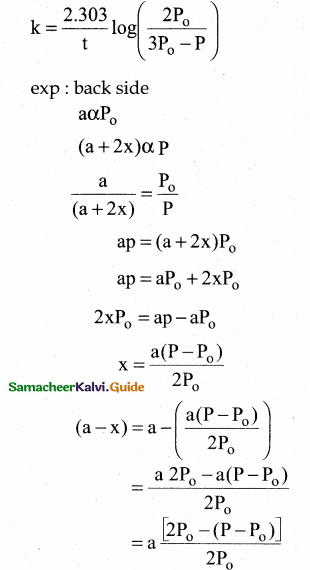
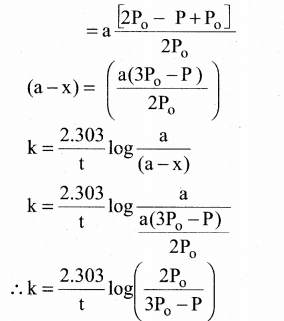
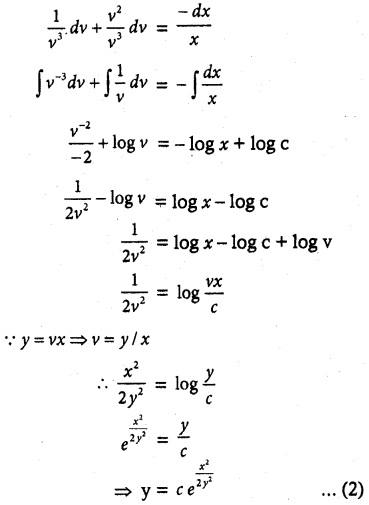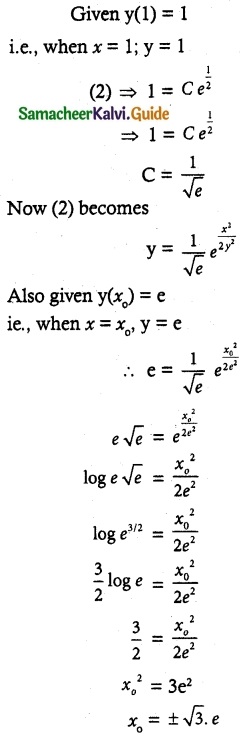Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Chemistry Guide Pdf Chapter 14 Biomolecules Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 14 Biomolecules
12th Chemistry Guide Biomolecules Text Book Back Questions and Answers
Part – I Text Book Evaluation
I. Choose the Correct Answer
Question 1.
Which one of the following rotates the plane polarized light towards left? (NEET Phase – II)
a) D(+) Glucose
b) L(+) Glucose
c) D(-) Fructose
d) D(+) Galactose
Answer:
c) D(-) Fructose
Question 2.
The correct corresponding order of names of four aldoses with configuration given below Respectively is, (NEET Phase I)1551
a) L-Erythrose, L-Threose, L-Erythrose, D-Threose
b) D-Threose,D-Ervthrose, L-Threose, L-Erythrose
c) L-Erythrose, L-Threose, D-Erythrose, D-Threose
d) D-Erythrose, D-Threose, L-Erythrose, L-Threose
Answer:
d) D-Erythrose, D-Threose, I-Erythrose, L-Threose
![]()
Question 3.
Which one given below is a non-reducing sugar? (NEET Phase – I)
a) Glucose
b) Sucrose
c) maltose
d) Lactose
Answer:
b) Sucrose
Question 4.
Glucose(HCN) → Product (hydroIysis) → Product (HI + Heat) A, the compoundA is
a) Heptanoic acid
b) 2-lodohexane
c)Heptane
d) Hep tanol
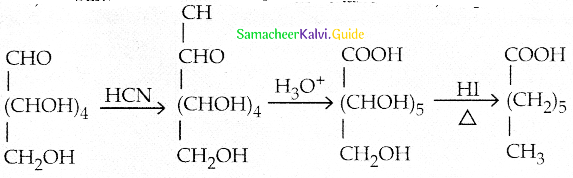
Answer:
a) Heptanoic acid
Question 5.
Assertion: A solution of sucrose in water is dextrorotatory. But on hydrolysis in the presence of little hydrochloric acid, it becomes levorotatory. (AIIMS)
Reason: Sucrose hydrolysis gives unequal amounts of glucose and fructose. As a result of this change in sign of rotation is observed.
a) If both assertion and reason are true and reason is the correct explanation of assertion
b) If both assertion and reason are true but reason is not the correct explanation of assertion
c) If assertion is true but reason is false
d) If both assertion and reason is false
Answer:
a) If both assertion and reason are true and reason is the correct explanation of assertion
![]()
Question 6.
The central dogma of molecular genetics states that the genetic information flows from (NEET Phase-II)
a) Amino acids Protein DNA
b) DNA Carbohydrates Proteins
c) DNA RNA Proteins
d) DNA RNA Carbohydrates
Answer:
c) DNA RNA Proteins
Question 7.
In a protein, various amino acids linked together by (NEET Phase – I)
a) Peptide bond
b) Dative bond
c) α – Glycosidic bond
d) β- Glycosidic bond
Answer:
a) Peptide bond
Question 8.
Among the following, the achiral amino acid is (AIIMS)
a) 2-ethylalanine
b) 2-methyl glycine
c) 2-hydroxymethylserine
d) Tryptophan
Solution:
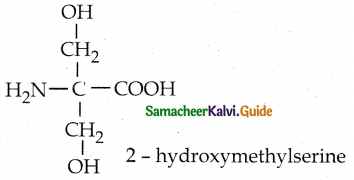
Answer:
c) 2-hydroxymethylserine
![]()
Question 9.
The correct statement regarding RNA and DNA respectively is (NEET Phase – I)
a) the sugar component in RNA is arabinose and the sugar component in DNA is ribose
b) the sugar component in RNA is 2′- deoxyribose and the sugar component in DNA is arabinose
c) the sugar component in RN A is arabinose and the sugar component in DNA is 2‘- deoxyribose
d) the sugar component in RNA is ribose and the sugar component in DNA is 2 – deoxyribose
Answer:
d) the sugar component in RNA is ribose and the sugar component in DNA is 2′-deoxyribose
Question 10.
In aqueous solution amino acids mostly exist in,
a) NH2-CH(R)-COOH
b) NH2-CH(R)-COO-
c) H3N+-CH(R)-COOH
d) H3N+-CH(R)-COO-
Answer:
d) H3N+-CH(R)-COO-
Question 11.
Which one of the following is not produced by the body?
a) DNA
b) Enzymes
c) Hormones
d) Vitamins
Answer:
d) Vitamins
![]()
Question 12.
The number of sp2 and sp 3 hybridised carbon in fructose are respectively
a) 1 and 4
b) 4 and 2
c) 5 and 1
d) 1 and 5
Answer:
d) 1 and 5
Question 13.
Vitamin B2 is also known as
a) Riboflavin
b) Thiamine
c) Nicotinamide
d) Pyridoxine
Answer:
a) Riboflavin
Question 14.
The pyrimidine bases present in DNA are
a) Cytosine and Adenine
b) Cytosine and Guanine
c) Cytosine and Thiamine
d) Cytosine and Uracil
Answer:
c) Cytosine and Thiamine
![]()
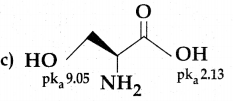
Question 15.
Among the following L-serine is
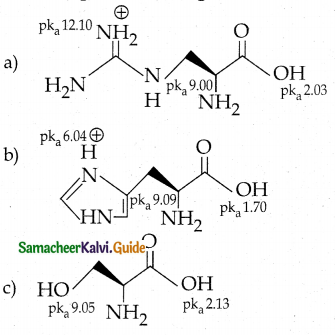
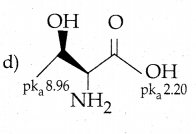
Answer:
Question 16.
The secondary structure of a protein refers to
a) fixed configuration of the polypeptide backbone
b) hydrophobic interaction
c) sequence of a-amino acids
d) α -the helical backbone
Answer:
d) α helical backbone
![]()
Question 17.
Which of the following vitamins is water-soluble?
a) Vitamin E
b) Vitamin K
c) Vitamin A
d) Vitamin B
Answer:
d) Vitamin B
Question 18.
Complete hydrolysis of cellulose gives
a) L-Glucose
b) D-Fructose
c) D-Ribose
d) D-Glucose
Answer:
d) D-Glucose
Question 19.
Which of the following statement is incorrect?
a) Ovalbumin is a simple food reserve in the egg- white
b) Blood proteins thrombin and fibrinogen are involved in blood clotting
c) Denaturation makes the protein more active
d) Insulin maintains the sugar level in the human body.
Answer:
c) Denaturation makes the protein more active
![]()
Question 20.
Glucose is an aldose. Which one of the following reactions is not expected with glucose?
a) It does not form an oxime
h) It does not react with the Grignard reagent
c) It does not form osazones
d) It does not reduce tollens reagent
Answer:
b) It does not react with the Grignard reagent
Question 21.
If one strand of the DNA has the sequence ‘ATGCTTGA’, then the sequence of complementary strand would be
a) TACGAACT
b) TCCGAACT
c) TACGTACT
d) TACGRAGT
Answer:
a) TACGAACT
Question 22.
Insulin, a hormone chemically is
a) Fat
b) Steroid
c) Protein
d) Carbohydrates
Answer:
c) Protein
![]()
Question 23.
α -D (+) Glucose and β -D (+) glucose are
a) Epimers
b) Anomers
c) Enantiomers
d) Conformational isomers
Answer:
b) Anomers
Question 24.
Which of the following are epimers
a) D(+)-Glucose and D(+)-Galactose
b) D(+)-Glucose and D(+)-Mannose
c) Neither (a) nor (b)
d) Both (a) and (b)
Answer:
d) Both (a) and (b)
Question 25.
Which of the following amino acids is achiral?
a) Alanine
b) Leucine
c) Proline
d) Glycine
Answer:
d) Glycine
![]()
II. Short Answer
Question 1.
What type of linkages holds together monomers of DNA?
Answer:
- Phospho diester linkages hold together monomers of DNA
- Phosphoric acid forms phospho diester bond between neucleotides
- The sugar – phosphate linkage forms the backbone of each strand of DNA.
Question 2.
Give the differences between primary and secondary structure of proteins
Answer:
| Primary structure of Proteins | Secondary Structure of Proteins |
| i) Linear Sequence of amino acids | Folding of the peptide chain into an α – helix or β- sheet |
| ii) Linear | Either an α- helix or β – sheet |
| iii) Composed of peptide bonds formed between amino acids | Amino acids in the polypeptide chain forms highly regular shapes through hydrogen bonds between the – C = O and – NH2 groups |
Question 3.
Name the Vitamins whose deficiency cause
- rickets
- scurvy
Answer:
- Rickets – deficiency of Vitamin D
- Scurvy – deficiency of Vitamin C
![]()
Question 4.
Write the Zwitter ion structure of alanine
Answer:
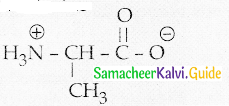
Question 5.
Give any three difference between DNA and RNA
Answer:
| DNA | RNA |
| i) Mainly present in nucleus, mitochondria and chloroplast | Mainly present in cytoplasm, nucleolous and ribosomes |
| ii) Contains deoxyribose sugar | Contains ribose sugar |
| iii) Base pair A = T G ≡ C | Base pair A = U C ≡ G |
Question 6.
Write a short note on peptide bond
Answer:
- Amino acids are linked covalently by peptide bonds.
- The carboxyl group of the first amino acid reacts with the amino group of the second amino acid to give an amide linkage between these two amino acids.
- This amide linkage is called a peptide bond.
- Peptide bond is –

- The resulting compound is called a dipeptide
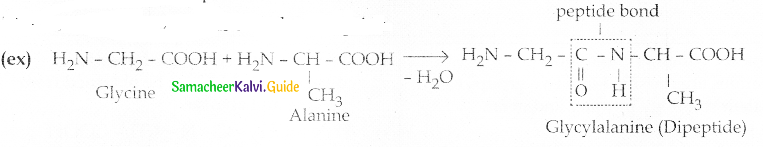
- If the number of amino acids attached is less it is called a polypeptide.
- If the number of amino acids attached is large it is called a protein.
![]()
Question 7.
Give two difference between Hormones and vitamins
Answer:
| Hormones | Vitamins |
| i) They are organic substances (eg. peptide or a steroid) that is secreted by one tissue into the bloodstream, | They are small organic compounds that cannot he synthesised by our body but must be obtained through diet. |
| ii) Induce physiological response. (eg) growth and metabolism in other tissues | Essential for certain functions, their deficiency or excess can cause diseases. |
Question 8.
Write a note on the denaturation of proteins.
Answer:
Denaturation of proteins.
1. In general, protein has a unique three – dimensional structure formed by interactions such as disuiphide bond, hydrogen bond, hydrophobic and electrostatic interactions.
2. These interactions can be disturbed when the protein is exposed to a higher temperature in certain chemicals such urea, alternation of pH, ionic strength, etc. It leads to the loss of the three – dimensional structure.
3. The process of a protein-losing its higher-order structure without losing the primary structure, It is called denaturation of the protein. When a protein denatures, its biological function is also lost.
4. Since the primary structure is intact, this process can be reversed in certain proteins. This can happen spontaneously upon restoring the original conditions or with the help of special enzymes called chaperons.
5. Example: Coagulation of egg white by the action of heat.
![]()
Question 9.
What is reducing and non – reducing sugars?
Answer:
| Reducing Sugars | Non – Reducing Sugars |
| Carbohydrates in which the aldehyde carbon is not involved in the glycosidic bond retain their reducing property are called reducing sugars | Carbohydrates in which the carbonyl carbons are involved in the glycosidic bonding lose their reducing property are called non – reducing sugars. |
Question 10.
Why carbohydrates are generally optically active?
Answer:
- Almost all carbohydrates are optically active because the’ have one or more chiral carbon.
- The number of optical isomers = 2n
where n = the total number of chiral carbons.
Question 11.
Classify the following into monosaccharides, oligosaccharides and polysaccharides.
i) starch
ii) fructose
iii) sucrose
iv) lactose
v) maltose
Answer:
| Monosaccharides | Oligo saccharides | Polysaccharides |
| Fructose | Sucrose Lactose Maltose |
Starch |
![]()
Question 12.
How are vitamins classified?
Answer:
Vitamins are classified into two groups based on their solubility in water and in fat. They are,
- Water-soluble vitamins
- Oil or fat-soluble vitamins
Water-soluble vitamins – Vitamins which dissolve in water are called water-soluble vitamins. Examples – Vitamins of B group and Vitamin C Oil or fat-soluble vitamins: Vitamins which dissolve in oils or fat are called oil or fat-soluble vitamins. Examples – Vitamin A, D, E and K.
Question 13.
What are hormones? Give Examples.
Answer:
The hormone is an organic substance that is sëcreted by one tissue into the bloodstream and induces a physiological response in other tissues. It is an intercellular signaling molecule. Virtually every process is a complex organism is regulated by one or more hormones. Examples: insulin, epinephrine, estrogen, androgen etc.
Question 14.
Write the structure of all possible dipeptides which can be obtained from glycine and alanine.
Answer:
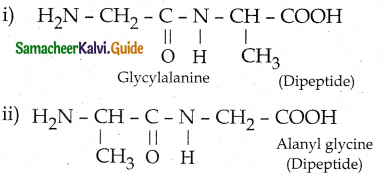
![]()
Question 15.
Define enzymes.
Answer:
There are many biochemical reactions that occur in our living cells. Digestion of food and harvesting the energy from them, and synthesis of necessary molecules required for various – cellular functions are examples for such reactions. All these reactions are catalysed by special proteins called enzymes.
(or)
Enzymes are biocatalysts produced by the living cells which catalyse many biochemical reactions in animal and plant bodies. They are more specific in their action.
Question 16.
Write the structure of α – D (+) glucophyranos
Answer:
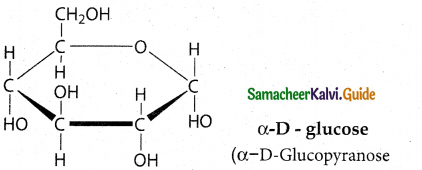
Question 17.
What are different types of RNA which are found in cell?
Answer:
- Ribosomal RNA (rRNA)
- Messenger RNA (rnRNA)
- Transfer RNA (tRNA)
Question 18.
Write a note on formation of α – helix
Answer:
- In the α – helix sub – structure, the aminoacids are arranged in a right handed helical (spiral) structure.
- This structure is stabiised by hydrogen bond between the carhonyl oxygen of one amino acid (nth residue) with amino hydrogen of the fifth residue (n + 4th residue).
- The side chains of the residues protrude outside of the helix.
- Each turn of an helix contains about 3.6 residues and in about 5.4 A long.
- The amino acid proline produces a kink in the helical structure and often called as a helix breaker due to its rigid cyclic structure.
![]()
Question 19.
What are the functions of lipids in living organisms?
Answer:
- Lipids are the integral component of cell membrane. They are necessary for the structural integrity of the cell.
- The main function of triglycerides in animals is as an energy reserve.
- They yield more energy than carbohydrates and proteins.
- They act as a protective coating in aquatic organisms.
- Lipids of connective tissue give protection to internal organs.
- Lipids help in the absorption and transport of fat-soluble vitamins.
- They are essential for the activation of enzymes such as lipases.
- Lipids act as emulsifiers in fat metabolism.
Question 20.
Is the following sugar, D – sugar or L – sugar?
Answer:
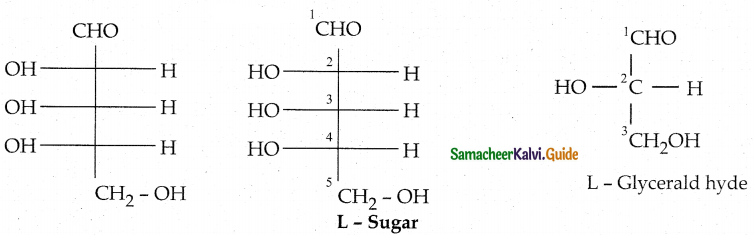
Because the H and OH on C4 carbon are in the same configuration as the H and OH on C2
carbon in L — Glyceraldehyde.
![]()
12th Chemistry Guide Biomolecules Additional Questions and Answers
Part – II – Additional Questions
Question 1.
Polyhydroxy aldehydes or ketones are called
a) Vitamins
b) Enzymes
c) Carbohydrates
d) Lipids
Answer:
c) Carbohydrates
Question 2.
The general molecular formula of Carbohydrates is
a) Cn(H2)O2n
b) Cn(H2O)n
c) Cn(H2O)2n + 2
d) Cn(H2O)2n -2
Answer:
b) Cn(H2O)n
Question 3.
Green leaves of plants, during photosynthesis synthesise
a) Vitamins
b) Enzymes
c) Carbohydrates
d) Lipids
Answer:
c) Carbohydrates
![]()
Question 4.
During photosynthesis, carbondioxide and water are converted into
a) Sucrose
b) Fructose
c) Maltose
d) Glucose
Answer:
d) Glucose
Question 5.
The number of optical isomers of a carbohydrate is given by the formula 2n, where n is the number of
a) Carbon atoms
b) Chiral carbon atoms
c) achiral carbon atom
d) hydroxy group
Answer:
b) Chiral Carbon atoms
Question 6.
Which among the following is not a monosaccharide?
a) Fructose
b) Erythrose
c) Maltose
d) Ribose
Answer:
c) Maltose – Disaccharide
![]()
Question 7.
The medicinal value of a drug is measured in terms of its
a) Deoxyribose
b) Goldnumber
c) Therapeutic index
d) Equilibrium constant
Answer:
c) Therapeutic index
Question 8.
Fructose is a
a) aldopentose
b) ketopentose
c) aldohexose
d) ketohexose
Answer:
d) ketohexose
Question 9.
Which is known as blood sugar?
a) Glucose
b) fructose
c) sucrose
d) maltose
Answer:
a) Glucose
![]()
Question 10.
Sucrose undergoes hydrolysis when boiled with dil.H2So4 to give
a) Glucose
b) fructose
c) both (a) & (b)
d) maltose
Answer:
c) both (a) & (b)
Question 11.
The number of asymmetric carbon atoms present in glucose are
a) 3
b) 4
c) 5
d) 6
Answer:
b) 4
Question 12.
Glucose is
a) dextro rotatory
b) laevorotatory
c) optically inactive
d) non – reducing sugar
Answer:
a) dextro rotatory
![]()
Question 13.
Glucose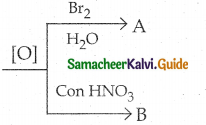
a) Saccharic acid, Gluconic acid
b) Gluconic acid, Saccharic acid
c) Glycollic acid, Tartaric acid
d) Tartaric acid, Glycollic acid
Answer:
b) Gluconic acid, Saccharic acid
Question 14.
The isomers called anomers differ in the configuration of
a) C1 carbon
b) C2 carbon
c) C4 carbon
d) C5 carbon
Answer:
a) C1 carbon
Question 15.
The cyclic structure of glucose is similar to
a) furan
b) pyran
c) pyridine
d) pyrrole
Answer:
(b) pyran
![]()
Question 16.
Sugars differing in configuration at an asymmetric centre are known as
a) anomers
b) epimers
c) enantimoers
d) diasteromers
Answer:
(b) epimers
Question 17.
D – mannose and D – glucose are
a) C – 2 epimers
b) C – 3 epimers
c) C – 4 epimers
d) C – 5 epimers
Answer:
a) C – 2 epimers
Question 18.
D – glucose and D – galactose are
a) C – 2 epimers
b) C – 3 epimers
c) C – 4 epimers
d) C- 5 epimers
Answer:
c) C – 4 epimers
![]()
Question 19.
In our body galactose is coverted into glucose by
a) hydrolysis
b) mutrarotation
c) fermentation
d) epimerisation
Answer:
d) epimerisation
Question 20.
Fructose is .
a) dexto rotatory
b) laevo rotatory
c) optically inactive
d) disaccharide
Answer:
b) laevo rotatory
Question 21.
Glucose and fructose are
a) Chain isomers
b) Position isomers
c) Functional isomers
d) tautomers
Answer:
c) Functional isomers
Hint: Glucose – Aldohexose
Fructose – Ketohexose
![]()
Question 22.
Fruit sugar is
a) glucose
b) fructose
c) ribose
d) erythrose
Answer:
b) fructose
Question 23.
Equal amount of glucose and fructose is termed as
a) Fruit sugar
b) blood sugar
c) invert sugar
d) cane sugar
Answer:
c) invert sugar
Question 24.
Sucrose is converted into glucose and fructose by the enzyme
a) Zymase
b) invertase
c) lactase
d) fructase
Answer:
b) invertase
![]()
Question 25.
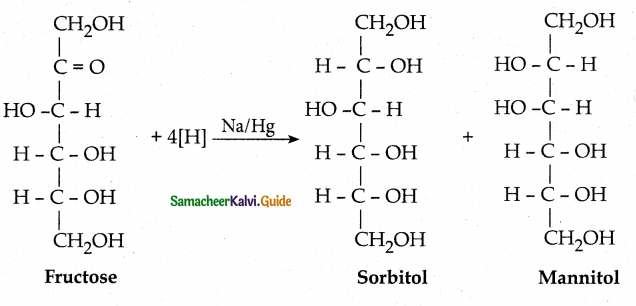
Partial reduction of fructose with sodium amalgam and water produces mixture of
a) D – Glucose and D – mannose
b) Sorbitol and Mannitol
c) GIvcollic acid and Tartaric acid
d) D – Glucose and D – Galactose
Answer:
b) Sorbitol and Mannitol
Question 26.
Sorbitol and Mannitol are
a) enantiomers
b) tautomers
c) epimers
d) functional isomers
Answer:
c) epimers
Question 27.
Which of the following statement incorrect?
a) Nucleoside + Phosphate → Nucleotide
b) Nucleoside + Base → Nucleotide
c) Sugar + Base → Nucleoside
d) n Nucleoside → Polynucleotide
Answer:
b) Nucleoside + Base → Nucleotide
![]()
Question 28.
The number of asymmetric carbon atom present in fructose is
a) 2
b) 3
c) 4
d) 5
Answer:
b) 3
Question 29.
Fructose forms a five membered ring similar to
a) Furan
b) Pyran
c) Pyridime
d) Pyrrole
Answer:
a) Furan
Question 30.
The general formula of disaccharides is
a) Cn(H2O)2n
b) Cn(H2O)n
c) Cn(H2O)n-1
d) Cn(H2O)2n + 2
Answer:
c) Cn(H2O)n-1
![]()
Question 31.
In disaccharides, two monosaccharides are linked by
a) hydrogen bond
b) ionic bond
c) peptide linkage
d) glvcosidic linkage
Answer:
d) glycosidic linkage
Question 32.
Honey is a mixture of
i) glucose
ii) fructose
iii) sucrose
iv) Maltose
a) (i) & (ii)
b) (ii) & (iii)
c) (i (ii) & (iii)
d) (i), (ii), (iv)
Answer:
c) (i), (ii), (iii)
Question 33.
Which carbon atoms of α -D – glucose and β – D – fructose are joined together in sucrose?
a) C1 and C2
b) C1 and C4
c) C1 and C5
d) C2 and C4
Answer:
a) C1 and C2
![]()
Question 34.
The disaccharide present in milk of mammals is
a) glucose
b) lactose
c) maltose
d) sucrose
Answer:
b) lactose
Question 35.
Which among the following is a non-reducing sugar
a) Lactose
b) Maltose
c) Sucrose
d) Glucose
Answer:
c) sucrose
Question 36.
On hydrolysis lactose gives
i) galactose
ii) glucose
iii) fructose
iv) maltose
a) (i) & (ii) h) (ii) & (iii)
b)(ii) & (iii)
c) (iii) & (iv) d) (i) & (iv)
d)(i)&(iv)
Answer:
a) (i) & (ii)
![]()
Question 37.
Which is produced during digestion of starch by the enzyme a – amylase?
a) Glucose
b) Maltose
c) Lactose
d) Sucrose
Answer:
b) Maltose
Question 38.
Which carbon atoms of a -D glucose are linked to form maltose?
a) C1 and C2
b) C1 and C3
c) C1 and C4
d) C3 and C4
Answer:
c) C1 and C4
Question 39.
Which among the following is a non-sugar?
a) glucose
b) cellulose
c) sucrose
d) fructose
Answer:
b) cellulose – polysaccharide which is a non – sugar
![]()
Question 40.
Starch contains
a) 80% amylopectin and 20% arnylose
b) 80% amylose and 20% arnylopectin
c) 50% amvlopectin and 50% amylose
d) 80% glucose and 20% fructose
Answer:
a) 80% amylopectin and 20% arnylose
Question 41.
Starch is a polymer of glucose molecules linked by
a) α (1, 2) glycosidic bonds
b) α (1, 3) glycosidic bonds
c) α (1, 4) glycosidic bonds
d) α (1, 5) glycosidic bonds
Answer:
c) α (1, 4) glycosidic bonds
Question 42.
Number of α- D glucose molecules joined by α (1,4) glycosidic bonds in amylose and amylopectin are respectively
a) up to 10,000 and 4000
b) up to 4000 and 10,000
c) up to 1000 and 400
d) up to 400 and 1000
Answer:
b) up to 4000 and 10,000
![]()
Question 43.
With iodine solution amylose and amylopectin give respectively
a) blue and red colour
b) violet and red colour
c) blue and purple colour
d) violet and yellow colour
Answer:
c) blue and purple colour
Question 44.
Major constituent of plant cell walls is
a) starch
b) cellulose
c) glycogen
d) amylose
Answer:
b) cellulose
Question 45.
In cellulose, glucose molecules are linked by
a) α (1, 2) glucosidic bond
b) α (1, 4) glycosidic bond
c) β (1, 2) glycosodic bond
d) β (1,4) glycosidic bond
Answer:
d) β (1,4) glycosidic bond
![]()
Question 46.
Cotton is almost pure
a) glucose
b) starch
c) cellulose
d) amylose
Answer:
c) cellulose
Question 47.
Gun cotton is
a) cellulose acetate
b) cellulose nitrate
c) ethyl cellulose
d) cellulose bromide
Answer:
b) cellulose nitrate
Question 48.
Animal starch is
a) glucose
b) glycogen
c) amylose
d) amylopectin
Answer:
b) glycogen
![]()
Question 49.
Protein are polymers of
a) α – amino acids
b) β – amino acids
c) α – hydroxy acids
d) β – hydroxy acids
Answer:
a) α – amino acids
Question 50.
The amino acids that can be synthesised by humans are called
a) essential amino acids
b) non – essential amino acids
c) polar amino acids
d) non – polar amino acids
Answer:
b) non – essential amino acids
Question 51.
At isoelectric point an amino acid exists as
a) positive ion
b) negative ion
c) zwitter ion
d) protein
Answer:
c) Zwitter ion
![]()
Question 52.
The amino acid which contains achiral carbon is
a) alanine
b) gylcine
c) phenyl alanine
d) valine
Answer:
b) glycine
Question 53.
Which is optically inactive?
a) alanine
b) glycine
c) phenyl alanine
d) valine
Answer:
b) glycine
![]()
Question 54.
A peptide bond is
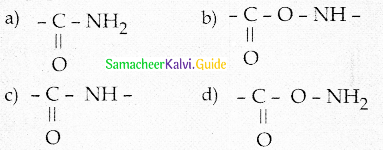
Answer:
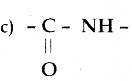
Question 55.
Number of peptide bonds present in a dipeptide is
a) 1
b) 2
c) 3
d) 4
Answer:
a) 1
Question 56.
In proteins α – helix and β – strands are two most common substructures present in
a) Primary structure
b) Secondary structure
c) Tertiary structure
d) Quaternary structure
Answer:
b) Secondary structure
![]()
Question 57.
Enzymes are
a) antibodies
b) transporters
e) bio catalysts
d) receptors
Answer:
c) bio catalysts
Question 58.
Which among the following is water soluble vitamin?
a) Vitamin A
b) Vitamin B
c) Vitamin D
d) Vitamin
Answer:
b) Vitamin B
Question 59.
Which is essential for blood clotting?
a) Vitamin A
b) Vitamin B
c) Vitamin D
d) Vitamin K
Answer:
d) Vitamin K
![]()
Question 60.
The chemical name of vitamin B6 is
a) Niacin
b) Biotin
c) Pyridoxine
d) Folic acid
Answer:
c) Pyridoxine
Question 61.
Glucose is an aldose. Which one of the following reactions is not expected with glucose?
a) It does not form oxime
b) It does not react with Grignard reagent
c) It does not form osazones
d) It does not reduce tollens reagent
Answer:
b) It does not react with Grignard reagent
Question 62.
The pyrimdine bases present in DNA are
(i) Cytosine
(ii) Thymine
(iii) Uracil
a) (i) & (ii)
b) (i) & (iii)
c) (ii) & (iii)
d) (i), (ii) & (iii)
Answer:
a) (i) & (ii)
![]()
Question 63.
A nucleotide is derived from a nucleoside by the addition of a molecule of
a) ribose
b) deoxy ribose
c) phosphoric acid
d) purine
Answer:
c) phosphoric acid
Question 64.
Which of the following is an intercellular signalling molecule?
a) Enzymes
b) Hormone
c) Protein
d) Vitamin
Answer:
b) Hormone
Question 65.
Which among the following is a steroidal hormone?
a) insulin
b) epinephrine
c) estrogen
d) adenine
Answer:
c) estrogen
![]()
Question 66.
The sweetest of all known sugars is
a) glucose
b) sucrose
c) fructose
d) ribose
Answer:
c) fructose
II. Pick out the Correct Statement
Question 1.
i) D – glucose is so named because the H and OH on C5 carbon are in the same configuration as the H and OH on C2
carbon in D – Glycerldehyde.
ii) Dextro rotatory compounds rotate the plane of polarised light in anti clock wise direction.
iii) Leavo rotatory compounds rotate the plane of polarised light in clockwise direction.
iv) The D or L isomers can either be dextro or leavo rotatory compounds.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Correct statements:
Answer:
d) (i) & (iv)
(ii) Dextro rotatory compounds rotate the plane of polarised light in clockwise direction.
(iii) Laevo rotatory compounds rotate the plane of polarised light in anti clockwise direction.
Question 2.
i) During hydrolysis of sucrose the optical rotation of the reaction mixture changes from levo to dextro.
ii) Honey bees have the enzyme invertase that catalyzes the hydrolysis of sucrose to glucose and fructose.
iii) In sucrose Cl of α – D – glucose is joined to C2 of β – D fructose
iv) Sucrose is a reducing sugar.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Correct statements :
Answer:
b) (ii) & (iii)
(i) During hydrolysis of sucrose the optical rotation of the reaction mixture changes from dextro to levo.
(iv) Sucrose is a non-reducing sugar.
![]()
Question 3.
i) Fibrous proteins are linear molecules similar to fibres.
ii) Fibrous proteins are generally soluble in water and are held together by disulphide bridges and weak intermolecular hydrogen bonds.
iii) Globular proteins have an over all spherical shape.
iv) Globular proteins are usually insoluble in water and have many functions including catalysis.
a) (i) & (ii)
b) (i) & (iii)
c) (ii) & (iii)
d) (iii) & (iv)
Answer:
b) (i) & (iii)
Correct statements :
(ii) Fibrous proteins are together by disulphide bridges and weak intermolecular hydrogen bonds.
(iv) Globular proteins are usually soluble in water and have many functions including catalysis.
Question 4.
i) Enzymes activate a reaction by increasing the activation energy by destabilizing the transition state.
ii) Many biochemical reactions are catalyzed by special proteins called enzymes.
iii) Sucrase enzyme catalyses the hydrolysis of lactose into glucose and galactose
iv) Enzymes are specific.
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (iv)
d) (i) & (iv)
Answer:
c) (ii) & (iv)
Correct statements :
(i) Enzymes activate a reaction by reducing the activation energy’- by stabilising the transition state.
(iii) Lactase enzyme catalyses the hydrolysis of lactose into glucose and galactose.
![]()
III. Pick out the InCorrect Statement
Question 1.
i) Mono-saccharides are carbohydrates that cannot be hydrolysed further and are called simple sugars.
ii) Glucose is a ketohexose.
iii) Glucose contain one primary alcoholic group and four secondary alcoholic group.
iv) Glucose is levulose
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (iv)
d) (iii) & (iv)
Answer:
c) (ii) & (iv)
Correct statements:
(ii) Glucose is an Aldohexose
(iv) Glucose is dextrose.
Question 2.
i) Amino acids obtained through diet are called non-essential amino acids.
ii) Amino acids have amphoteric behaviour.
iii) Iso electric point is the pH value at which the Zwitter ion of amino acid won’t move towards an electrode.
iv) pH above the isoelectric point the amino acid is positively charged.
a) (i) & (ii)
b) (ii) & (iii)
c)(iii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statements :
(i) Amino acids obtained through diet are called essential amino acids.
(iv) pH above the isoelectric point the amino acid is negatively charged.
![]()
Question 3.
i) The relative arrangement of the amino acids in the poly peptide chain is called the primary structure of the protein,
ii) α- Helix and ( β – strands are two most common sub structures formed by proteins.
iii) In the α- helix sub structure, the amino acids are arranged in a left handed helical structure.
iv) Each turn of an α- helix contains about 6.3 residues and is about 4.5 A long.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
c) (iii) & (iv)
Correct statements :
(iii) In the α-helix sub structure, the amino acids are arranged in a right handed helical structure.
(iv) Each turn of an α- helix contains about 3.6 residues and is about 5.4 A long.
Question 4.
i) Each vitamin has a specific function in the living system, mostly as co-enzymes.
ii) Water soluble vitamins can be stored in fatty tissues and livers.
iii) Excess of fat soluble vitamins will be excreted through urine and not stored in our body.
iv) Vitamins are small organic compounds that can not be synthesised by our body.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statements :
(ii) Fat soluble vitamins can be stored in fatty tissues and livers.
(iii) Excess of water soluble vitamins will be excreted through urine and not stored in our body.
![]()
IV. Assertion & Reason
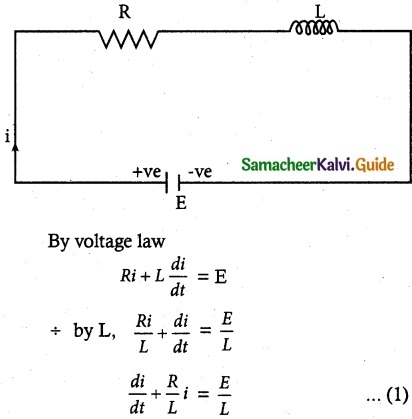
Question 1.Assertion : Thymine pairs with Adenine whereas Cytosine pairs with Guanine in DNA molecule.
Reason : The hydrogen bonding between bases of two strands is highly specific.
a) Both assertion and reason are true and reason is the correct explanation of assertion.
b) Both assertion and reason are true but reason is not the correct explanation of assertion.
c) Assertion is true and reason is false.
d) Both assertion and reason are false.
Answer:
c) Assertion is true and reason is false.
Question 2.
Assertion (A): Glycyl alanine is a dipeptide
Reason (R): In glycyl alanine the amino group of glycine react with the carboxyl group of alanine to form a peptide linkage.
a) Both A and R are correct, R explains A.
b) Both A and R are correct but R does not explain A.
c) A is correct but R is wrong.
d) A is wrong but R is correct
Answer:
c) A is correct but R is wrong.
Correct Reason (R): In glycyl alanine the carbonyl group of glycine react with the amino group of alanine to form a peptide linkage.
![]()
Question 3.
Assertion (A) : A nucleotide is derived from a nucleoside by the addition of a molecule of phosphoric acid.
Reason (R) : A nucleoside is a molecule without the phosphate group.
a) Both A and R are correct, R explains A.
b) Both A and R are correct but R does not explain A.
c) A is correct but R is wrong.
d) A is wrong but R is correct
Answer:
a) Both A and R are correct, R explains A.
Question 4.
Assertion (A) : The specific association of bases A and T, C and G of the two chains of the double helix is known as complementary base pairing.
Reason (R): There are 10.5 base pairs per turn of the helix of DNA.
a) Both A and R are correct, R explains A.
b) Both A and R are correct but R does not explain A.
c) A is correct but R is wrong.
d) A is wrong but R is correct
Answer:
b) Both A and R are correct, R does not explain A.
V. Match the following
Question 1.
| Type of Sugar | Example |
| i. Aldotetrose | Glucose |
| ii. Ketotriose | Ribose |
| iii. Aldotetrose | Fructose |
| iv. Ketotetrose | Ribulose |
| v. Aldopentose | Glyceraldehyde |
| vi. Keto pentose | Erythrulose |
| vii. Aldo hexose | Dihydroxy acetone |
| viii. Keto hexose | Erythrose |
Answer:
i. – Glyceraldehyde
ii – Dihydroxy acetone
iii – Erythrose
iv – Erythrulose
v – Ribose
vi – Ribulose
vii – Glucose
viii – Fructose
![]()
Question 2.
| Vitamin | Deficiency disease |
| i. Thiamine | Depression |
| ii. Ribo florin | Scurvy |
| iii. Cobalamin | Beri – beri |
| iv. Biotin | Pernicious anaemia |
| v. Ascorbic acid | Cheilosis |
Answer:
i. – Beri – beri
ii. – Cheilosis
iii – Pernicious anaemia
iv. -Depression
v. – Scurvy
Question 3.
| Vitamin | Chemical Name |
| i. B1 | Pyridoxine |
| ii. B2 | Folic acid |
| iii. B3 | Riboflovin |
| iv. B6 | Thiamine |
| v. B9 | Niacin |
Answer:
i. – Thiamine
ii. – Riboflovin
iii. – Niacin
iv. – Pyridoxine
v. – Folic acid
![]()
Question 4.
| Biomolecule | Example |
| Homo polysaccharide | heparin |
| ii. Hetero poly saccharide | phylloquinone |
| iii. α – amino acid | androgen |
| iv. Vitamin | glycogen |
| v. Hormone | histidine |
Answer:
i. – glycogen
ii. – heparin
iii. – histidine
iv. – phylloquinone
v. – androgen
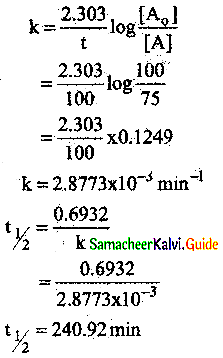
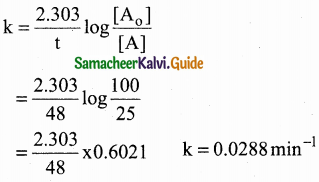
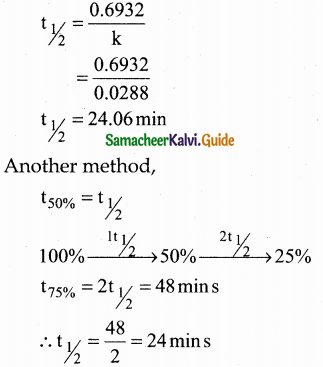
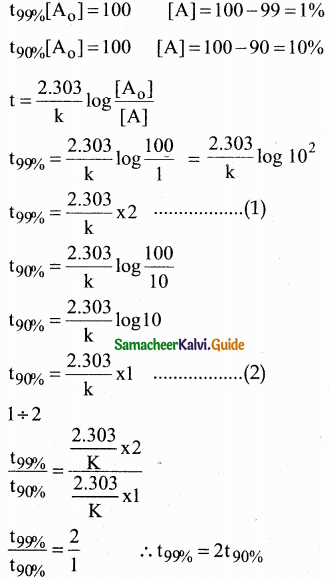
VI. Two Mark Questions
Question 1.
How do plants synthesise glucose?
Answer:
- Plants synthesise glucose by a complex process called photosynthesis.
- During photosynthesis green leaves of plants use sunlight to convert carbon-dioxide and water into glucose and oxygen.
- Glucose is then converted into other carbohydrates
![]()
![]()
Question 2.
What is muta rotation?
Answer:
- The specific rotation of β – and (3- D glucose are 1120 and 18.70 respectively.
- When pure form of any one of these sugars is dissolved in water, slow inter conversion of α- D Glucose and (β – D Glucose via open chain structure occurs until equilibrium is established with constant specific
- rotation + 530.
- This phenomenon is called muta rotation.
Question 3.
What are epimers? What is epimerisation?
Answer:
- Sugars differing in configuration at an asymmetric centre are known as epimers.
- The process by which one epimer is converted into other is called epimerisation.
- It requires the enzymes epimerase.
- (ex) Galactose is converted into glucose by this manner in our body.
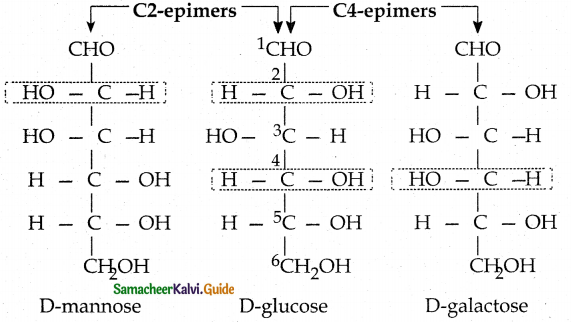
Epimers: glucose and mannose are epimers at C2 carbon and glucose and galactose are epimers at C4 carbon
![]()
Question 4.
What is inversion of sucrose?
Answer:
- Fructose is obtained from sucrose by heating with dilute H2SO4 or with the enzyme invertase
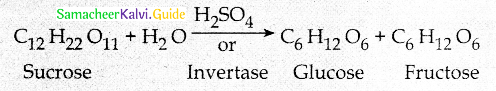
- The solution having equal amount of Glucose and Fructose is termed as invert sugar and the process is known as inversion of sucrose.
Question 5.
What happens when Inulin is hydrolyzed?
Answer:
Fructose is prepared commercially by the hydrolysis of Inuiin in acidic medium
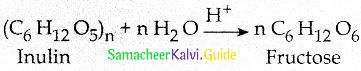
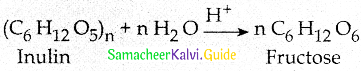
![]()
Question 6.
Why sucrose is called as invert sugar?
Answer:
- On hydrolysis sucrose yields equal amount of glucose and fructose units.
![]()
- Sucrose ( + 66.60) and glucose (+ 52.50) are dextrorotatory.
- Fructose is levorotatory (- 92.40)
- During hydrolysis of sucrose the optical rotation of the reaction mixture changes from dextro to levo.
- Hence, sucrose is called invert sugar.
Question 7.
Write about the structure of sucrose?
Answer:
- In sucrose C – 1 of α – D – Glucose is joined to C – 2 of β- D fructose
- The glycosidic bond thus formed is called a -1, 2- glycosidic bond.
- Since both the carbonyl carbons (reducing groups) are involved in the glycosidic bonding, sucrose is a non – reducing sugar.
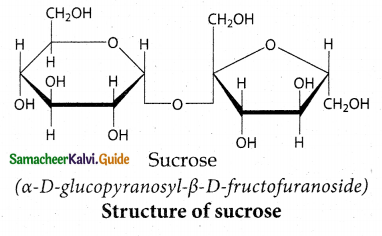
![]()
Question 8.
Write notes on lactose.
Answer:
- Lactose is a disaccharide which on hydrolysis yields galactose and glucose.
- It is found in the milk of mammals and hence called as milk sugar.
- The β-D- Galactose and β -D-Glucose are linked by b-1, 4 – glycosidic bond.
- The aldehyde carbon is not involved in the glycosidic bond.
- Hence it retains its reducing property and is called a reducing sugar.
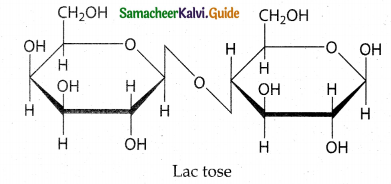
(β-D-galactopyranosyl-( 1 → 4) β-D-glucopyranose)
Structure of Lactose
Question 9.
Write a note on maltose?
Answer:
- Maltose is extracted from malt and hence called as malt sugar.
- Malt from sprouting barley is the major source of maltose.
- Maltose is produced during digestion of starch by the enzyme a- amylase.
- Maltose consists of two units of α -D-glucose linked by an α -1, 4 – glycosidic bond between anomeric carbon of one unit and C – 4 of the other unit.
- Since one of the glucose has the aldehydic group intact it acts as a reducing sugar.
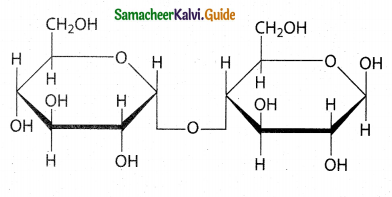 Maltose
Maltose
(α -D-glucopymnosyl-( 1 → 4) α -D-glucopyranose)
Structure of Maltose
![]()
Question 10.
What are Polysaccharides? How are they classified?
Answer:
- Polysaccharides consist of largo number of monosaccharide units bonded together by glycosidic bonds.
- They are tire most common form of carbohs drates.
- They do not have sweet taste and are called as non – sugars.
- They form linear and branched chain molecules.
- They are of two types.
i) Homopolysaccharides :
They are composed of only one type of monosaccharides, (ex) Starch, cellulose, glycogen.
ii) Hetero polysaccharide:
They are composed of more than one type of monosacchardes. (ex) hyaluronic acid, heparin.
Question 11.
Write about starch.
Answer:
- Starch is used for energy storage in plants.
- Potatoes, corn, wheat and rice are the rich sources of starch.
- It is a polymer of glucose in which glucose molecules are linked by α-(1, 4) – glycosidic bonds.
- Starch can be separated into twro fractions namely water soluble amylose and water insoluble amylopectin.
- Starch contains 20% of amylose and about 80% of amylopectin.
- Amylose is composed of unbranched chains upto 4000 α-D-Glucose molecules joined by α -(1, 4) – glycosidic bonds.
- Amylopectin contains chains upto 10000 α- D-Glucose molecules linked by a-(1, 4) – glycosidic bonds.
- In addition, there is a branching from linear chain.
- At branch points, new chains of 24 to 30 glucose molecules are linked by α -(1, 6)- glycosidic bonds.
- With iodine solution amylose give blue colour and amylopectin gives a purple colour.
![]()
Question 12.
Write briefly about cellulose.
Answer:
- Cellulose is the major constituent of plant cell walls.
- Cotton is almost pure cellulose.
- On hydrolysis cellulose gives D- glucose molecules.
- Cellulose is a straight chain polysaccharide.
- Glucose molecules are linked by (3 (1, 4) glycosidic bond.
- Cellulose is used in the manufacture of paper., cellulose fibres, rayon, explosive gun cotton.
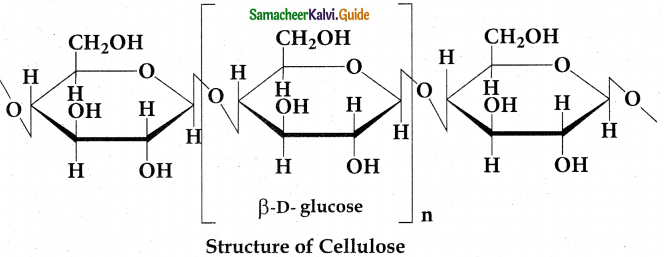
Question 13.
Write a short notes on glycogen.
Answer:
- Glycogen is the storage polysaccharide of animals.
- It is present in the liver and muscles of animals.
- Glycogen is also called as animal starch.
- On hydrolysis it, gives glucose molecules.
- Structurally, glycogen resembles amylopectin with more branching.
- In glycogen the branching occurs every 8 – 14 glucose units opposed to 24 – 30 units in amylopectin.
- The excessive glucose in the body is stored in the form of glycogen
![]()
Question 14.
What are essential and non – essential amino acids?
Answer:
| Essential amino acids | Non Essential amino acids |
| 1. These cannot be synthesised by our body and must be supplied through diet | These can be synthesised by our body |
| 2. (ex) Phenyl alanine, Valine, Threonine | (ex) Glycine, Alanine, Glutamic acid |
Question 15.
What is isoelectric point?
Answer:
The pH value at which the net charge of an amino acid is neutral is called isoelectric point.
Question 16.
What are Zwitter ions?
Answer:
- In aqueous solution the proton from carboxyl group can be transferred to the amino group of an amino acid leaving these groups with opposite charges.
- Despite having both positive and negative charges this molecule is neutral and has amphoteric behaviour.
- These ions are called Zwitter ions.
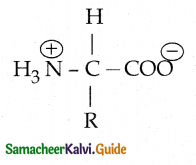
![]()
Question 17.
What are proteins? How are they classified?
Answer:
- Proteins are polymers of amino acids.
- They are classified into two major types based on their structure.
| Fibrous Proteins | Globular Proteins |
| i) Linear molecules similar to fibres. | Have an overall spherical shape |
| ii) Generally insoluble in water | Usually soluble in water |
| iii) Held together by disulfide bridges and weak inter molecular hydrogen bonds | Have many functions including catalysis
|
| iv) (ex) Keratin, Collagen | (ex) enzymes, myoglobin, insulin |
Question 18.
What is complementary base pairing in DNA?
Answer:
- In the double helix of DNA each base is hydrogen bonded to a base in opposite strand to form a planar base pair.
- Two hydrogen bonds are formed between adenine and thymine.
- Three hydrogen bonds are formed between guanine and cytosine.
- This specific association of the two chains of the double helix is known as complementary base pairing.
- Other pairing tends to destabilize the double helical structure.
![]()
Question 19.
Name the biological functions of nucleotides.
Answer:
- Energy carriers (ATP)
- Components of enzyme cofactors (co- enzyme A, NAD+, FAD)
- Chemical messengers (cyclic AMP, cAMP)
Question 20.
Name the vitamins whose deficiency cause
i) Pellagra
ii) Beri – Beri
iii) Night blindness
Answer:
- Pellagra (photo sensitive dermatitis) – Vitamin B3 (Niacin)
- Ben – Ben – Vitamin B1 (Thiamine)
- Night blindness – Vitamin A (Retinol)
Question 21.
What is glycosidic linkage?
Answer:
- The oxide linkage by which two monosaccharides are linked in disaccharides is called as a
glycosidic linkage. - A glycosidic linkage is formed by the reaction of the hydroxyl group of the anomeric carbon of one
mono saccharide with a hydroxyl group of another mono saccharide.
![]()
VII. Three Mark Questions
Question 1.
Write about the configuration of carbohydrates.
Answer:
- Almost all carbohydrates are optically active as they have one or more chiral carbon.
- Number of optical isomers = 2n (where n = no of chiral carbons)
- Fischer has devised a projection formula to relate the structure of a carbohydrate to one
of the two enatiomeric forms of glyceraldehyde.
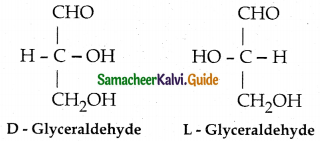
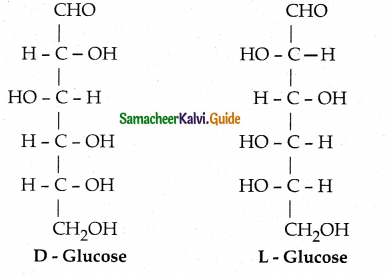
- Based on the above structures, carbohydrates are named as D or L.
- For example D – Glucose is so named because the H and OH on C5 carbon are in the same configuration as H and OH on C2 carbon is D – glyceraldehyde.
- Dextro rotatory compounds rotate the plane of plane polarised light in clockwise direction and represented as (+)
- Laevo rotatory compounds rotate the plane of plane polarised light in anticlockwise direction and represented as (-).
- D or L isomers can be dextro or laevo rotatory.
- Dextro rotatory compounds are represented as D (+) or L (+) and the laevo rotatory compounds as D (-) or L ( -).
Question 2.
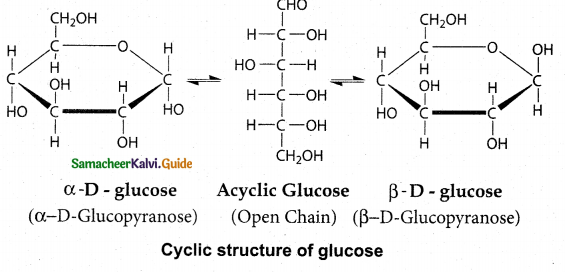
Explain the cyclic structure of glucose.
Answer:
- Glucose was found to crystallise in two different forms depending upon the crystallisation conditions with different melting points (419 and 423 K).
- In order to explain these it was proposed that one of the hydroxyl group reacts with the aldehyde group to form a cyclic structure (hemiacetal form) as shown in figure.
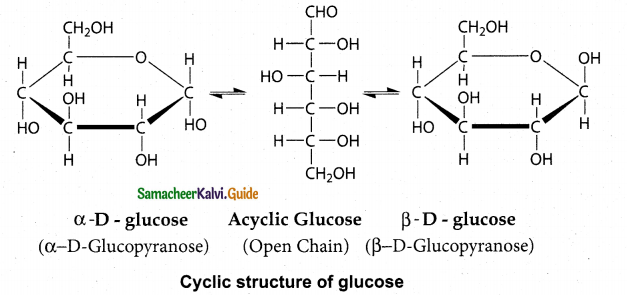
- This also results in the conversion of the achiral aldehyde carbon into a chiral one with the possibility of two isomers.
- These two isomers which differ only in the configuration of C1 carbon are called anomers.
- The two anomeric forms of glucose are called α – and β – forms.
- This cyclic structure is similar to pyran, a cyclic compound with 5 carbon and one oxygen atom, and hence is called pyranose form.
- The specific rotation of α- and (β- (D) Glucose are 1120 and 18.70 respectively.
- When pure form of any one of these sugars is dissolved in water, slow interconversion of α- D Glucose and β- D Glucose via open chain structure occurs until equilibrium is established with constant specific rotation +530
- This phenomenon is called mutarotation.
![]()
Question 3.
Write about the cyclic structure of fructose.
Answer:
- Like glucose, fructose also forms a cyclic structure.
- Unlike glucose, fructose forms a five membered ring similar to furan.
- Hence it is called furanose form.
- When fructose is a component of a saccharide as in sucrose, it usually occurs in furanose form.
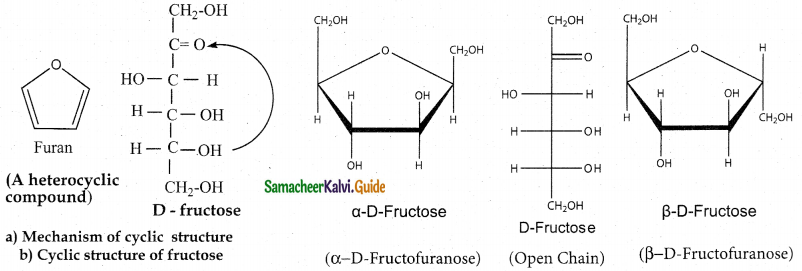
Question 4.
What is a peptide bond? How is it formed?
Answer:
- The carboxyl group of the first amino acid react with the amino group of the second amino acid to give an amide linkage between these amino acids.
- This amide linkage is called peptide bond.
- The resulting compound is called a dipeptide.
- Peptide bond is

(ex) Glycine and alanine combine to form glycyl alanine a dipeptide through one peptide bond.
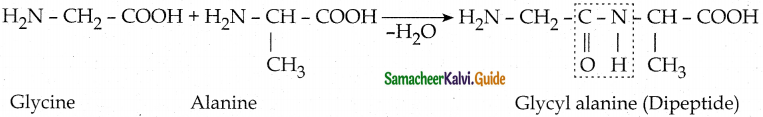
![]()
Question 5.
Differentiate tertiary and quaternary structure of proteins
Answer:
|
Tertiary structure of proteins |
Quaternary structure of proteins |
| i. Secondary structure elements a-helix and b-sheets further folds to form the three dimensional structure called tertiary structure | In some proteins with more than one polypeptide chain the individual poh peptide units interact with eac li other to form multimeric structure known as quaternary structure. |
| ii. Stabilised by interactions between the side chains of the amino acids. | (ex) haemoglobin contains four polypeptide chains. |
| iii. nteractions include the disulfide bridges between cysteine residues, electrostatic, hydrophobic, hydrogen bonds and vanderwaals interactions
|
Iinteractions include the disulfide bridges between cysteine residues, electrostatic,
hydrophobic, hydrogen bonds and vender waals interactions.
|
Question 6.
What are lipids? How are they classified?
Answer:
- The word lipids is derived from the Greek word ‘lipos’ meaning fat.
- Lipids are organic molecules that are soluble in organic solvents such as chloroform and methanol.
- Lipids are insoluble in water.
- They are the principal components of cell membranes including cell walls.
- They act as energy source for living systems.
- Fat, a lipid provide 2-3 fold higher energy compared to carbohydrates or proteins.
Types : Simple lipids, compound lipids and derived lipids.
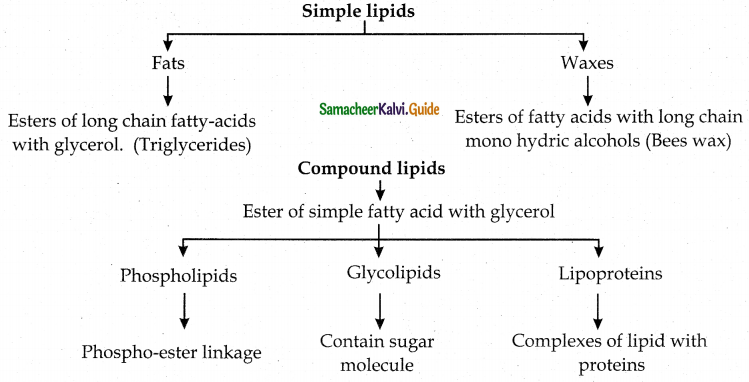
![]()
Question 7.
How are hormones classified?
Answer:
Hormones are classified according to the distance over which they act as endocrine, paracrine and autocrine hormones.
| Endocrine Hormones | Paracrine Hormones | Autocrine Hormones |
| Act on cells distant from the site of their release | Act only on cells close to the cell that released them | Act on the same cell that released them |
| (ex) Insulin, epinephrine | Interleukin -1 (IL-1) | Interleukin – 2 (IL – 2) |
VIII. Five Mark Questions
Question 1.
What are carbohydrates? How are they classified?
Answer:
- Carbohydrates are defined as polyhydroxy aldehydes or Ketones.
- General formula Cn(H2O)nCarbohydrates
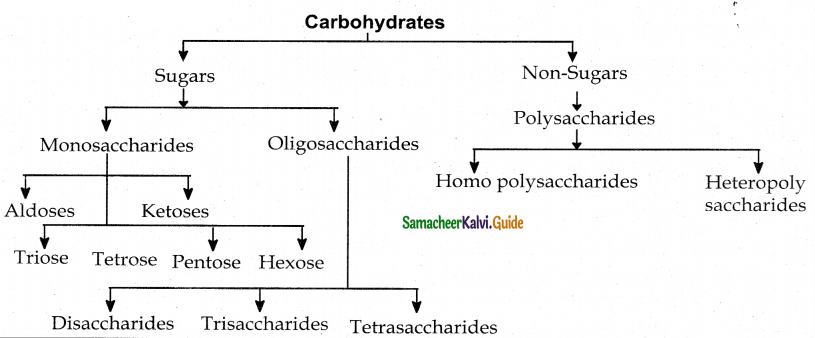
Monosaccharides : These are simple sugars that cannot be hydrolysed further.
Aldoses : Contain aldehyde functional group (ex) Glucose
ketoses : Contain Ketone functional group (ex) Fructose
| Trioses | Tetrose | Pen tose | Hexose |
| 3C – atoms | 4C – atoms | 5C – atoms | 6C – atoms |
Disaccharides : On hydroysis give two monosaccharides. (ex) Sucrose.
Polysaccharides : On hydrolysis give large number of monosaccharides. (ex) Starch.
Homo polysaccharides : Consist of only one type of monosaccharides. (ex) Starch.
Hetero polysaccharides: Consist of more than one type of monosaccharides. (ex) heparin.
![]()
Question 2.
Elucidate the structure of glucose.
Answer:
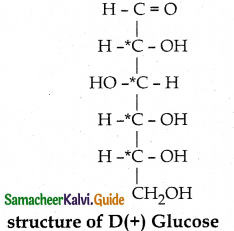
- The molecular formula of glucose is C6H1206.
- On reduction with conc.HI and red phosphorus at 373K, glucose gives a mixture of n-hexane and 2-
iodohexane. This indicates that the six carbon atoms are bonded linearly.
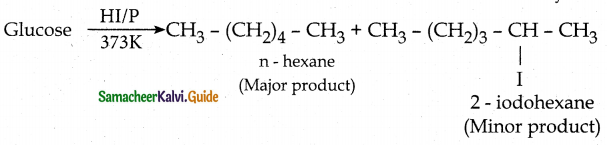
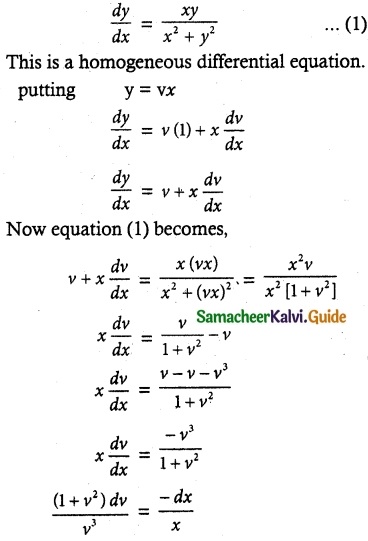
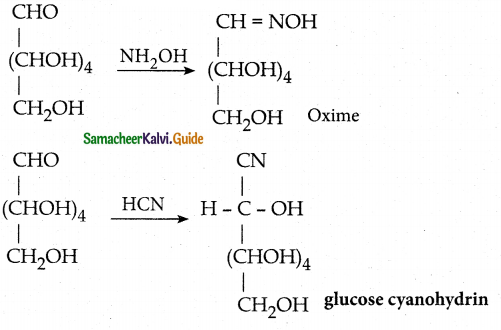
- Glucose reacts with hydroxyl amine forms oxime, with HCN to form cyanohydrin. This shows the presence of carbonyl of group
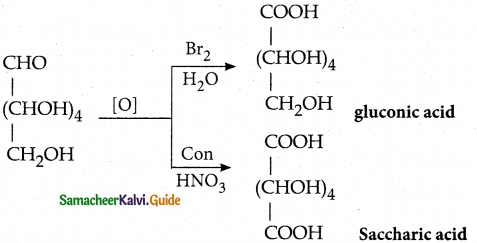
- Glucose is oxidîzed by bromine water to form glucoriic acid. This suggests that glucose contains an aldehyde
group. With strong oxidising agent like con.HNO3 Saccharic acid is obtained. This shows the presence of primary alcoholic group at one end
- Glucose reduces Tollens reagent, and Fehling’s solution – Hence it contains an aldehyde group.
- Glucose forms penta acetate with acetic anhydride. This shows the presence of – OH groups in glucose.
- Glucose is a stable compound and does not undergo dehydration. This shows the presence of 5 – OH groups on different carbons.
- The glucose is referred to as D(+) glucose.
- Thus the structure of D(+) Glucose can be written as
![]()
Question 3.
Elucidate the structure of fructose.
Answer:
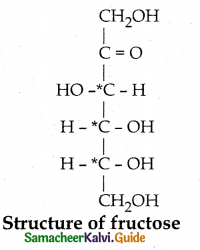
- The molecular formula of fructose is C6H12O6.
- On reduction with HI and red phosphorus gives a mixture of n-hexane and 2-iodohexane. This indicates that the six carbon atoms are bonded linearly.
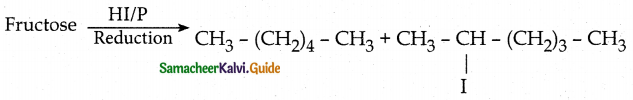
- Fructose reacts with NH2OH and HCN to form Oxime and cyanohydrin respectively. This shows the presence of carbonyl group.
- Fructose reacts with acetic anhydride in the presence of pyridine to form pentaacetate. This shows the presence of five hydroxyl groups.
- Fructose is not oxidised by bromine water. This shows the absence of aldehyde group.
Fructose reduced with Sodium amalgam and water. - Partial reduction with sodium amalgam and water gives epimeric alcohols sorbitol and mannitol. New asymmetric carbon is formed at C2. This confirms the presence of Keto group at C2.
Oxidation with nitric acid gives glycollic acid and tartaric acid which contain
smaller number of carbon atoms than in fructose.
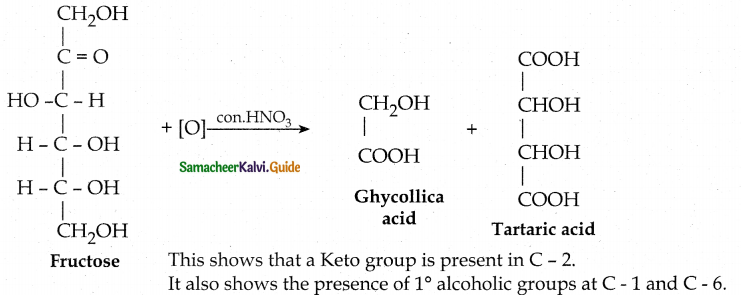
![]()
Question 4.
Explain the composition and structure of nucleic acids.
Answer:
- Particles in nucleus of the cell are responsible for the transmission of inherent characteristics of each and every species form one generation to the next.
- They are called chromosomes.
- They are made up of proteins and a type of molecules called nucleic acids.
- Nucleic acids are biopolymers of nucleotides.
- They are of two types (i) deoxyribonucleic acid (DNA) (ii) ribonucleic acid (RNA).
- They are the molecular repositories that carry genetic information in every organism.
Composition and structure :
Controlled hydrolysis of DNA and RNA yields three components namely a nitrogenous base, a pentose sugar and phosphate group.
Nitrogen base:
These are organic nitrogen compounds which are derivatives of two parent compounds, pyrimidine and purine.
| DNA | RNA |
| Contains purine bases adenine (A) and guanine (G) | Contains purine bases adenine (A) and guanine (G) |
| Contains pyrimidine bases cytosine (C) and thymine (T) | Contains pyrimidine bases cytosine (C) and uracil (U) |
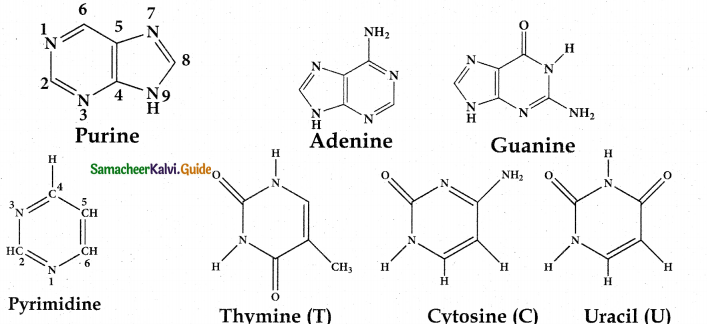
Pentose Sugar:
- DNA contains 2 – deoxy – D – ribose.
- RNA contains D – ribose.
- In nucleotides both the types of pentoses are in their b- Furanose (closed five membered ring) form.
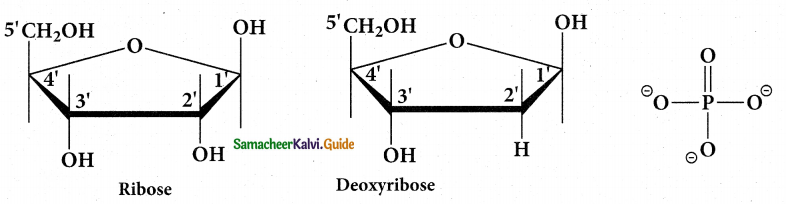
Phosphate group:
- Phosphoric acid forms phosphor diester bond between nucleotides.
- Based on the number of phosphate group present in the nucleotides, they are classified into mono nucleotide, dinucleotide and trinucleotide.
Nucleosides and nucleotides :
- The molecule without the phosphate group is called a nucleoside.
- A nucleotide is derived from a nucleoside by the addition of a molecule of phosphoric acid.
- Phosphorylation occurs generally in the 5′ OH group of the sugar.
- Nucleotides are linked in DNA and RNA by phospho diester bond between 5′ OH group of one nucleotide and 3′ OH group on another nucleotide.
Sugar + base → Nucleoside
Nucleoside + Phosphate → Nucleotide
n Nucleotide → Polynucleotide (Nucleic acid)
![]()
Question 5.
Explain the double strand helix structure of DNA.
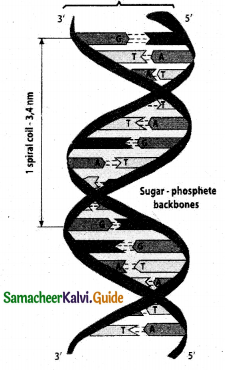
- The structure elucidation of DNA by Watson and Crick in 1953 was a momentous event in science.
- They postulated a 3 – dimensional model of DNA structure.
- This model consisted of two anti-parallel helical DNA chains wound around the same axis to form
a right – handed double helix. - The hydrophilic back bones of alternating deoxyribose and phosphate groups are on the outside of the double helix, facing the surrounding water.
- The purine and pyrimidine bases of both strands are stacked inside the double helix, with their hydrophobic and ring structures very close together and perpendicular to the long axis.
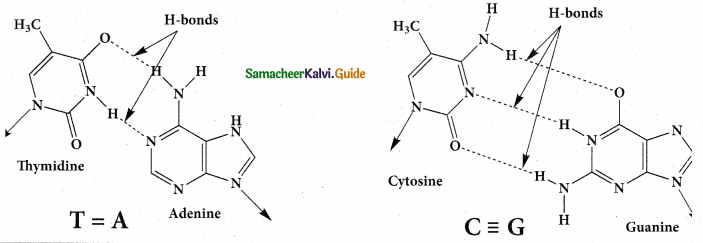
- This reduces the repulsions between the charged phosphate groups.
- The offset pairing of the two strands creates a major groove and minor groove on the surface of the duplex.
- This model revealed that, there are 10.5 base pairs (36A0) per turn of the helix and 3.4 AO between the stacked bases.
- Each base is hydrogen bonded to a base in opposite strand to form a planar base pair.
- Two hydrogen bonds are formed between adenine and thymine.
- Three hydrogen bonds are formed between guanine and cytosine.
- Other pairing tends to destabilise the double helical structure.
- This specific association of the two chains of the double helix is known as complementary base pairing.
- The DNA double helix or duplex is held together by two forces.
a) Hydrogen bonding between complementary base pairs.
b) Base – stacking interactions.
![]()
Question 6.
What are the differences between DNA and RNA?
Answer:
| DNA | RNA |
| Double-stranded molecules | Single-stranded molecules |
| Its lifetime is high | It is short-lived |
| It is stable and not hydrolysed easily by alkalis | It is unstable and hydrolyzed easily by alkalis |
| It can replicate itself | It is formed from DNA. |
Question 7.
Explain DNA fingerprinting.
Answer:
- DNA fingerprinting was first invented by Sir Alec Jeffreys in 1984.
- The DNA fingerprint is unique for every person.
- It can be extracted from traces of samples from blood, saliva, hair, etc.
- By using this method the individual-specific variation in human DNA can be detected.
- The extracted DNA is cut at specific points along the strand with restriction enzymes.
- This results in the formation of DNA fragments of varying lengths which were analysed by a technique called gel electrophoresis.
- This method separates the fragments based on their size.
- The gel containing the DNA fragments are then transferred to a nylon sheet using a technique called blotting.
- Then the fragments will undergo auto-radiography in which they were exposed to DNA probes (pieces of synthetic DNA that were made radioactive and that bound to the fragments)
- A piece of X-ray film was then exposed to the fragments, and a dark mark was produced at any point where a radioactive probe had become attached.
- The resultant pattern of marks could then be compared with other samples.
- DNA fingerprinting is based on slight sequence differences (usually single base – pair changes) between individuals.
- These methods are proving decisive in court cases worldwide