Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Chemistry Guide Pdf Chapter 3 p-Block Elements – II Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 3 p-Block Elements – II
12th Chemistry Guide p-Block Elements – II Text Book Questions and Answers
Part – I – Text Book Evaluation
I. Choose the correct answer
1. In which of the following, NH3 is not used?
a) Nessler’s reagent
b) Reagent for the analysis of IV group basic radical
c) Reagent for the analysis of III group basic radical
d) Tollen’s reagent
Answer:
a) Nessler’s reagent
2. Which is true regarding nitrogen?
a) least electronegative element
b) has low ionisation enthalpy than oxygen
c) d – orbitals available
d) ability to form pπ -pπ bonds with itself
Answer:
d) ability to form pπ -pπ bonds with itself
3. An element belongs to group 15 and 3rd period of the periodic table, its electronic configuration would be
a) Is² 2s² 2p4
b) Is² 2s² 2p³
c) Is² 2s² 2p6 3s2² 3p²
d) Is² 2s² 2p6 3s² 3p³
Answer:
d) Is² 2s² 2p6 3s² 3p³
4. Solid (A) reacts with strong aqueous NaOH liberating a foul smelling gas(B) which spontaneously burn in air giving smoky rings. A and B are respectively
a) P4(red) and PH3
b) P4 (white) and PH3
c) S8 and H2S
d) P4(white) and H2S
Answer:
b) P4 (white) and Ph3
5. On hydrolysis, PCl3 gives
a) H3PO3
b) PH3
c) H3PO4
d) POCl3
Answer:
a) H3PO3
6. P4O6 reacts with cold water to give
a) H3PO3
b) H4P2O7
c) HPO3
d) H3PO4
Answer:
a) H3PO3
![]()
7. The basicity of pyrophosphorous acid (H4P2O3) is
a) 4
b) 2
c) 3
d) 5
Answer:
b) 2
8. The molarity of given orthophosphoric acid solution is 2M. Its normality is
a) 6N
b) 4N
c) 2N
d) none of these
Answer:
a) 6N
Normality = M x basicity =2×3 = 6
9. Assertion : bond dissociation energy of fluorine is greater than chlorine gas
Reason : chlorine has more electronic repulsion than flourine
a) Both assertion and reason are true and reason is the correct explanation of assertion.
b) Both assertion and reason are true bu t reason is not the correct explanation of assertion
c) Assertion is true bu t reason is false
d) Both assertion and reason are false
Answer:
d) Both assertion and reason are false The converse is true
10. Among the following, which is the strongest oxidizing agent?
a) Cl2
b) F2
C) Br2
d) l2
Answer:
b) F2
11. The correct order of the thermal stability of hydrogen halide is (PTA – 4)
a) Hl > HBr > HCl > HF
b) HF > HCl > HBr > HI
c) HCl > HF > HBr > HI
d) HI > HCl > HF > HBr
Answer:
b) HF > HCl > HBr > HI
12. Which one of the following compounds is not formed?
a) XeOF4
b) XeO3
c) XeF2
d) NeF2
Answer:
d) NeF2
13. Most easily liquefiable gas is
a) Ar
b) Ne
c) He
d) Kr
Answer:
c) He
![]()
14. XeFg on complete hydrolysis produces
a) XeOF4
b) XeO2F2
c) XeO3
d) XeO2
Answer:
c) XeO3
15. Which of the following is strongest acid among all?
a) HI
b) HF
c) HBr
d) HCl
Answer:
a) HI
Reason : H-I bond is weakest.
16. Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules? (NEET)
a) Br2 > I2 > F2 > Cl2
b) F2 > Cl2 > Br2 > I2
c) I2 > Br2 > Cl2 > F2
d) Cl2 > Br2 > F2 > I2
Answer:
d) Cl2 > Br2 > F2 > I2
17. Among the following the correct order of acidity is (NEET)
a) HClO2 < HClO < HClO3 < HClO4
b) HClO4 < HClO2 < HClO < HClO3
c) HClO3 < HClO4 < HClO2 < HClO
d) HClO < HClO2 < HClO3 < HClO4
Answer:
d) HClO < HClO2 < HClO3 < HClO4
18. When copper is heated with cone HNOs it produces
a) Cu (NO3)2, NO and NO2
b) Cu (NO3)2 and N2O
c) Cu (NO3)2 and NO2
d) Cu (NO3)2 and NO
Answer:
c) Cu (NO3)2 and NO2
II. Answer the following questions.
Question 1.
What is the inert pair effect?
Answer:
In p-block elements, as we go down the group, two electrons present in the valence s-orbital become inert and are not available for bonding (only p-orbital involves chemical bonding). This is called inert pair effect.
Question 2.
Chalcogens belong to p-block.
Answer:
Give reason.
- Chalcogens belong to p-block elements.
- Because their outer electronic configuration is ns² np4.
- In these elements the last electron enters np orbital.
- Hence they belong to p-block elements.
![]()
Question 3.
Explain why fluorine always exhibits an oxidation state of -1?
Answer:
Fluorine the most electronegative element than other halogens and cannot exhibit any positive oxidation state. Fluorine does not have a d-orbital while other halogens have d-orbitals. Therefore fluorine always exhibits an oxidation state of-1 and others in the halogen family shows +1, +3, +5 and +7 oxidation states.
Question 4.
Give the oxidation state of halogen in the following
Answer:
a) OF2
b) O2F2
c) Cl2O3
d) I2O4
| Halogen | Oxidation State |
| OF2 | -1 |
| O2F2 | -1 |
| Cl2O3 | +3 |
| I2O4 | +4 |
Question 5.
What are interhalogen compounds? Give examples
Answer:
Each halogen combines with other halogens to form a series of compounds called interhalogen compounds. For example, Fluorine reacts readily with oxygen and forms difluorine oxide (F2O) and difluorine dioxide (F2O2).
Question 6.
Why fluorine is more reactive than other halogens? (PTA – 1, 3)
Answer:
Fluorine is the most reactive element among halogens. This is due to the low value of F – F bond dissociation energy.
Question 7.
Give the uses of helium. (PTA – 2)
Answer:
- Helium and oxygen mixture is used by divers in place of air oxygen mixture. This prevents the painful dangerous condition called bends.
- Helium is used to provide an inert atmosphere in the electric arc welding of metals
- Helium has the lowest boiling point hence used in cryogenics (low-temperature science).
- It is much less denser than air and hence used for filling air balloons.
8. What is the hybridisation of iodine in IF7? Give its structure. (PTA – 5)
Answer:
| Inter halogen | Hybridisation | Structure |
| IF7 | Sp3d3 | Pentagonal bipyramidal |
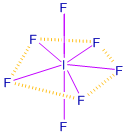
9. Give the balanced equation for the reaction between chlorine with cold NaOH and hot NaOH
Answer:
Chlorine reacts with cold NaOH to give sodium hypochlorite
![]()
Chlorine reacts with hot NaOH to give sodium chlorate
![]()
10. How will you prepare chlorine in the laboratory? (PTA – 2)
Answer:
1. Chlorine is prepared by the action of cone, sulphuric acid on chlorides in presence of manganese dioxide.
4NaCl + MnO2 + 4H2SO4 → Cl2 + MnCl2 + 4NaHSO4 + 2H2O
2. It can also be prepared by oxidising hydrochloric acid using various oxidising agents such as manganese dioxide, lead dioxide, potassium permanganate or dichromate.
PbO2 + 4HCl → PbCl2 + 2H2O + Cl2
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
2KMnO4 + 16HCl → 2KCl + 2MnCl + 8H2O + 5Cl2
K2Cr2O7 + 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
3. When bleaching powder is treated with mineral acids chlorine is liberated
CaOCl2 + 2HCl → CaCl2 + H2O + Cl2
CaOCl2 + H2SO4 → CaSO4 + H2O + Cl2
![]()
11. Give the uses of sulphuric acid.
Answer:
Sulphuric acid is used
- In the manufacture of fertilisers, ammonium sulphate and superphosphates.
- In the manufacture of other chemicals such as hydrochloric acid, nitric acid etc.,
- as a drying agent.
- in the preparation of pigments, explosives, etc.,
12. Give a reason to support that sulphuric acid is a dehydrating agent. (PTA – 1)
Answer:
- Sulphuric acid is highly soluble in water.
- It has a strong affinity towards water.
- Hence it can be used as a dehydrating agent.
- When dissolved in water, it forms mono (H2SO4.H2O) and dihydrates (H2SO4.2H2O) and the reaction is exothermic.
ex
C12H22O11 + H2SO4 → 12C + H2SO4.11H2O
HCOOH + H2SO4 → CO + H2SO4.H2O
13. Write the reason for the anomalous behaviour of Nitrogen.
Answer:
1. Due to its small size, high electronegativity, high ionisation enthalpy and absence of d-orbitals.
2. N, has a unique ability to form pπ – pπ multiple bond whereas the heavier members of this group (15) do not form pπ – pπ bond, because their atomic orbitals are so large and diffused that they cannot have effective overlapping.
3. Nitrogen exists a diatomic molecule with triple bond between the two atoms whereas other elements form single bond in the elemental state.
4. N cannot form dπ – pπ bond due to the absence of d – orbitals whereas other elements can.
14. Write the molecular formula and structural formula for the following molecules
Answer:
a) Nitric add
b) dinitrogen pentoxide
c) phosphoric acid
d) phosphine
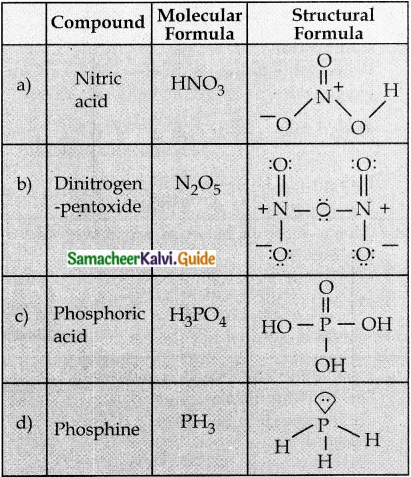
15. Give the uses of argon.
Answer:
Argon prevents the oxidation of hot filament and prolongs the life in filament bulbs.
16. Write the valence shell electronic configuration of group -15 elements.
Answer:
Valence shell electronic configuration of group 15 elements is ns²np³
| Elements | Valence Shell Electronic configuration |
| N | 2s2p3 |
| P | 3s2p3 |
| S | 4s24p3 |
| Sb | 5s25p3 |
| Bi | 6s2p3 |
17. Give two equations to illustrate the chemical behaviour of phosphine.
Answer:
Basic Nature :
Phosphine is weakly basic and forms phosphonium salts.
PH3 +HI → PH4 I
PH4I + H2O \(\underrightarrow { \triangle } \) PH3 + H3O+ + I–
It react with halogen to give phosphorous penta halidae
PH3 +4Cl2 → PCl5 + 3HCl
Combustion:
When phosphine is heated with air or oxygen it burns to give metaphosphoric acid
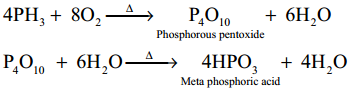
18. Give a reaction between nitric acid and a basic oxide.
Answer:
Nitric acid rects with a basic oxide to form salt and water.
3 FeO + 10HNO3 → Fe (NO3)3 + NO + 5H2O
![]()
19. What happens when PCl5 is heated?
Answer:
On heating Phoshorous pentachloride decomposes into phosphorus trichloride and chlorine
![]()
20. Suggest a reason why HF is a weak acid, whereas binary acids of all other halogens are strong acids.
Answer:
- HF is only slightly ionised, hence it is a weak acid
- Other halogen acids are almost completely ionised, hence they are strong acids.
- Among halogen acids, the electronegativity difference is maximum (1.9) in HF acid.
- Hence the bond between H and F is stronger and the acid HF is weaker.
21. Deduce the oxidation number of oxygen in hypofluorous acid – HOF.
Answer:
Oxidation number of F = -1
Oxidation number of H = +1
Oxidation number of O in HOF = x
(+1) + x + (-1) = 0
x = 0
Oxidation number of O in HOF = 0
22. What type of hybridisation occur in (PTA – 5)
a) BrF5
b) BrF3
23. Complete the following reactions
1. NaCl+ MnO2 + H2SO4 →
2. NaNO2 + HCl →
3. P4 + Na0H + H2O →
4. AgNO3 + PH3 →
5. Mg + HNO3 →
8. Sb + Cl2 →
9. HBr + H2SO4 →
10. XeF6 + H2O →
11. XeO64- + Mn2+ + H+ →
12. XeOF4 + SiO2 →
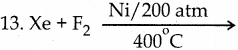
Answer:
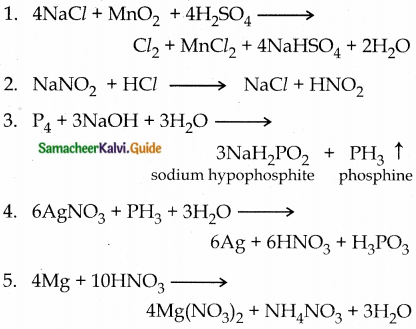
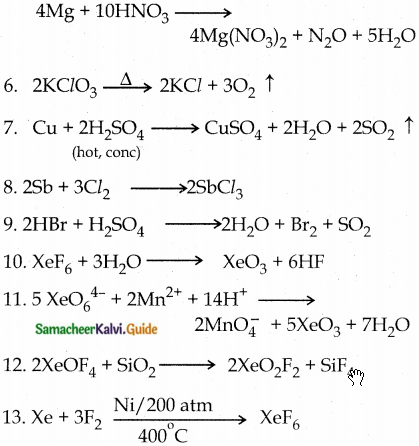
III. Evaluate yourself
1. Write the products formed in the reaction of nitric acid (both dilute and concentrated) with zinc.
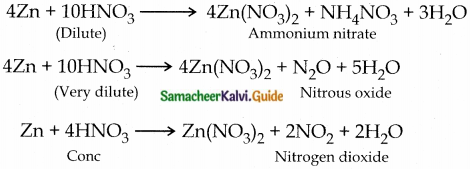
12th Chemistry Guide p-Block Elements – II Additional Questions and Answers
Part II – Additional Questions
I. Match the following
1.
| Compound | The oxidation state of Nitrogen |
| i) NH3 | +2 |
| ii) N22 | +5 |
| ii) N2O | +4 |
| iv) NO | +3 |
| v) HNO2 | 0 |
| vi) NO2 | +1 |
| vii) HNO3 | -3 |
Answer:
| Compound | Oxidation state of Nitrogen |
| i) NH3 | -3 |
| ii) N22 | 0 |
| ii) N2O | +1 |
| iv) NO | +2 |
| v) HNO2 | +3 |
| vi) NO2 | +4 |
| vii) HNO3 | +5 |
2.
| Name | Molecular Formula |
| i) Sulpurous acid | H2S2O8 |
| ii) Thiosulphuric acid | H2S2O7 |
| iii) Pyrosulphuric acid | H2S2O6 |
| iv) Marshall’s acid | H2SO3 |
| v) Dithionic acid | H2S2O3 |
Answer:
| Name | Molecular Formula |
| i) Sulpurous acid | H2SO3 |
| ii) Thiosulphuric acid | H2S2O3 |
| iii) Pyrosulphuric acid | H2S2O7 |
| iv) Marshall’s acid | H2S2O8 |
| v) Dithionic acid | H2S2O6 |
3.
| Inter Halogen compound | Hybridisation |
| i) IF5 | Sp3d3 |
| ii) BeF3 | Sp3 |
| iii) IF7 | Sp3d2 |
| iv) ClF | Sp3d |
Answer:
| Inter Halogen compound | Hybridisation |
| i) IF5 | Sp3d2 |
| ii) BeF3 | Sp3d |
| iii) IF7 | Sp3d3 |
| iv) ClF | Sp3 |
4.
| Compound | Structure |
| XeOF2 | Linear |
| XeO3 | Square planar |
| XeF | Pyramidal |
| XeOF4 I | Distorted octahedron |
| XeF4T | T shaped |
| XeP6 | Square pyramidal |
Answer:
| Compound | Structure |
| XeOF2 | T shaped |
| XeO3 | Pyramidal |
| XeF | Linear |
| XeOF4 | Square pyramidal |
| XeF4T | Square planar |
| XeP6 | Distorted octahedron |
II. Assertion and Reason
i) Both A and R are correct, R explains A.
ii) A is correct, R is wrong
iii) A is wrong, R is correct
iv) Both A and R are correct, but R does not explain A.
1. Assertion (A) : Aqueous solution of potash Alum is acidic
Reason (R) : Aluminium sulphate undergo hydrolysis. (PTA – 2)
Answer:
i) Both A and R are correct, R is explanation of A
2. Assertion (A) : Elements belonging to group 16 are called chalcogens
Reason (R) : Group 16 elements are saltforming elements
Answer:
ii) A is correct, R is wrong
Correct Reason : Group 16 elements are ore forming elements
3. Assertion (A) : Among halogen acids, HF has low melting and boiling points
Reason (R) : In HF hydrogen bond is present.
Answer:
iii) A is wrong, R is correct.
Correct A : Among halogen acids HF has high melting and boiling points.
4. Assertion (A) : A small piece of Zinc dissolved in dilute nitric acid but hydrogen gas is not evolved. (PTA – 3)
Reason (R) : HNO3 is an oxidising agent and this oxidizes hydrogen.
Answer:
ii) A is correct but R is wrong.
III. Pick out the Correct statement
1. i) Oxygen is diamagnetic
ii) Oxygen forms hydrogen bonds
iii) Oxygen exists in two allotropic forms
iv) Oxygen exists as a triatomic gas
a) (i) &(ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statement: (i) Oxygen is paramagnetic (iv) Oxygen exists as a diatomic gas
2. i) Sulphur exists in crystalline as well as an amorphous form
ii) Rhombic sulphur has a characteristic yellow colour and composed of Sg molecules.
iii) When heated slowly above % C monoclinic sulphur is converted into Rhombic sulphur
iv) At around 140°C Rhombic sulphur melts to form mobile pale yellow liquid called X sulphur.
a) (i) &(ii)
b) (i) & (iii)
c) (ii) & (iii)
d) (iii) & (iv)
Answer:
a) (i) & (ii)
Correct statement : (iii) When heated slowly above 96°C, Rhombic sulphur is converted into monoclinic sulphur
(iv) At around 140°C the monoclinic sulphur melts to form mobile pale yellow liquid called X sulphur
![]()
3. i) H2SO4 is a dibasic acid
ii) H3PO3 is a tribasic acid
iii) H3PO4 is a dibasic acid
iv) H3PO2 is a monobasic acid
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statement:
(ii) H3PO3 is a dibasic acid
(iii) H3PO4 is a tribasic acid
4. i) Krypton is used in cryogenics.
ii) Neon is used in high-speed electronic flashbulbs used by photographers.
iii) Helium is used to provide an inert atmosphere in electric arc welding of metals.
iv) Radon is used as a source of gamma rays.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
c) (iii) & (iv)
Correct statement: (i) Helium is used in cryogenics
(ii) Xenon is used in high speed electronic flash bulbs used by photographers.
IV. Pick out the incorrect statement
1. i) In inter halogen compounds the central atom will be the smaller halogen
ii) Interhalogen compounds can be formed only between two halogen atoms.
iii) Flourine can act as a central atom.
iv) Interhalogens are strong oxidising agents
a) (i) & (ii)
b) (i) & (iii)
c) (ii) & (iii)
d) (i) & (iv)
Answer:
b) (i) & (iii)
Correct statements:
(i) In interhalogen compounds the central atom will be the larger halogen
(iii) Flourine cannot act as a central atom.
2. i) Nitrogen reacts with group 2 metals to form ionic nitrides.
ii) Ammonia is less soluble in water.
iii) Liquid nitrogen is used in biological preservation.
iv) In the conversion of metal oxides to metal ammonia acts as an oxidising agent.
a) (i) & (ii)
b) (ii) & (iii)
c) (i) & (iii)
d) (ii) & (iv)
Answer:
d) (ii) & (iv)
Correct statements:
(ii) Ammonia is extremely soluble in water.
(iv) In the conversion of metal oxides to metal ammonia acts as a reducing agent
![]()
3. i) When reacted with metals, nitric acid liberates hydrogen
ii) Chromium when reacted with nitric acid becomes passive due to the formation of nitrate on its surface.
iii) In most of the reactions nitric acid acts as an oxidising agent
iv) Fuming nitric acid contains oxides of nitrogen
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (iv)
d) (i) & (iv)
Answer:
a) (i) & (ii)
Correct statement : (i) When reacted with metals, nitric acid does not liberate hydrogen
(ii) Chromium when reacted with nitric acid becomes passive due to the formation of oxide on its surface
4. The rate of decomposition of ozone increases sharply in alkaline solution.
ii) In acidic solution ozone exceeds the oxidising power of fluorine and atomic oxygen
iii) Considerable amount of ozone is formed in the upper atmosphere by the action of UV light
iv) The shape of the Ozone molecule is linear
a) (i) &(ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statement : (i) The rate of decomposition of ozone drops sharply in alkaline solution.
(iv) The shape of the ozone molecule is bent.
V. Pick out the odd man out
1. w.r.t oxidation number pick the odd man out.
a) HPO3
b) H3PO3
c) H3PO4
d) H4P2O7
Answer:
b) H3PO3 – O.N is +3 while in others the O.N of phosphorous is +5
2. w.r.t the reaction with sulphuric acid pick the odd man out
a) Gold
b) Silver
c) Platinum
d) Copper
Answer:
d) Copper – copper reacts with sulphuric acid while others do not
![]()
3. w.r.t reactivity pick the odd man out
a) F2
b) Cl2
c) Br2
d) I2
Answer:
a) F2 – F2 is more reactive than other halogens
4. w.r.t the ability to form oxoacids pick the odd man out
a) fluorine
b) chlorine
c) bromine
d) iodine
Answer:
a) Flour – Fluorine forms only one oxoacid where as other halogens form more than one oxoacid.
VI. Choose the best answer.
1. The principal gas present in atmosphere is
a) O2
b) N2
C) H2
d) CO2
Answer:
b) N2
2. The basicity of hypophosphorous acid is (PTA – 2)
a) 1
b) 2
c) 3
d) 4
Answer:
a) 1
3. Chile salt petre is
a) NaNO2
b) NaNO3
c) KNO2
d) KNO3
Answer:
b) NaNO3
4. Indian salt petre is
a) NaNO2
b) NaNO3
c) KNO2
d) K.NO3
Answer:
d) KNO3
5. Inert character of nitrogen is due to its
a) high electronegativity
b) low electro negativity
c) high bonding energy
d) low bonding energv
Answer:
c) high bonding energy
![]()
6. Which of the following is caro’s acid?
a) H2S2O8
b) H2S2O7
c) H2SO3
d) H2SO3
Answer:
c H2SO5
7. Haber’s process is used for the synthesis of
a) NO2
b) HNO3
c) NH3
d) N2O
Answer:
a) NH3
8. The substance used in cryosurgery for producing low temperature is
a) liquid oxygen
b) liquid nitrogen
c) liquid hydrogen
d) liquid ammonia
Answer:
b) liquid nitrogen
9. Urea on hydrolysis gives
a) NO2
b) HNO3
c) NH3
d) N2O
Answer:
a) NH3
10. The catalyst used in Haber’s process is
a) Ni
b) Fe
c) Co
d) Pt
Answer:
b) Fe
11. The smell of ammonia is
a) rotten egg
b) rotten fish
c) pungent
d) garlic
Answer:
c) pungent
12. Like water, ammonia is a fairly good ionising solvent, because its dielectric constant is
a) considerably low
b) considerably high
c) equal to zero
d) equal to one
Answer:
b) considerably high
13. The process used for the manufacture of nitric acid is known as
a) Haber’s process
b) Deacon’s process
c) contact process
d) Ostwald’s process
Answer:
d) Ostwald’s process
14. With excess of chlorine, ammonia reacts to give an explosive substance
a) N2
b) NH4NO3
c) NH4Cl
d) NCl3
Answer:
d) NCl3
15. The deep blue colour compound formed when excess of ammonia is added to aqueous solution of copper sulphate is
a) [Cu(NO3)2]
b) [Cu(NH3)2]2+
c) [Cu(NH3)4]2+
d) [Cu(NH3)2]+
Answer:
c) [Cu(NH3)4]2+
16. The shape of ammonia molecule is
a) tetrahedral
b) pyramidal
c) square planar
d) octahedral
Answer:
b) pyramidal
17. The bond angle in ammonia is
a) 104°
b) 104°28′
c) 107°
d) 180°
Answer:
c) 107°
![]()
18. The colour of Pure nitric acid is
a) colourless
b) brown
c) pale green
d) green
Answer:
a) colourless
19. Fuming nitric acid contains oxides of
a) sulphur
b) hydrogen
c) nitrogen
d) carbon
Answer:
c) nitrogen
20. Nitric acid can act as
a) an acid
b) an oxidising agent
c) nitrating agent
d) all of the above
Answer:
d) all the above
21. The formula of hyponitrous acid is (MARCH 2020)
a) H2N2O2
b) H4N2O4
c) HOONO
d) HNO2
Answer:
a) H2N2O2
22. The oxidising power of oxo acids follows the order
a) HOX > HXO2 > HXO3 > HXO4
b) HXO4 > HXO3 > HXO2 > HOX
c) HXO3 > HXO4 > HXO2 > HOX
d) HOX > HXO4 > HXO3 > HXO2
Answer:
a) HOX > HXO2 > HXO3 > HXO4
23. White phosphorous is kept under
a) kerosene
b) water
c) alcohol
d) ether
Answer:
b) water
24. White phosphorous becomes yellow phosphorous due to
a) hydrolysis
b) reduction
c) oxidation
d) displacement
Answer:
c) oxidation
25. In the conversion of yellow phosphorous into phosphine, phosphorous acts as
a) oxidising agent
b) reducing agent
c) catalyst
d) hydrolysing agent
Answer:
b) reducing agent
26. In the conversion of phosphorous into orthophosphoric acid, the catalyst used is
a) Cl2
b) Br2
c) I2
d) F2
Answer:
c) I2
27. Which is used in match boxes?
a) White phosphorous
b) Red phosphorous
c) Black phosphorous
d) Scarlet phosphorous
Answer:
b) Red Phosphorous
28. The acid having O-O bond in its structure (PTA – 6)
a) H2SO3
b) H2S2O6
c) H2S2O8
d) H2S4O6
Answer:
c) H2S2O8
29. The smell of phosphine is
a) rotten egg
b) rotten fish
c) pungent
d) garlic
Answer:
b) rotten fish
30. The compounds used in Holme’s signal are
a) CaC2 & Ca3P2
b) AlP & Ca3P2
c) CaC2 & P4
d) AlP & P4
Answer:
a) CaC2 & Ca3P2
31. The gases liberated in Holme’s signal are
a) C2H2 & CH4
b) C2H2 & Ph3
c) C2H4 & PH3
d) CH4 & Ph3
Answer:
b) C2H2 & Ph3
32. The formula of pyrophosphoric acid is
a) H4P2O6
b) H4P2O7
c) H3PO2
d) H3PO3
Answer:
b) H4P2O7
![]()
33. Thermodynamically stable allotrophic form of sulphur is
a) Rhombic sulphur
b) Monoclinic sulphur
c) Plastic sulphur
d) Colloidal sulphur
Answer:
a) Rhombic sulphur
34. The gas found in volcanic eruptions is
a) NO2
b) NO
c) SO2
d) SO3
Answer:
c) SO2
35. The hybridisation of sulphur in SO2 is
a) sp
b) sp²
c) sp³
d) dsp²
Answer:
b) sp²
36. The gas liberated when dilute sulphuric acid reacts with metals is
a) SO2
b) SO3
c) H2
d) O2
Answer:
c) H2
37. The gas liberated when cone, sulphuric acid reacts with metals is
a) SO2
b) SO3
c) H2
d) O2
Answer:
a) SO2
38. When sulphuric acid reacts with barium chloride solution, the white precipitate formed is
a) PbSO4
b) BaSO4
c) (CH3COO)2SO4
d) PbCl2
Answer:
b) BaSO4
39. The halogen which exists as a liquid is
a) flourine
b) chlorine
c) bromine
d) iodine
Answer:
c) bromine
40. The halogen which exists as a solid is
a) flourine
b) chlorine
c) bromine
d) iodine
Answer:
d) iodine
41. Chlorine is manufactured by
a) Haber’s process
b) Deacon’s process
c) Contact process
d) Ostwald’s process
Answer:
b) Deacon’s process
![]()
42. The colour of chlorine gas is
a) colourless
b) brown
c) greenish yellow
d) pale green
Answer:
c) greenish yellow
43. Aqua regia is a mixture of cone. HCl and cone. HNO3 in the ratio
a) 1 : 3
b) 3 : 1
c) 2 : 3
d) 3 : 2
Answer:
b) 3 :1
44. The halogen acid which forms hydrogen bond is
a) HF
b) HCl
c) HBr
d) HI
Answer:
a) HF
45. Among halogen acids, the strongest bond is present in
a) HF
b) HCl
c) HBr
d) HI
Answer:
a) HF
46. Among halogen acids, the weakest bond is present in
a) HF
b) HCl
c) HBr
d) HI
Answer:
d) HI
47. Among halogen acids, the strongest acid is
a) HF
b) HCl
c) HBr
d) HI
Answer:
d) HI
48. Among halogen acids, the weakest acid is
a) HF
b) HCl
c) HBr
d) HI
Answer:
a) HF
49. The correct order of acid strength is
a) HF > HCl > HBr > HI
b) HF < HCl < HBr < HI
c) HF > HCl < HBr > HI
d) HF < HCl > HBr < HI
Answer:
b) HF < HCl < HBr < HI
50. Which is more reactive towards hydrogen?
a) flourine
b) chlorine
c) bromine
d) iodine
Answer:
a) flourine
![]()
51. The number of bond pair and lone pair of electrons present in the interhalogen compound BrF3 is
a) 1 & 3
b) 3 & 2
C) 5 & 1
d) 7 & 0
Answer:
b) 3 & 2
52. The oxidation number of oxygen in F2O is
a) -2
b) -1
c) +2
d) +1
Answer:
c) +2
53. The oxidation number of chlorine in Cl2O7 is
a) +1
b) +4
c) +6
d) +7
Answer:
d) +7
54. The strongest oxidising agent among the following is
a) chlorous acid
b) chloricacid
c) hypochlorous acid
d) perchloric acid
Answer:
c) hypochlorous acid
55. The first ionisation energy of noble gases is in the order
a) He < Ne < Ar < Kr
b) He > Ne > Ar > Kr
c) He < Ne > Ar < Kr
d) He > Ne < Ar > Kr
Answer:
b) He > Ne > Ar > Kr
56. Among noble gases, chemical reactivity is shown by
a) He & Ne
b) Ar & Kr
c) Kr & Xe
d) Xe & Rn
Answer:
c) Kr & Xe
57. Which among the following is used in cryogenics?
a) He
b) Ne
c) Ar
d) Kr
Answer:
a) He
58. Which is used for filling air balloons?
a) He
b) Ne
c) Ar
d) Kr
Answer:
a) He
![]()
59. Which is used in advertisement sign boards?
a) He
b) Ne
c) Ar
d) Kr
Answer:
b) Ne
60. Lamps used in airports as approaching lights is filled with
a) He
b) Ne
c) Ar
d) Kr
Answer:
d) Kr
VII. Two Mark Questions
Question 1.
How is pure nitrogen gas prepared?
Answer:
Pure nitrogen gas is prepared by the thermal decomposition of sodium azide at about 575 K
2NaN3 \(\underrightarrow { 573K } \) 2Na + 3N2
Question 2.
Nitrogen does not form any penta halides like phosphorus, why?
Answer:
Nitrogen does not form pentahalide although it exhibit +5 oxidation state. Due to the absence of d-orbitals.
It cannot undergo sp3d hybridization and hence cannot form pentahalides.
Question 3.
What is Haber’s process?
Answer:
The synthesis of ammonia from nitrogen and hydrogen at high pressure and optimum temperature in presence of iron catalyst is known as Haber’s process.
![]()
∆Hf = -46.2 Kjmol-1
Question 4.
Write the uses of nitrogen
Answer:
Nitrogen is used
In the manufacture of ammonia, nitric acid, and calcium cyanamide etc.
Liquid nitrogen is used for producing low temperature required in cryosurgery and so used in biological preservation.
Question 5.
How is ammonia prepared in the laboratory?
Answer:
Ammonia is prepared in the laboratory by heating an ammonium salt with a base
2NH4Cl + CaO → CaCl2 + 2NH3 + H2O
Question 6.
Write about the reducing property of ammonia.
Answer:
When passed over heated metallic oxides Ammonia reduces metal oxides into metal
3PbO + 2NH3 → 3Pb + N2 + 3H2O
Question 7.
What happens when ammonia reacts with excess of chlorine?
Answer:
With excess of chlorine ammonia reacts to give an explosive substance nitrogen trichloride
2NH3 + 6Cl2 → 2NO3 + 6HO
Question 8.
On standing nitric acid becomes yellow in colour why?
Answer:
- Pure nitric acid is colourless
- Fuming nitric acid contains oxides of nitrogen
- It decomposes on exposure to sunlight or on being heated into nitrogendioxide, water and oxygen.
4HNO3 → 4NO2 + 2H2O + O2 - Due to this reaction, pure nitric acid or its concentrated solution becomes yellow on standing
Question 9.
Prove that nitric acid is an oxidising agent.
Answer:
Non metals like carbon, sulphur are oxidised by nitric acid.
C + 4HNO3 → CO2 + 4NO2 + 2H2O
S + 2HNO3 → H2SO4 + 2NO
![]()
Question 10.
Prove that nitric acid is a nitrating agent.
Answer:
Nitric acid replaces hydrogen atom from organic compounds with nitronium ion NO2+. This is called nitration.
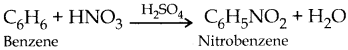
Question 11.
Write the uses of nitric acid is used
Answer:
- as an oxidising agent.
- in the preparation of aqua regia.
- Salts of nitric acid are used in photography (AgNO3) and gunpowder for fire arms (NaNO3)
Question 12.
How is nitrous oxide prepared?
Answer:
By Heating ammonium nitrate nitrous oxide is prepared
NH4NO3 → N2O + 2H2O
Question 13.
How is nitrous acid prepared?
Answer:
By treating nitrite salt with acids, nitrous acid is prepared
Ba(NO2)2 + H2SO4 → 2HNO2 + BaSO4
Question 14.
What is phosphorescence?
Answer:
White phosphorous undergoes spontaneous slow oxidation in air giving a greenish yellow glow which is visible in the dark. This is known as phosphorescence. The main product of this slow oxidation is P2O3.
Question 15.
How is phosphine prepared?
Answer:
Phosphine is prepared by the action of sodium hydroxide with white phosphorous in an inert atmosphere of carbon dioxide
![]()
Question 16.
How is orthophosphoric acid prepared in ‘ the laboratory?
When phosphorous is treated with cone, nitric acid in the presence of iodine catalyst, it is oxidised to orthophosphoric acid.
Question 17.
Write the uses of phosphorous Phosphorous is used
Answer:
- in match boxes
- For the production of certain alloys such as phosphor bronze.
Question 18.
What happens when phosphine is heated in the absence of air?
Answer:
Phosphine decomposes into its elements when heated in the absence of air at 317 K
4PH3 \(\underrightarrow { 317K } \) P4 + 6H2
Question 19.
Write about the reducing property of phosphine?
Answer:
Phosphine reduces silver nitrate into silver
PH3 + 6AgNO3 + 3H2O → 6Ag + 6HNO3 + H3PO3
Question 20.
How is phosphorous trichloride prepared?
Answer:
- When a slow stream of chlorine is passed over white phosphorous, PCl3 is obtained.
- It is also prepared by treating white phosphorous with thionyl chloride.
P4 + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2
Question 21.
Ozone (O3) acts as a powerful oxidizing agent why? (PTA – 5)
Answer:
Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen.
Nascent oxygen, being a free radical, is very reactive
O3 \(\underrightarrow { \triangle } \) O2 + [O]
Question 22.
Write the uses of oxygen
Answer:
- Oxygen is one of the essential components for the survival of living organisms.
- Oxygen is used in oxyacetylene welding.
- Liquid oxygen is used as a rocket fuel.
Question 23.
How is sulphur dioxide prepared in the laboratory?
Answer:
SO2 is prepared in the laboratory by treating a metal or metal sulphite with sulphuric acid
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
SO32- + 2H+ → SO2 + H2O
![]()
Question 24.
Illustrate the oxidising property of SO2.
Answer:
SO2 oxidises hydrogen sulphide to sulphur and magnesium to magnesium oxide.
2H2S + SO2 → 3S + 2H2O
2Mg + SO2 → 2MgO + S
Question 25.
Write about contact process.
Answer:
- In contact process SO2 is oxidised to SO3
- It is used in the manufacture of sulphuric acid.
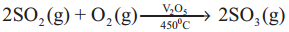
Question 26.
Write the uses of sulphurdioxide.
Answer:
- SO2 is used in bleaching hair, silk, wool etc.
- SO2 is used for disinfecting crops and plants in agriculture
Question 27.
Write about the structure of sulphr dioxide.
Answer:
- Sulphur undergoes sp² hybridisation.
- A double bond arises between S and O due to pπ – dπ overlapping

Question 28.
Illustrate the dehydrating property of sulphuric acid.
Answer:
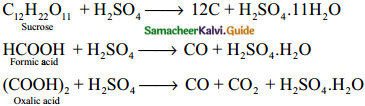
Question 29.
Show that sulphuric acid is a dibasic acid.
Answer:
H2SO4 forms two types of salts with NaOH Hence it is dibasic.
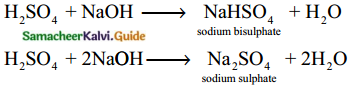
Question 30.
How is chlorine is manufactured by Deacon’s process?
Answer:
- A mixture of air and hydrochloric acid is passed up a chamber containing a number of shelves, containing pumice stones soaked in cuprous chloride.
- Hot gases at about 723 K are passed through a jacket that surrounds the chamber.
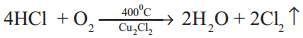
- Chlorine obtained is dilute and used for the manufacture of bleaching powder
Question 31.
Write about the bleaching action of chlorine.
Answer:
Chlorine is a strong oxidising and bleaching agent since it produces nascent oxygen.
H2O + Cl2 → HCl + HOCl (Hypochlorous acid)
HOCl → HCl +[0]
Colouring matter + Nascent oxygen → Colourless oxidation product.
The bleaching of chlorine is permanent.
Question 32.
How is bleaching powder prepared? (MARCH 2020)
Answer:
Bleaching powder is prepared by passing chlorine gas through dry slaked lime (calcium hydroxide)
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Question 33.
Write the uses of chlorine Chlorine is used
Answer:
- In the purification of drinking water.
- In the bleaching of cotton textiles, paper, and rayon.
- In the extraction of gold and platinum.
Question 34.
How is hydrochloric and prepared in the laboratory?
Answer:
Hydrochloric add is prepared by the action of sodium chloride and cone, sulphuric acid
NaCl + H2SO4 → NaHSO4. + HCl
NaHSO4 + NaCl → Na2SO4. + HCl
Dry hydrochloric acid is obtained by passing the gas through cone, sulphuric acid
Question 35.
How is xenon trioxide prepared?
Answer:
2XeF6 + SiO2 \(\underrightarrow { 50°C } \) 2XeOF4 + SiF4
2XeOF4 + SiO2 → 2XeO2F2 + SiF4
2XeO2F2 + SiO2 → 2XeO3 + SiF4
(or)
XeF6 + 3H2 → XeO3 + 6HF
Question 36.
How is sodium per xenate obtained?
Answer:
When XeF6 reacts with 2.5 M NaOH, sodium per xenate is obtained.
2XeF6 + 16NaOH → Na4XeO6 + Xe + O2 + 12NaF + 8H2O
![]()
Question 37.
Show that sodium per xenate is a strong oxidising agent
Answer:
Sodium per xenate oxidises manganese (II) ion into permanganate ion even in the
absence of a catalyst
5XeO4-6 + 2Mn2+ + 14H+ → 2MnO–4 + 5XeO3 + 7H2O
Question 38.
Give reason: ICl is more reactive than l2 (PTA – 3)
Answer:
- This is because inter-halogen compounds are in general more reactive than halogens due to w eaker inter-halogen X-X bond than X-X bond.
- So, I2 is more stable and less reactive than ICl.
VIII. Three Mark Questions
Question 1.
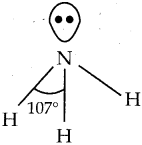
Write about the structure of ammonia.
Answer:
- Ammonia molecule is pyramidal in shape.
- Hybridisation of nitrogen is sp³.
- The shape must be tetrahedral but in one of the tetrahedral positions a lone pair of electrons from the nitrogen atom is present, hence it is pyramidal.
- The N – H bond distance is 1.016 A0.
- The H – H bond distance is 1.645 A°.
- The bond angle is 107°
Question 2.
How does red phosphorous react with oxygen?
Answer:
Red phosphorous reacts with oxygen on heating to give phosphorous trioxide and phosphorous pentoxide.
P4 + 3O2 → P4O6
P4 + 5O2 → P4O10
Question 3.
How is pure phosphine prepared?
Answer:
Pure phosphine is prepared by heating phosphorous acid
![]()
A pure sample of phosphine is prepared by heating phosphonium iodide with caustic soda solution.
![]()
Question 4.
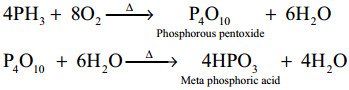
What happens when phosphine is heated with air?
Answer:
When phosphine is heated with air or oxygen, it undergoes combustion to give meta phosphoric acid.
Question 5.
Write about Holmes signal
Answer:
- In a ship during distress, a container with calcium carbide and calcium phosphide mixture is pierced and thrown into the sea.
- The mixture reacts with seawater liberating acetylene and phosphine gases.
- The liberated phosphine catches fire and ignites acetylene.
- These burning gases with lot of smoke serves as a signal to the approaching ships.
- This is known as Holme’s signal.
Question 6.
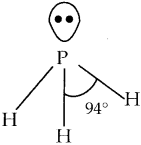
Write about the structure of phosphine
Answer:
- Phosphorous shows sp³ hybridisation.
- Three orbitals are occupied by bond pair electrons.
- Fourth orbital is occupied by lone pair of electrons.
- Hence instead of tetrahedral, PH3 has a pyramidal shape.
- Bond angle is reduced to 94°
Question 7.
How is oxygen prepared in the laboratory?
Answer:
Oxygen is prepared in the laboratory by the decomposition of hydrogen peroxide in presence of Mn02 catalyst or by the oxidation of potassium permanganate.
![]()
5H2O2 + 2MnO4– + 6H+ → 5O2 + 8H2O + 2Mn2+
![]()
Question 8.
Write about ozone
Answer:
- Ozone is an allotropic form of oxygen
- Ozone is triatomic gas
- Although negligible amounts of ozone occurs at sea level, it is formed in the upper atmosphere by the action of UV light.
- In the laboratory ozone is prepared by passing electrical discharge through oxygen.
- At a potential of 20,000 V about 10% of oxygen is converted into ozone, it gives a mixture known as ozonised oxygen.
- Pure ozone is obtained as a pale blue gas by the fractional distillation of liquefied ozonised oxygen.
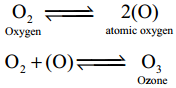
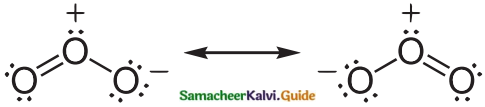
- Ozone molecule has a bent shape and symmetrical with delocalised bonding between the oxygen atoms.
Question 9.
Write about the reducing property of sulphur dioxide
Answer:
SO2 reduces chlorine into hydrochloric acid
SO2 + 2H2O + Cl2 → H2SO4 + 2HCl
SO2 reduces potassium permanganate into manganese sulphate (Mn2+).
2KMnO4 + 5SO2 + 2H2O → K2SO4 + 2MnSO4 + 2H2SO4
SO2 reduces potassium dichromate into chromic sulphate (Cr3+)
K2Cr2O7 + 3SO2 + H2SO4 → K2SO4 + Cr2(SO4)3 + H2O
Question 10.
Write about the bleaching action of sulphur dioxide.
Answer:
In presence of water, sulphur dioxide bleaches coloured wool, silk, sponges and straw into colourless due to its reducing property
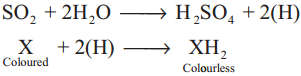
When the bleached product (Colourless) is allowed to stand in air, it is reoxidised by atmospheric oxygen to its original colour.
Hence bleaching action of sulphurdioxide is temporary
Question 11.
Explain the manufacture of sulphuric acid
Answer:
- Sulphur dioxide is produced by burning sulphur or iron pyrites in oxygen / air
S + O2 → SO2
4FeS2 + 11O2 → 2Fe2O3 + 8SO2 - SO2 is oxidised to SO3 by air in presence of V2O5 or platinised absestos
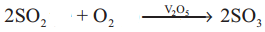
- SO3 is absorbed in cone H2SO4 and oleum is produced
SO3 + H2SO4 H2S2O7 - Oleum is converted into sulphuric acid by diluting it with water.
H2S2O7 + H2O → 2H2SO4 - To maximize the yield the plant is operated at 2 bar pressure and 720 K.
96% pure H2SO4 is obtained.
Question 12.
Show that sulphuric acid is an oxidising agent
Answer:
Sulphuric acid is an oxidising agent as it produces nascent oxygen
H2SO4 → H2O + SO2 + [O] (Nascert oxygen)
Sulphuric acid oxidises carbon into carbon dioxide
C + 2H2SO4 → 2SO2 + 2H2O + CO2
Sulphuric acid oxidises phosphorous into orthophosphoric acid
P4 + 10H2SO4 → 4H3PO4 + 10SO2 + 4H20
Sulphuric acid oxidises iodide into iodine.
H2SO4 + 2HI → 2SO2 + 2H2O + I2
![]()
Question 13.
What is the action of sulphuric acid on metals?
Answer:
Dilute sulphuric acid reacts with metals liberating hydrogen gas.
Zn + H2SO4 ZnSO4 + H2 ↑
Hot cone. Sulphuric acid reacts with metals to give sulphates and sulphur dioxide
Cu + 2H2SO4 → CuSO4 + 2H2O + SO2 ↑
Sulphuric acid does not react with noble metals like gold, silver and platinum.
Question 14.
Give the test for sulphate / sulphuric acid
Dilute solution of sulphuric acid / Sulphates react with barium chloride or lead acetate solution to give a white precipitate
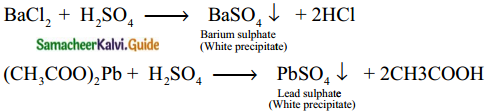
Question 15.
How is chlorine manufactured by the electrolytic process?
Answer:
When brine solution (NaCl) is electrolyzed, Na+ and Cl– ions are formed.
Na+ ions react with OH– ions of water forming sodium hydroxide.
Hydrogen and chlorine are liberated as gases.
NaCl → Na+ +Cl–
H2O → H+ +OH–
Na+ + OH– → NaOH
At cathode : H+ + e– → H
H + H → H2
At anode : Cl– → Cl + e–
Cl + Cl → Cl2
Question 16.
What is aqua regia? What is its action on gold?
Answer:
Aqua regia is a mixture of three parts of cone, hydrochloric acid and one part of cone, nitric acid.
This is used for dissolving gold, platinum etc.
AU + 4H+ + NO3– + 4Cl– → AuCl4– +NO + 2H2O
Question 17.
HF acid is a weaker acid at low concentration, but becomes stronger as the concentration increases why?
Answer:
0.1 M Solution HF is 10% ionised, hence it is a weak acid.
But 5 M, 15 M solution of HF is stronger due to the equilibrium.
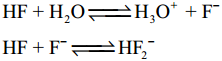
At high concentration, the equilibrium involves the removal of flouride ions and increases the hydrogen ion concentration, HF becomes stronger acid as the concentration increases.
![]()
Question 18.
HF acid is not stored in glass bottles why? (MARCH 2020)
Answer:
HF attacks silica and silicates present in glass bottles.
Hence HF is not stored in glass bottles. But HF is stored in Teflon bottles.
SiO2 + 4HF → SiF4 + 2H2O
Na2SiO3 + 6HF → Na2SiF6 + 3H2O
Question 19.
Mention the characteristic of interhalogen compounds (PTA – 2)
Answer:
- The central atom will be the larger halogen.
- It can be formed only between two halogens and not more than two halogens.
- Fluorine can’t act as a central atom because it is the smallest among halogens and highly electronegative.
- They are strong oxidising agents and undergo auto ionization.
Question 20.
Give the preparation of xenon fluorides
Answer:
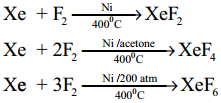
Question 21.
What is the hybridisation in XeOF2? Give its structure. (PTA – 1)
Answer:
| Element | hybridisation | structure |
| XeOF2 | Sp3d | T-shaped |
IX. Five Mark Questions
Question 1.
How is nitric acid manufactured using Ostwald’s process?
- Ammonia prepared by Haber’s process is mixed about 10 times of air.
- This mixture is preheated and passed into the catalyst chamber where they come in contact with platinum gauze.
- The temperature rises about 1275 K.
- The metallic gauze brings about the rapid catalytic oxidation of ammonia resulting in the formation of NO.
- NO is oxidized to NO2
4NH3 +5O2 → 4NO + 6H2O +120 KJ
2NO + O2 → 2NO2 - NO2 produced is passed through a series of adsorption towers.
- NO2 reacts with water to give nitric acid.
- Nitric acid formed is bleached by blowing air.
6NO2 + 3H2O → 4HNO3 + 2NO + H2O
![]()
Question 2.
Explain the action of nitric acid on metals with one example.
Primary reaction:
Metal nitrate is formed with the release of nascent hydrogen.
3Cu + 6HNO3 → 3CU(NO3)2 + 6(H)
Secondary reaction:
Nascent hydrogen produces the reduction products of nitric acid
6(H) + 3HNO3 → 3HNO2 + 3H2O
Tertiary reaction:
With dilute acid, the secondary products decompose to give final products.
3HNO2 → HNO3 + 2NO + H2O
Hence overall reaction is
3Cu + 8HNO3 → 3CU(NO3)2 + 2NO + 4H2O
With concentrated acid the secondary products react to give the final products.
HNO2 + HNO3 → 2NO2 + H2O
Hence overall reaction is
Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O
Question 3.
Write the preparation of nitrogen oxides.
Answer:
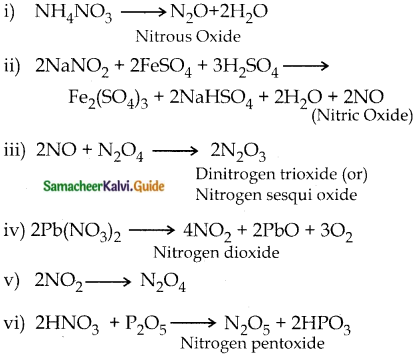
Question 4.
Write the preparation of oxoacids of nitrogen
Answer:
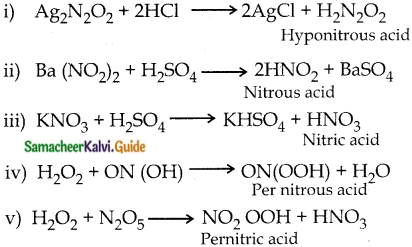
Question 5.
Explain the structure of oxides of phosphorus
Answer:
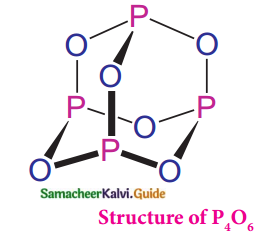
Phosphorus trioxide :
- In P4O6, four phosphorous atoms lie at the corners of a tetrahedron and six oxygen atoms along the edges.
The P – O bond distance is 165.6 pm which is shorter than the single bond distance of the P-O bond (184 pm) - This is due to Pπ – dπ bonding
- This results in the considerable double bond character
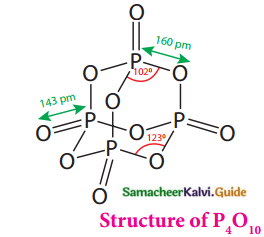
Phosphorous Pentoxide:
- In P4O10 each P atom forms a single bond with three oxygen atoms and a coordinate bond with one oxygen atom.
- Terminal coordinate P-O bond length is 143 pm
- This is less than the expected single bond distance
- This may be due to lateral overlap of filled
p – Orbitals of an oxygen atom with empty
d – Orbital on phosphorous.
Question 6.
Write the structure of and basicity oxoacids of phosphorous. (PTA – 3)
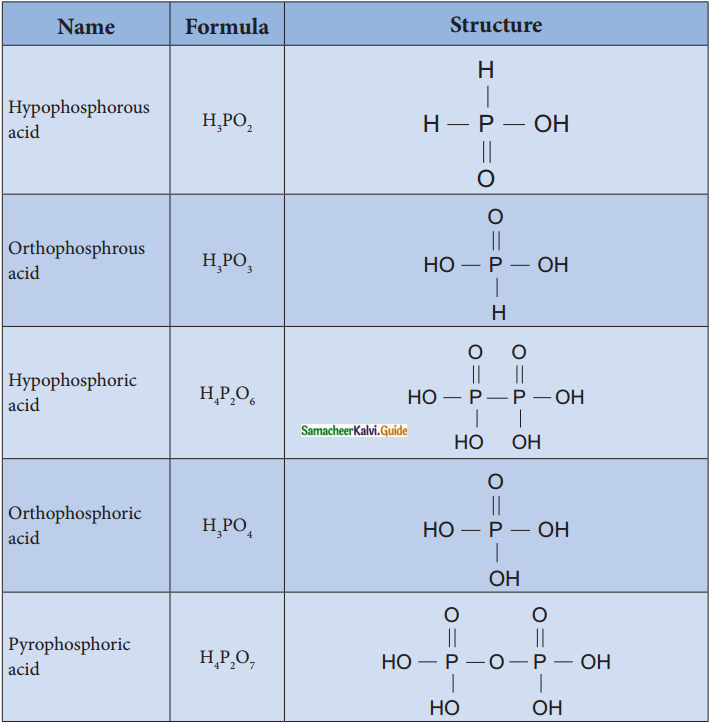
Answer:
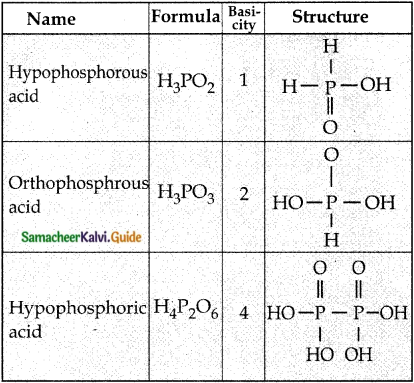
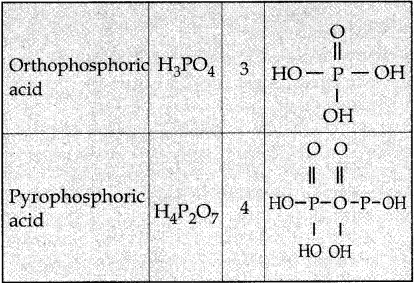
Question 7.
Write the preparation of oxoacids of phosphorous
Answer:
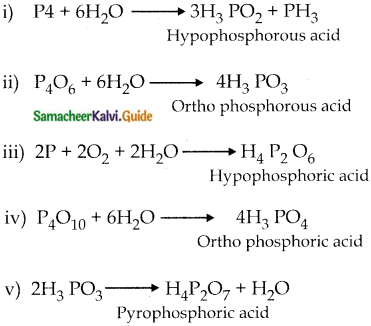
Question 8.
Write the structure of oxo acids of sulphur.
Answer:
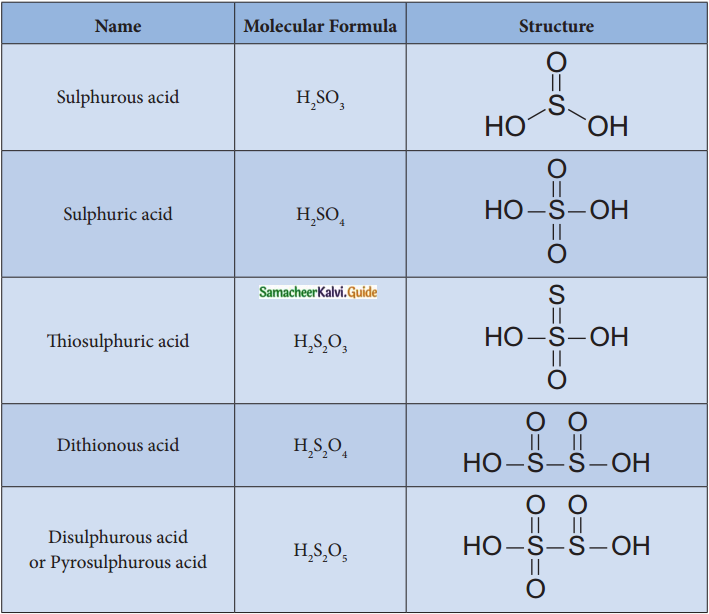
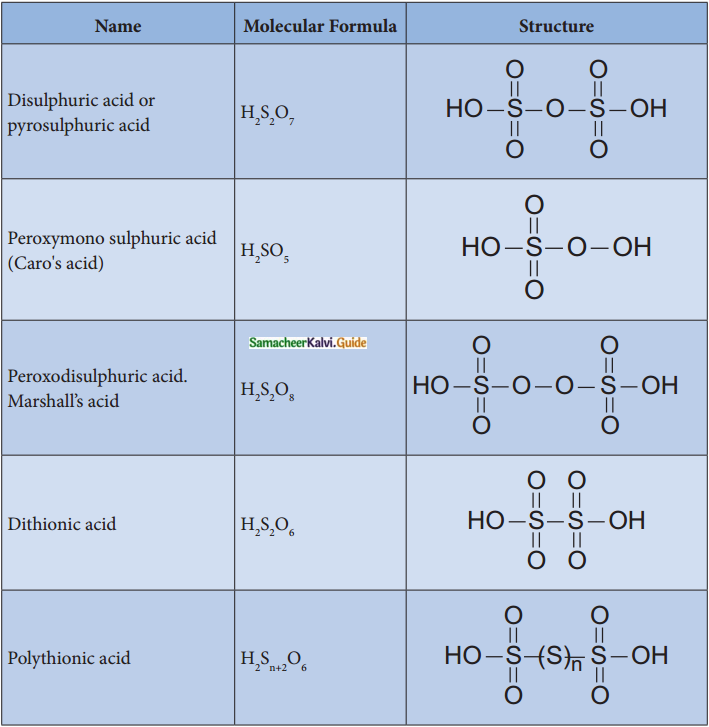
Question 9.
List any five compounds of Xenon and mention the type of hybridization and structure of the compounds (PTA – 6)
Answer:
| Compound | Hybridisation | shape/structure |
| 1. XeP2 | sp3d | Linear |
| 2. XeF4 | sp3d2 | Square planar |
| 3. XeF6 | sp3d3 | Distorted octahedron |
| 4. XeOF2 | sp3d | T-shaped |
| 5. XeOF4 | sp3d2 | Square pyramidal |
| 6. XeO3 | sp3 | Pyramidal |