Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Bio Botany Guide Pdf Chapter 9 Tissue and Tissue System Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 9 Tissue and Tissue System
11th Bio Botany Guide Tissue and Tissue System Text Book Back Questions and Answers
Part-I.
Question 1.
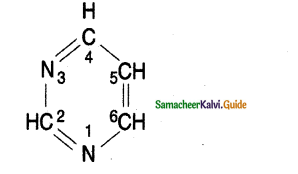
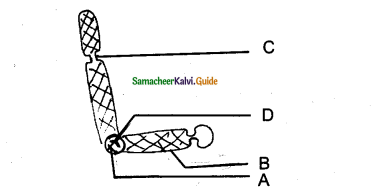
Refer to the given figure and select the correct statement.
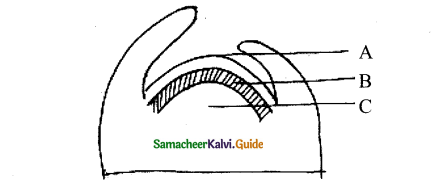
i) A, B, and C are histogen of shoot apex,
ii) A Gives rise to medullary rays.
iii) B Gives rise to cortex.
iv) C Gives rise to epidermis
a) I and ii only
b) ii and iii only
c) i and iii only
d) iii and iv only
Answer:
c) i and iii only
Question 2.
Read the following sentences and identify the correctly matched sentences.
i) In exarch condition, the protoxylem lies outside of the metaxylem.
ii) In endarch condition, the protoxylem lies towards the centre.
iii) In centrarach condition, metaxylem lies in the middle of the protoxylem
iv) In mesarch condition, protoxylem lies in the middle of the metaxylem
a) i, ii, and iii only
b) ii, iii, and iv only
c) i, ii, and iv only
d) All of these
Answer:
c) i, ii and iv only
![]()
Question 3.
In Gymnosperms, the activity of sieve tubes are controlled by.
a) Nearby sieve tube members
b) Pholem parenchyma cells
c) Nucleus of companion cell
d) Nucleus ofalbuminous cells
Answer:
d) Nucleus of albuminous cells
Question 4.
When a leaf trace extends from a vascular bundle in a dicot stem, what would be the arrangement of vascular in the veins of the leaf?
a) Xylem would be on top and the pholem on the bottom
b) Pholem would be on the top and the xylem on the bottom
c) Xylem would encircle the pholem
d) Pholem would encircle the xylem
Answer:
a) Xylem would be on top and the pholem on the bottom
![]()
Question 5.
Grafting is successful in dicots but not in monocots because the dicots have
a) Vascular bundles arranged ina ring
b) Cambium for secondary growth
c) Vessels with elements arranged end to end
d) Cork cambium
Answer:
b) Cambium for secondary growth
Question 6.
Why the cells of sclerenchyma and tracheids become dead?
Answer:
The cells of sclerenchyma and tracheids become dead because they lack protoplasm.
![]()
Question 7.
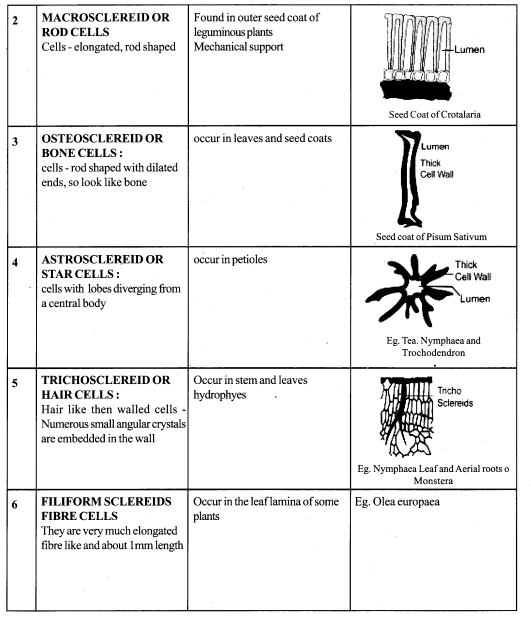
Explain sclereids with their types
Answer:
1. Sclereids – dead cells Isodiametric – but some elongated.
2. Cell wall is very thick due to lignification
3. Lumen – much reduced
4. Pits – may be simple or branched.

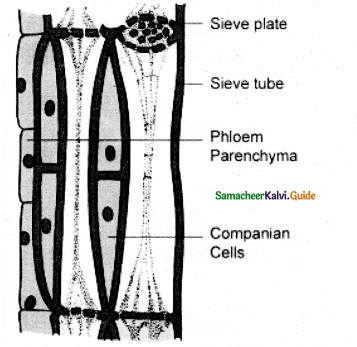
Question 8.
What are sieve tubes? Explain.
Answer:
Sieve tubes are long tube-like conducting elements in the phloem. These are formed from a series of cells called sieve tube elements. The sieve tube elements are arranged one above the other and form vertical sieve tube. The end wall contains a number of pores and it looks like a sieve. So it is called as sieve plate. The sieve elements show nacreous thickenings on their lateral walls. They may possess simple or compound sieve plates.
The function of sieve tubes are believed to be controlled by campanion cells In mature sieve tube, Nucleus is absent. It contains a lining layer of cytoplasm. A special protein (P. Protein = Phloem Protein) called slime body is seen in it. In mature sieve tubes, the pores in the sieve plate are blocked by a substance called callose (callose plug). The conduction of food material takes lace through cytoplasmic strands. Sieve tubes occur only in Angiosperms.
Question 9.
Distinguish the anatomy of dicot root from monocot root
Answer:
|
Characters |
Dicot root |
Monocot root |
| 1. Pericycle | Gives rise to lateral roots, phellogen and a part of vascular cambium | Gives rise to lateral roots only. |
| 2. Vascular tissue | Usually limited number of xylem and phloem strips. | Usually more number of xylem and phloem strips, |
| 3. Conjunctive tissue | Parenchymatous; Its cells are differentiated into vascular cambium. | Mostly sclerenchymatous but sometimes parenchymatous. It is never differentiated in to vascular cambium. |
| 4. Cambium | It appears as a secondary meristem at the time of secondary growth. | It is altogether absent. |
| 5. Xylem | Usually tetrach | Usually poly arch |
| 6. Pith | Absent | Present at the centre |
Question 10.
Distinguish the anatomy of dicot stem from monocot stem
Answer:
|
Characters |
Dicot root |
Monocot root |
| 1. Hypodermis | collenchymatous | Sclerenchymatous |
| 2. Ground tissue | Differentiated into cortex, endodermis and pericycle and pith | Not differentiated, but it is a continuous mass of parenchyma. |
| 3. StarchSheath | Present | Absent |
| 4. Medullary rays | Present | Absent |
| 5. Vascular bundles | a) Collateral and open | a) Collateral and closed |
| b) Arranged in a ring | b) Scattered in ground tissue | |
| c) Secondary growth occurs | c) Secondary growth usually does not occur. |
Part-II
11th Bio Botany Guide Tissue and Tissue System Additional Important Questions and Answers
I. Choose the correct answer
Question 1.
Who is the father of plant anatomy?
(a) David Muller
(b) Katherine Esau
(c) Nehemiah Grew
(d) Hofmeister
Answer:
(c) Nehemiah Grew
Question 2.
Father of Anatomy, as well as the scientist, who coined the term Meristem is
a) Hofmeister
b) Mettemius
c) Nehemiah Grew
d) Bloch
Answer:
c. Nehemia Grew
![]()
Question 3.
The book “Anatomy of seed plants” is written by:
(a) Hanstein
(b) Schmidt
(c) Nicholsen
(d) Katherine Esau
Answer:
(d) Katherine Esau
Question 4.
The fibres in which lignin is less and cellulose is more in the cell walls is known as
a) Gelatinous fibres,
b) Septate fibres
c) Libriform fibres
d) Hard fibres
Answer:
a. Gelatinous fibres
![]()
Question 5.
Which of the statement is not correct?
(a) Meristematic cells are self-perpetuating
(b) Meristematic cells are the most actively dividing cells
(c) Meristematic cells have large vacuoles
(d) Meristematic cells have dense cytoplasm with a prominent nucleus
Answer:
(c) Meristematic cells have large vacuoles
Question 6.
In mature sieve tubes, the pores in the sieve plates are blocked by a substance called
a) gum & nesins
b) Callose
c) Callus
d) Pectinose
Answer:
b. Callose
![]()
Question 7.
The tunica is:
(a) the peripheral zone of shoot apex, that forms cortex
(b) the inner zone of shoot apex, that forms stele
(c) the peripheral zone of shoot apex, that forms the epidermis
(d) the inner zone of shoot apex, that forms cortex and stele
Answer:
(c) the peripheral zone of shoot apex, that forms the epidermis
Question 8.
The tissue, that provide mechanical support and elasticity to the growing parts of the plant is
a) Sclerenchyma
b) Sclereids
c) Fibres
d) Collenchyma
Answer:
d. Collenchyma
![]()
Question 9.
The quiescent centre concept was proposed by:
(a) Lindall
(b) Clowes
(c) Holstein
(d) Sanio
Answer:
(b) Clowes
Question 10.
A meristem which divide in all planes is called
a) Lateral meristem
b) Apical meristem
c) Plate meristem
d) Mass meristem
Answer:
d. Mass meristem
![]()
Question 11.
Petioles of banana is composed of:
(a) storage parenchyma
(b) stellate parenchyma
(c) angular collenchyma
(d) prosenchyma
Answer:
(b) stellate parenchyma
Question 12.
The term ‘Hadrome’ for xylem and ‘Leptome’ for phloem were coined by
a) Sachs
b) Nageli
c) Hanstein
d) Haberlandt
Answer:
d. Haberlandt
![]()
Question 13.
The seed coat of groundnut is made up of:
(a) stone cells
(b) osteosclereids
(c) macrosclereids
(d) parenchyma cells
Answer:
(b) osteosclereids
Question 14.
The theory equivalent to Tunicia Corpus theory is
a) Histogen theory
b) Korperkappe theory
c) Apical cell theory
d) Quiescent center concept
Answer:
b. Korper Kappe theory
![]()
Question 15.
The term xylem was introduced by:
(a) Alexander
(b) Nageli
(c) Holstein
(d) Schmidt
Answer:
(b) Nageli
Question 16.
Trichoblasts are
a) Long cells seen in the root epidermis
b) the hair-like appendages seen on stem epidermis
c) the short cells seen in the piliferous layer of roots
d) the cells helping in the dispersal of seeds and fruits
Answer:
c. the short cells seen in the piliferous layer of roots
![]()
Question 17.
In cross-section, the tracheids are:
(a) hexagonal in shape
(b) rectangular in shape
(c) triangular in shape
(d) polygonal in shape
Answer:
(d) polygonal in shape
Question 18.
Stele include
a) Endodermis, pericycle, & Vascular bundle
b) Pericycle, Vascular bundle & pith
c) Cortex, endodermis, & Percycle
d) Xylem, phloem, cambium, & Pith
Answer:
b. Pericycle, Vascular bundle & Pith
![]()
Question 19.
Bulliform cells are present in:
(a) mango
(b) grasses
(c) groundnut
(d) potato
Answer:
(b) grasses
Question 20.
Water stomata occur in
a) Mangrove plants
b) Grass plants
c) Monocotyledon plants
d) Aquatic plants
Answer:
b. Grass Plants
![]()
Question 21.
In Ocimum the trichomes are:
(a) non – glandular
(b) fibrous
(c) glandular
(d) none of these
Answer:
(c) glandular
Question 22.
Sunken stomata is an adaptation seen in
a) Cycas
b) Neem
c) Ficus
d)Nerium
Answer:
d. Nerium
![]()
Question 23.
Casparian strips contain thickenings of:
(a) calcium carbonate and calcium oxalate
(b) carbohydrate, protein and lignin
(c) crystal of calcium oxalate
(d) lignin, suberin and some other carbohydrates
Answer:
(d) lignin, suberin and some other carbohydrates
Question 24.
The extension of pith cells that are involved in radial conduction of food and water is known as
a) Amphivasal vascular rays
b) Radial vascular parenchyma
c) Medullary ray
d) Inter fascicular parenchyma
Answer:
c. Medullary ray
![]()
Question 25.
Secondary phloem is derived from:
(a) apical meristem
(b) vascular cambium
(c) primary phloem
(d) none of the above
Answer:
(b) vascular cambium
Question 26.
Ground tissue includes all tissues except
a) Vascular bundles and pith
b) Epidermis and vascular strands
c) Cortex and vascular strands
d) Pith and conjunctive tissue
Answer:
b. Epidermis and vascular strands
![]()
Question 27.
In beans, the metaxylem vessels are generally:
(a) polygonal in shape
(b) circular in shape
(d) rectangular in shape
(d) triangular in shape
Answer:
(a) polygonal in shape
Question 28.
The thickening of which substance make endodermis impervious to water
a) Hemicellulose, cellulose, and pectin
b) Lignin, suberin, or cutin
c) Cellulose, Pectin, and Lignin
d) Pectin, Hemicellulose, and Suberin
Answer:
b. Lignin, Suberin, or Cutin
II. Match The Following & Find Out The Correct Order:
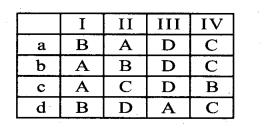
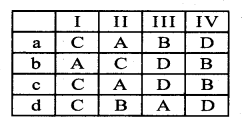
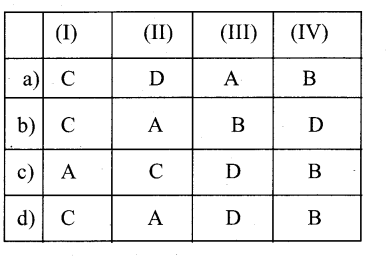
Question 1.
(I) Protoxylem lacuna – A. Liriodendron
(II) Multiple perforation plates – B. Gnetum
(III) Fibre like sclereids occur in – C. Zeamaysstem
(IV) Vessels occur in – D. Olea europaea

Answer:
a) C-A-D-B
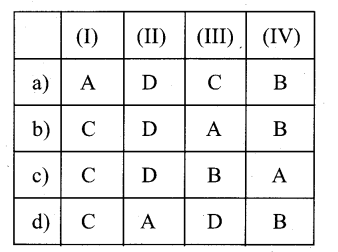
Question 2.
(I) Apical Meristem – A. Cambium
(II) Lateral Meristem – B. Intemode
(III) Intercalary meristem – C. Root Apex
(IV) Secondary meristem – D. Cork cambium

Answer:
d) C-A-B-D
![]()
Question 3.
Name of the cell Occurence
(I) Bulliform cells or Motor cells – A. Rose&Ocimum
(II) Multilayered epidermis – B. Styrax & Hibiscus
(III) Glandular trichomes – C. Nerium & Ficus
(IV) Stellate hairs – D. Chloris & Grass

Answer:
a) D-C-A-B
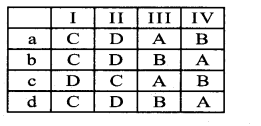
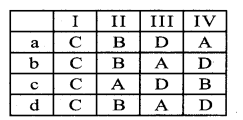
Question 4.
Nature of vascular bundle Example
(I) Conjoint, Collateral & closed – A. Dicot root
(II) Conjoint, Collateral open Endarch – B. Monocot stem
(III) Radial, Tetrarch & Exarch – C. Dicot leaf & Monocot leaf
(IV) Conjoint, Collateral Close & Endarch- D. Dicot stem
Answer:
b) C-D-A-B
![]()
Question 5.
(i) Surface fibre – A. Jute
(II) Soft fibre – B. Agave
(III) Leaf fibre – C. Coconut
(IV) Septate fibre – D. Cofton
(V) Mesocarp fibre – E. Teak

Answer:
b) D – A – B – E
Question 6.
Lateral roots originate
(i) Endo genously
(ii) From pericycle cells
(iii) Exogenously
(iv) From endodermal cells
a) I & II
b) II & III
c) III & IV
d) I & IV
Answer:
a. I & II
![]()
Question 7.
Monocot stem has
(I) Medulla or pith
(II) Atactostele
(III) Cambium – present
(IV) Scattered & skull-shaped bundles occur
a) I & II
b) II & III
c) II & IV
d) I & III
Answer:
c. II & IV
Question 8.
Which of the following statements are correct with reference to monocot stem
(I) Starch sheath is absent
(II) Pith is absent
(III) Pericycle absent
(IV) Phloem parenchyma is present
a) I, II, III
b) I and IV
c) II and IV
d) III & IV
Answer:
a. I, II,& III
III. State True Or False & On That Basis Choose The Right Answer
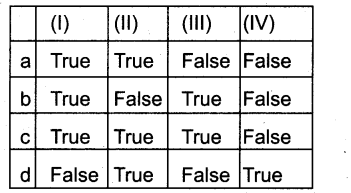
Question 1.
I) Lateral meristem – It occurs between the mature tissues, responsible for elongation of intemodes.
II) Inter calary meristem – It occurs along the longitudinal axis of stem and root, responisble for secondary growth
III) Protoderm – It gives rise to epiderminal tissue system, (i.e) epidermis, stomata & hairs
IV) Ground meristem – It gives rise to all tissues except Vascular strands and epidermis

Answer:
b) False – False – True – True
![]()
Question 2.
I) Phloem fibres and phloem parenchyma, are absence in primary phloem of monocot stem.
II) Phloem fibres are also known as Libriform fibres.
Ill Sieve cells are main food conducting elements of Angiosperms
IV) Phloem fibres are absent in primary phloem of Dicot stem

Answer:
a) True – False – False – True
Question 3.
I) The bundle cap of Dicto stem is known as Hard bast.
II) The bundle cap of Dicot stem is parenchymatous
III) The bundle sheath of Dicot leaf is sclerenchymatous walls of Endodermis in Endodermis is known as the outermost layer of stele.
IV) In Angiosperms pericycle gives rise to lateral roots

Answer:
b) False – True – False – True
![]()
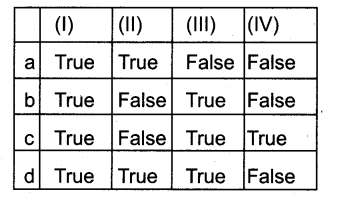
Question 4.
I) Prickles are one type of epidermal emergences with vascular supply
II) Albuniinous cells! straburger cells – in conifers are analogous to companian cells of Angiosperm but
III) Piliferous layer, Epiblema are other names of Endodermis.
IV) Hypodermis of Dicot stem is living, whereas the Hypodermis of Moncot stem is dead.

Answer:
d) False – True – False – True
Question 5.
I) The inner most layer of cortex is known as pericycle
II) Suberin, lignin, and some other carbohydrates are present as strips in the radial and inner tangentious walls of the endodermis
III) Endodermis is known as the outer most layer of stele.
IV) InAngiosperms pericycle gives rise to lateral roots

Answer:
b) False – True – False – True
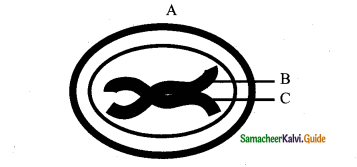
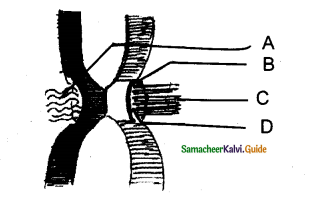
IV. With Reference To The Given Diagram, Identify The Incorrect Option Given Below:
Question 1.
a) Living cells with cell wall made up of more of hemicellulose and pectin besides cellulose
b) The type of tissue is of common occurrence in the hypodermis of Helianthus stem
c) Here cells are compactly arranged with thickening on the intercellular spaces
d) Here cells compactly arranged with thickening appear as successive tangential layers

Answer:
c) Here cells are compactly arranged with thickening on the intercellular spaces.
![]()
Question 2.
With reference to the given diagram/ figure of section of the plant organ, identify the in correct.
a) There is no epidermal growth, and hypodermis is sclerenchymatous
b) Cortex is absent but ground tissue is present
c) Endodermis, pericycle and pith are absent
d) Vascular bundles are scattered, skull shaped conjoint, collateral open and endarch
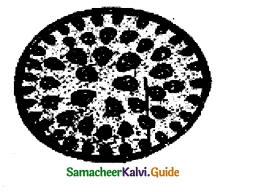
Answer:
d) Vascular bundles are scattered skull-shaped conjoint, collateral open and endarch
Question 3.
With reference to the given figure of the section of the plant part, identify the incorrect option given.
a) The vascular bundles are radial, tetrarch and endarch
b) The vascular bundles are radial tetrarch, and exarch
c) This is the cross-section of the primary structure root of Beam
d) Here xylem and phloem are arranged alternate to one another

Answer:
a) The vascular bundles are radial, tetrarch and endarchces.
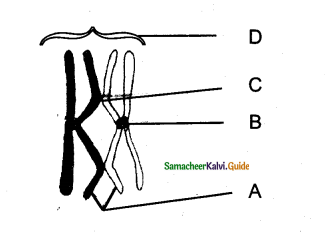
Question 4.
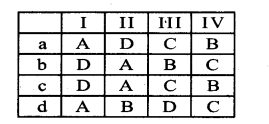
With reference to the figure choose the right option.
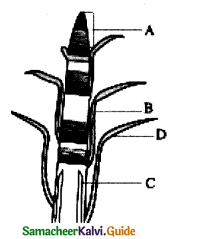
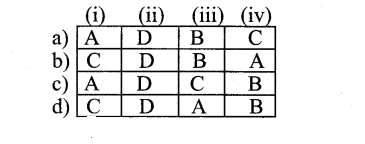
|
A |
B | C |
D |
|
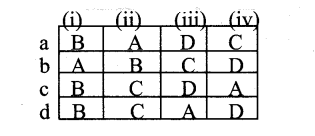
| a | Lateral meristem | LeafPrimordia | Intercalary meristem | Apical meristem |
| b | Apical meristem | Lateral meristem | Intercalary meristem | Leaf primordia |
| c | Apical meristem | Intercalary meristem | Lateral meristem | Leaf primordia |
| d | Intercalary meristem | Lateral meristem | Leaf primordia | Apical meristem |
V. Out of the given four options, find out the three relevant statements with reference to Quiescent centre.
Question 1.
a) It is the peripheral zone of shoot apex
b) This is the appearance in the active region of cells in root promeristem
c) It is located between calyptrate and other differentiating cells
d) It is the site of hormone synthesis.
i) a, b & C
ii) b, c & d
iii) a, c & d
iv) a, b & d
Answer:
ii) b, c & d
Question 2.
Out of the given four, find out the three relevant statements with reference to sclereids
a) These are dead cells, isodiametric, but some elongated to
b) The cell wall is very thick due to lignification
c) These are living, lignified cells with elongated tapering ends
d) These are only mechanical in function.
i) a,b& c
ii) a,c& d
iii) a,b,& d
iv) b,c, & d
Answer:
iii) a,b, & d
![]()
Question 3.
Generally, Ground tissue include
a) Cortex
b) Pericycle
c) Pith
d) Vascular bundle
i) a,b& d
ii) b,c,& d
iii) a,c,& d
iv) a,b,& c
Answer:
iv) a,b& c
VI. Find out the incorrect statement.
Question 1.
a) A stoma is surrounded by pa ir of guard cells
b) Each stoma opens into an air chamber
c) Guard cells contain no chloroplasts
d) The cuticle helps to check transpiration
Answer:
c. Guard cells contain no chloroplasts
Question 2.
a) Sieve cells occur in gymnosperms
b) Sieve tubes occur in Angiosperms
c) Sieve cells are absent in Angiosperms
d) Vessels are absent in Gnetum
Answer:
d. Vessels are absent in Gneturn
![]()
Question 3.
Read the following statements having two blank A and B
Collenchyma cell walls contain and find the correct option for A and B
| Blank A | Blank B |
| a) Pectin | 1. Lignin |
| b) cellulose | 2. Aminosugar |
| e) Lignin | 3. Cellulose |
| d) Pectin | 4. Hemicellulose |
Answer:
d. Pectin Hemicellulose
Question 4.
Read the following statements having two blank A and B Collenchyma cell walls contain A and B find the correct option for A and B
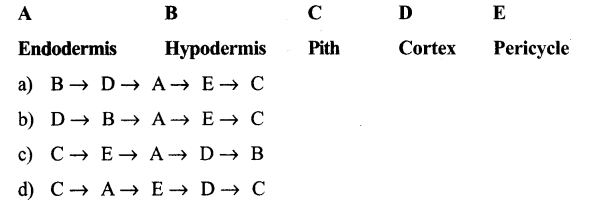
Answer:
C. C → E → A → D → B
VII. From the given choose the correct answer – Regarding Assertion & Reason
Question 1.
ASSERTION: – A The endodermis of root is homologous to starch sheath of dicot stem.
REASON -R The cells of endodermis are rich in starch grain and so-referred as starch sheath.
a. A & R correct and R is explaining A
b. A&R correct but R is not explaining A
c. A-correct but R is false
d. A – correct and R is not explaining ‘A’
Answer:
a. A&R correct and R is explaining A
Question 2.
ASSERTION: – A In Gymnosperm – plants show well developed vessels & fibres
REASON -R Companian cells are absent in Gymnosperm plants.
a. BothA&Rture, ‘R’is giving correct explanation of‘A’
b. Both A&R- true, but ‘ R’ is not correct explanation of ‘ A’
c. Both A & R are false
d. ‘A’ is false and ‘R’ is true.
Answer:
d. ‘A’ is false and ‘R’ is true.
![]()
Question 3.
ASSERTION:-A In grasses the bundle sheath is called kranz sheath
REASON -R It is involved in photsynthesis
a. ‘A’ and ‘R’ are right
b. A and R are wrong
c. R does not explain A
d. A is right and ‘R’ is wrong
Answer:
a. ‘A’ and ‘R’ are right
VIII. 2 Marks Questions
Question 1.
What is the Use of the study of Anatomy?
Answer:
- The organisation of cells and different kinds of tissues is understood.
- It is studied by means of dissection and microscopic examination.
- The organisation of cells and different kinds of tissues is understood by the study of anatomy
- The anatomical structure of different organs of plants can be compared
- The anatomical knowledge play an important role in taxonomical studies too.
Question 2.
What are the different types of plant tissue?
Answer:
The two types of principal groups are:
- Meristematic tissues
- Permanent tissues.
Question 3.
The pulp of pear is stony & gritty, whereas the seed coat of Pisum sativum seed coat is bony & shiny give reasons.
Answer:
The pulp of pear has Brachysclereids that make it stony and gritty, whereas the seed Coat of Peas seed coat is bony and shiny due to the presence of Osteosclereids
![]()
Question 4.
Mention the function of the apical meristem.
Answer:
Present in apices of root and shoot. It is responsible for the increase in the length of the plant, it is called primary growth.
Question 5.
Differentiate between Centrach and Mesearch xylem
Answer:
|
Centrach |
Mesarch |
| Protoxylem lies in the centre, surrounded by metaxylem | Protoxylem lies in the centre, surrounded by metaxylem |
| Only one Vascular stand is developed Eg – Selaginella sp |
Here unlike cent reach many vascular bundles are developed Eg – Ophioglossum sp. |
Question 6.
Differentiate between Trichoblast and Trichomes
Answer:
| Trichoblast | Trichomes |
| The root epidermis is made up of single layer of parenchyma, with big and small cells – The root hair are the extension of small cells known as trichoblast. | The epidermal layers of stems and leaves have unicellular or multicellular appendages that originate from the epidermal cells, known as trichomes, can be branched or unbranched, glandular or non glandular, helpful m dispersal of fruits & seeds. They are also protective infunction. |
Question 7.
Differentiate between Exarch and Endarch condition.
Answer:
|
Exarch |
Endarch |
| Protoxylem lies towards the periphery and metaxylem towards the centre is called Exarch condition. Eg. – Root Anatomy |
Protoxylem lies towards the centre and metaxylem, towards the periphery is known as Endarch condition. Eg. Stem Anatomy |
Question 8.
Explain briefly Branchysciereids or Stone cells.
Answer:
Isodiametric sclereids, with hard cell wall. It is found in bark, pith cortex, hard endosperm and fleshy portion of some fruits. eg: Pulp of Pyrus.
Question 9.
What is Protoxylent lacuna?
Answer:
- In Monoeoi stem, Xylem vessels occur in the form of letter ‘ Y’. The upper two arms of has two metaxylem vessels and at the base on or two protoxylem vessels occur.
- At maturity, the lowes, basal protoxylem disintegrates and form a cavity known as Protoxylem lacuna.
Question 10.
Distinguish between Eustele and Atactostele
Answer:
|
Eustele |
Atactostele |
| Vascular bundles are arranged in the form of a ring around the pith is known as Eustele Eg. Dicot Stem (Sun flower) |
Vascular bundles are simply scattered in the ground tissue. This condition is known as Atactostele Eg. Monocot Stem (Maize) |
Question 11.
What are bast fibres?
Answer:
These fibres are present in the phloem. Natural Bast fibres are strong and cellulosic. Fibres obtaining from the phloem or outer bark of jute, kenaf, flax and hemp plants. The so-called pericyclic fibres are actually phloem fibres.
![]()
Question 12.
What is the significance of Quiescent centre?
Answer:
- The apparently inactive centre in the root anatomy, located between root cap and differentiating cells of the root.
- It is the site of hormone synthesis and also the ultimate source of all meristematic cells of the meristem.
Question 13.
Differentiate between Meristematic Tissue and Permanent tissue.
Answer:
|
Meristematic tissue |
Permanent tissue |
| Cells divide repeatedly | Donot divide but develop from meristematic tissue. |
| Cells are undifferentiated | Cells are differentiated |
| Produce other tissues | Perform specific functions. |
Question 14.
Differentiate between xylary fibres and Extra xylary fibres (Phloem fibres)
Answer:
|
Xylary fibres |
Bast fibres |
| Associated with sec xylem tissue | Present in phloem |
| Derived from the vascular cambium | Derived from phloem or outer bark |
| Many types Eg. Teak |
Eg. Jute, Kenaf, Flax & hemp plant fibres |
Question 15.
Explain bulliform cells in grasses.
Answer:
Some cells of the upper epidermis (eg: Grasses) are larger and thin-walled. They are called bulliform cells or motor cells. These cells are helpful for the rolling and unrolling of the leaf according to the weather change.
Question 16.
What is meant by Sunken Stomata?
Answer:
In some Xerophytic plants (eg: Cycas, Nerium), stomata are sunken beneath the abaxial leaf surface within stomatal crypts. The sunken stomata reduce water loss by transpiration.
![]()
Question 17.
Distinguish, Protoxylem and Metaxylem from Protophloem and Metaphloem
Answer:
|
Proto & Metaxylem |
Proto & Metaphloem |
| From the primary Xylem derived from procambium, the first formed elements are known as protoxylem and the later formed are known as metaxylem. | From the primary phloem derived from procambium, the first formed elements are known as proto phloem are known as meta phloem. |
Question 18.
Distinguish the Bundle sheath of stem and leaf
Answer:
|
Bundle sheath of stem |
Bundle sheath of leaf |
| 1. Bundle sheath is the surrounding tissue of the vascular bundle | 1. The sheath surrounding the dicot leaf and monocot leaf is known as bundle sheath |
| 2. In monocot stem it is sclerenchymatous | 2. It is parenchymatous both in Dicot and Monocot leaf |
| 3. It is protective in function. | 3.It is also known as border parenchyma, protective in function. |
Question 19.
Distinguish Guard Cells and Subsidiary Cells
Answer:
|
Guard Cells |
Subsidiary Cells |
| 1. The two kidney-shaped cells in dicot leat and the two dumbbell-shaped cells in monocot leaf, which flank the stoma are called Guard Cells | 1. These are specialised epidermal cells, distinct from other cells of the epidermis |
| 2. Chloroplasts are present in the cells | 2. Chloroplasts are absent in the cells |
| 3.Help in opening and closing of stoma | 3.She subsidiary ceils assist guard cells in the opening and closing of stoma |
Question 20.
Differential between Radial and Collateral Vascular bundle.
Answer:
|
Radial |
Collateral |
| 1. Here, the xylem and phloem are arranged at different radius, (i.e) alternating with one another. Eg. Root Anatomy |
1. this condition, the phloem and xylem lie in the same radius, one below another. 2. Here phloem is above and xylem lies below, this condition is known as conjoint, collateral Eg. Stem Anatomy |
Question 21.
Describe briefly radial types of vascular Bundles.
Answer:
Xylem and phloem are present on different radii alternating with each other. The bundles are separated by parenchymatous tissue. (Monocot and Dicot roots).
![]()
Question 22.
What are Halophiles?
Answer:
- Plants adapted to grow in salty environmental conditions are known as Halophytes
- The secretion of ions by the salt glands, present in the leaves is the best mechanism to regulate the salt content of plant shoots.
- Eg. Mangrove Plants-Avicennia
Question 23.
Write down the function of Sclerenchyma.
Answer:
- Main function is to provide mechanical strength.
- Grittiness in the pulp of fruits like Guava, the presence of Pear, Pyrus etc is due to the presence of Sclerenchyma tissue.
- Provide rough and stiffness to seed coats nuts etc.
- Give various types of commercially useful fibres. Eg. Jute, hemp, cotton.
Question 24.
What are the special aspects of the trichomes on the leaves of insectivorous plants?
Answer:
The trichomes on the leaves of the insectivorous plants secrete mucopolysaccharides that help to trap bisects in the insectivorous plants living in marshy plants.
![]()
Question 25.
Define, hydathode?
Answer:
A hydathode is a type of epidermal pore, commonly found in higher plants. Structurally, hydathodes are modified stomata, usually located at leaf tips or margins, especially at the teeth. Hydathodes occur in the leaves of submerged aquatic plants such as ranunculus fluitans as well as in many herbaceous land plants.
Question 26.
Notes on multilayered epidermis multiseriate epidermis.
Answer:
- In some leaves the upper and lower epidermis remain multilayered.
- The outer most layer has cuticle.
- In Nerium these multilayers and the culicle help to reduce the rate of transpiration.
- In Ficus the upper epidermal layer contain cystoliths made up of calcium carbonate crystals.
- These are plants that grow in dry climatic conditions and these are the Anatomical adaptations seen in xerophytic plants.
Question 27.
Notes on Medulla or Pith.
Answer:
- In the Dicot stem, Dicot root and Monocot root the central part is made up of ground tissue known as pith.
- Usually, starch, fatty substances, tannin, phenol, calcium oxalate crystals are stored in the pith.
- Function: storage
Question 28.
State Tunica corpus theory.
Answer:
- The theory was proposed by A. Schmidt (1924)
- There are two zones of tissues are found in apical meristem.
- Tunica-It is the peripheral zone of shoot apex that forms epidermis.
- Corpus – It is the inner zone of shoot apex that forms cortex and stele of the shoot.
![]()
Question 29.
State Koroperkappe theory.
Answer:
- The Korper Kappe theory was proposed by schuepp.
- This theory is equivalent to Tunica corpus theory of shoot apex.
- The two divisions are distinguished by the type of T division.
- Korper is characterised by inverted T divisions
- Kappe is characterised by straight T divisions.
Question 30.
Name the 4 types of xylary fibres.
Answer:
- Xylary fibres are associated with the secondary xylem tissue.
- These fibres are derived from the vascular cambium.
There are 4 types of xylary fibres.
- Libriform fibres – Long, narrow fibres with simple pits and lignified secondary walls
- Fibre tracheids – Shorter with moderate thickening pits-simple or bordered
- Septate fibres – Fibres have thin septa separating the lumen in to distinct chambers. Eg. Teak.
- Gelatinous fibres – Fibres with less lignin and more cellulose in the cell wall.
Question 31.
Distinguish single perforation plate from multiple perforation plate.
Answer:
Xylem vessels are perforated at the end walls.
If the entire cell wall is dissolved – and, give rise one pore then it is known as single perforation plate. Eg. Mangifera.
If the perforation plate has many pores it is called Multiple perforation plate. Eg. Liriodendron.
![]()
Question 32.
State Apical cell theory.
Answer:
- Proposed by Nagel
- Single apical cell-composes the Root meristem
- The apical initial is tetrahedral in shape and produces the root cap from one side.
- The remaining 3 sides produces epidermis cortex and vascular tissue.
- Fg. Vascular cryptogams.
IX. Identify the diagram & Label the parts.
Question 1.
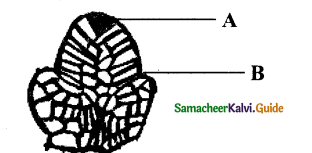
Answer:
The given diagram is shoot apical meristem
A – Apical cell
B – Leaf Primordium
Question 2.
Answer :
Brachysciereid
A – Lumen Cell B – Thick cell wall
Question 3.
Name the tissue found ¡n these fruits Name the fruits a, b, c

Answer :
Sclerenchyma is the tissue found in these fruits
A – Pear fruit
B – Strawberry
C – Guava
Question 4.

Answer :
Asteroscie Reid
A – Thick cell wall
B – Lumen
X. 3 Mark Questions
Question 1.
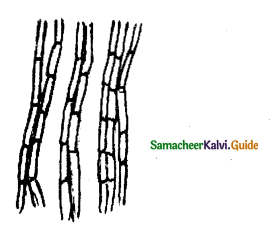
Give an account of Prosenchyma and Chiorenchyma
Answer:
These are the two types of Parenchyma tissue
Prosenchyma:
Here the parenchyma cells become elongated, pointed and slightly thick-walled it provide mechanical support
Chiorenchyma:
Parenchyma cells with chiorephyll is known as chiorenchyma.
Eg. Mesophyll of leaves. it can be divided in Palisade tissue and spongy tissue in dicot leaf.
Question 2.
Distinguish libre and sclereids?
Answer:
|
Fibre |
Sclereids |
| 1. Long Cells | Short Cells |
| 2. Narrow, Elongated pointed ends | Usually short and broad |
| 3. Occurs in bundles | Occurs individually or in small groups |
| 4. Commonly unbranched | Maybe branched |
| 5. Derived directly from meristematic tissue | Develops from secondary sclerosis parenchyma cells |
Question 3.
What is meant by the quiescent centre concept?
Answer:
Quiescent centre concept was proposed by Clowes (1961) to explain root apical meristem activity. This centre is located between the root cap and differentiating cells of the roots. The apparently inactive region of cells in root promeristem is called quiescent centre. It is the site of hormone synthesis and also the ultimate source of all meristematic cells of the meristem.
![]()
Question 4.
Difference Between Meristernatic Tissue and Permanent Tissue.
Answer:
|
Meristematic tissue |
Permanent tissue |
| 1. Cells divide repeatedly | 1. Do not divide |
| 2. Cells are undifferentiated | 2. Cells are fully differentiated |
| 3. Cells are small and Isodiametric | 3. Ceils are variable in shape and size |
| 4. Intercellular spaces are absent | 4. Intercellular spaces are present |
| 5. Vacuoles are absent | 5. Vacuoles are present |
| 6.Cell walls are thin | 6.Cell walls are may be thick or thin |
| 7. Inorganic inclusions are absent | 7. Inorganic inclusions are present |
Question 5.
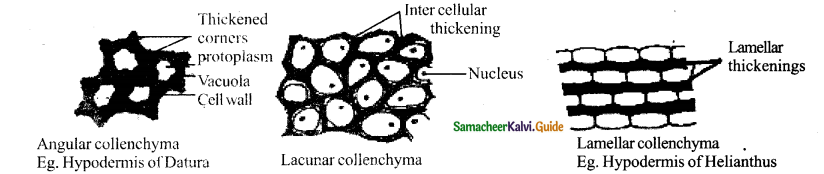
Draw and label three types of collenchyma
Answer:
Question 6.
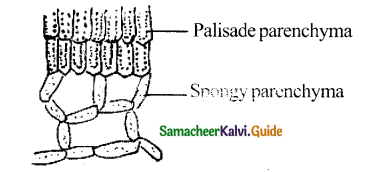
Differentiate between Dicot leaf and Monocot leaf.
Answer:
|
Dicot Leaf |
Monocot Leaf |
| 1. Dorsiventral leaf | 1. Isobilateral leaf |
| 2. The mesophyll is differentiated into palisade and spongy parenchyma | 2. Palisade parenchyma is present on both sides of the leaf and spongy parenchyma lies in the centre |
| 3. Eg. Sunflower | 3. Eg. Grass |
Question 7.
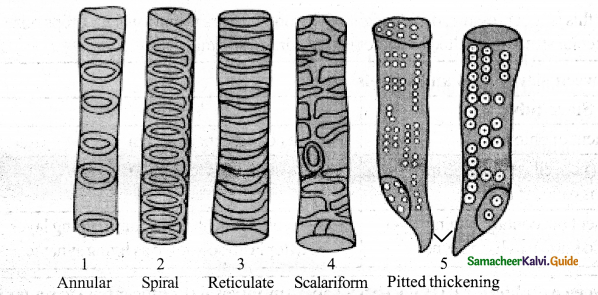
Define tracheids & Draw the different types of cell wall thickening seen in tracheids & vessels
Answer:
Tracheids are dead, lignified and elongated cells with tapering ends. Its lumen is broader than that of fibres. In cross section, the tracheids are polygonal.
Question 8.
Draw the structure of stomata & label the parts.
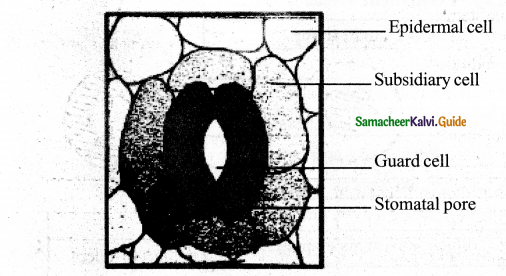
Question 9.
Give a brief answer on subsidiary cells in plant leaves.
Answer:
Stomata are minute pores surrounded by two guard cells. The stomata occur mainly in the epidermis of leaves. In some plants addition to guard cells, specialised epidermal cells are present which are distinct from other epidermal cells. They are called Subsidiary cells. Based on the number and arrangement of subsidiary cells around the guard cells, the various types of stomata are recognized, The guard cells and subsidiary cells help in the opening and closing of stomata during gaseous exchange and transpiration.
![]()
Question 10.
Distinguish between Bulliform or motor cells, and silica cells
Answer:
|
Bulliform or motor cells |
Silica cells |
| 1. Some cells of upper epidermis in Grasses are larger and thin-walled, are known as bulliform or motor cells | 1. Some of the epidermal cells of grass are filled with silica. They are called silica cells. |
| 2. These cells are helpful for the rolling and unrolling of the leaf- according to the weather change in order to check transpiration | 2. They provide mechanical stability and protection to the tissues |
Question 11.
Explain the piliferous layer as epiblema.
Answer:
The outermost layer of the root is known as piliferous layer. It consists of single row of thin-walled parenchymatous cells without any intercellular space. Epidermal pores and cuticle are absent in the piliferous layer. Root hairs that are found in the piliferous layer are always unicellular. They absorb waer and mineral salt from the soil. Root hairs are generally short-lived. The main function of piliferous layer is protection of the inner tissues.
Question 12.
Differentiate between sieve tubes and vessels
Answer:
|
Sieve tube |
Vessels |
| 1. It is a component of phloem | 1. It is a component of xylem |
| 2. It is a syncyte (i.e) cell which is formed by fusion of cells is called syncyte | 2. It is also a syncyte |
| 3. Nucleus is absent but contain a lining layer of cytoplasm so known as living syncyte | 3. Nucleus is absent but contain a lining layer of cytoplasm so known as living syncyte |
Question 13.
Differentiate between Amphicribral (Halocentric) and Amphivasal (Leptocentric) vascular bundle.
Answer:
The above two come under concentric type of vascular bundle. Here xylem and phloem are present in concentric circles one around the other, in some stems.
Amphicribral – (Halocentric)
Here xylem lies in the centre and phloem surrounding it.
Eg. Ferns – (Polypodium) dicots – aquatic Amphivasal – (Leptocentric)
Here phloem lies in the centre and xylem surrounding it.
Eg. Dragon plant – Dracena & Yucca
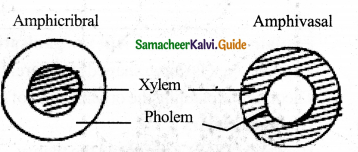
Question 14.
Bring out the different between vascular bundles of Dicot and Monocot roots.
Answer:
|
Dicot roots |
Monocot root |
|
| 1. Vascular tissue | Usually limited number of xylem and phloem strips | Usually more number of xylem and phloem strips. |
| 2. Conjunctive tissue | Parenchymatous; Its cells are differentiated into vascular cambium | Mostly sclerenchymatous but sometimes parenchymatous. It is never differentiated in to vascular cambium |
| 3. Cambium | It appears as a secondary meristem at the time of secondary growth | It is altogether absent |
| 4. Xylem | Usually tetrarch | Usually poly arch |
Question 15.
What is meant by kranz Anatomy? What is its importance.
Answer:
- In C4 plants like maize, the tissue outside the vein, (vascular bundle), the bundle sheath is with large chloro- plasts where as its spongy tissue have few if any chloroplast.
- This anatomical uniqueness is known as Kranz anatomy. The border parenchyma has chloro plasts with out grana.
This kranz sheath help in efficient CO2, fixation in C4 plants than C3 plants.
![]()
Question 16.
Explain the nature of phloem in dicot stem.
Answer:
Primary phloem lies towards the periphery. It consists of protpphloem and metaphloem. Phloem consists of sieve tubes, companion cells and phloem parenchyma. Phloem fibres are absent in primary phloem. Phloem conduct organic foods material from the leaves to other parts of the plant body.
XI. 5 Marks Questions
Question 1.
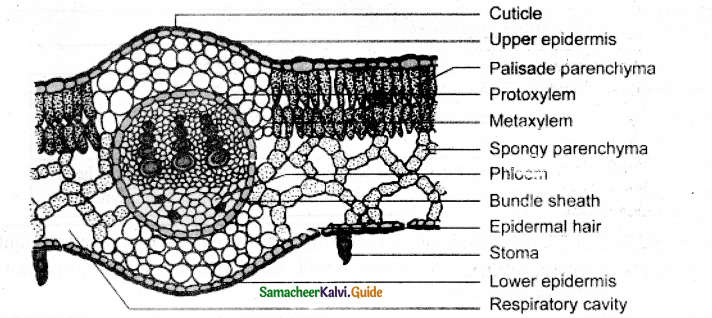
A section enlarged – T.S. of Dicot leaf (Helianthus)
Answer:
Question 2.
Explain in detail about the vascular bundles of monocot stem.
Answer:
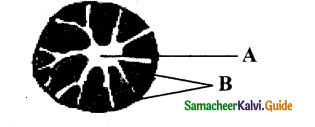
1. Vascular bundles: Vascular bundles are scattered (atactostele) in the parenchyma ground tissue. Each vascular bundle is surrounded by a sheath of sclerenchymatous fibres called bundle sheath. The vascular bundles are conjoint, collateral, endarch and closed. Vascular bundles are numerous, small and closely arranged in the peripheral portion. Towards the centre, the bundles are comparatively large in size and loosely arranged. Vascular bundles are skull or oval-shaped.
2. Phloem: The phloem in the monocot stem consists of sieve tubes and companion cells. Phloem parenchyma and phloem fibres are absent. It can be distinguished into an outer crushed protophloem and an inner metaphloem.
3. Xylem: Xylem vessels are arranged in the form of ‘Y’ the two metaxylem vessels at the base. In a mature bundle, the lowest protowylem disintegrates and forms a cavity known as protoxylem lacuna.
Question 3.
Korper Kappe theory:
Answer:
- Schuepp (1917)- proposed it
- According to it, Root system has 2 zones – Korper and Kappe
- Korper – zone forms body and Kappe forms the cap
- This theory is comparable to Tunica – corpus theory of shoot apex.
Question 4.
Compare and contrast simple and complex tissues by tabulation.
Answer:
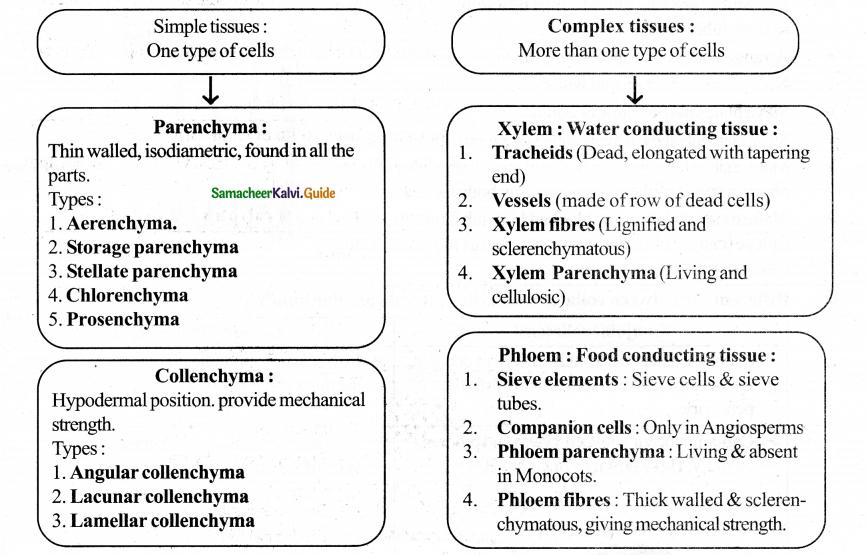

Question 5.
Draw the different types of phloem elements and add a note on sieve tubes.
Answer:
- Sieve tubes are long tubes formed by a series of cells known as sieve tube elements.
- Arranged one above another to form vertical sieve tube.
- No. of pores occur on end walls – known as sieve plate.
- Sieve plates may be simple or compound.
- Sieve elements show nacreous thickening on their lateral walls.
- Mature sieve tube, nucleus is absent, only lining layer of cyto plasm, a special phloem protein (slimy body) is seen in it.
- Mature sieve tubes pores blocked by a substance known as callose (callose plug) sieve tube function as food conducting tissue Angiosperm.
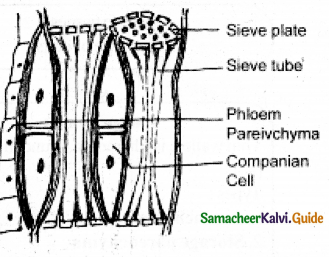
Question 6.
Differentiate between collateral and Bicollateral vascular bundles.
Answer:
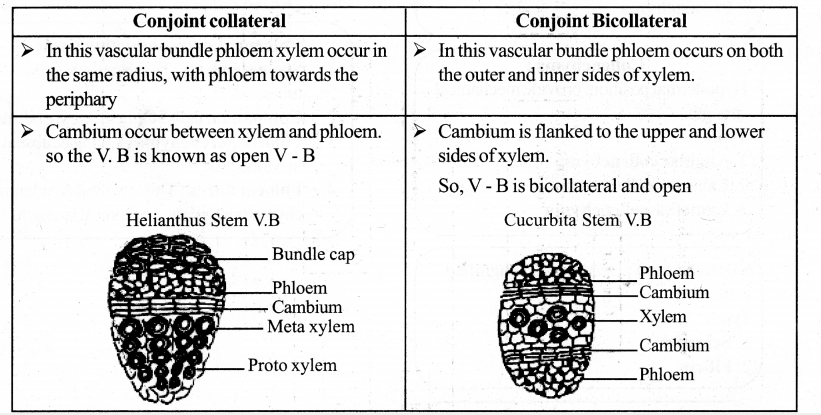
Question 7.
Tabulate the Anatomical differences between root and stem
Answer:
|
Characters |
Root |
Stem |
| 1. Epidermis | Absence of Cuticle and epidermal pores. | Presence of cuticle and epidermal pores. |
| Presence of unicellular root hairs. | Presence of unicellular and multicellular trichomes. | |
| 2. Outer cortical cells | Chlorenchyma absent | Chlorenchyma present |
| 3. Endodermis | Well defined | ill-defined or absent |
| 4. Vascular bundles | Radial arrangement | Conjoint arrangement |
| 5. Xylem | Exarch | Endarch |
Question 8.
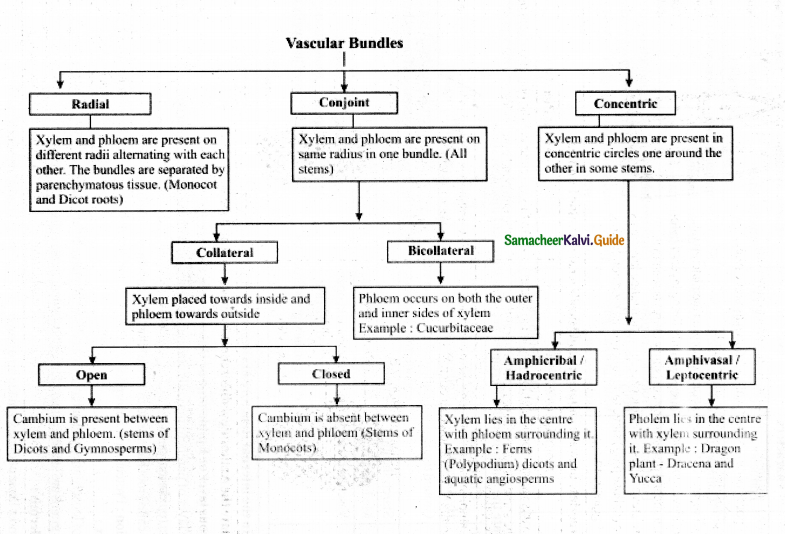
Explain the various types of vascular bundles in a tabulation form.
Answer:
Question 9.
Tabulate the types and characteristics of tissue systems.
Answer:
|
Types/characters |
Epidermal tissue system | Ground or fundamental tissue system |
Vascular or conduction tissue system |
| 1.Formation | Forms the outermost covering protoderm | Forms the ground meristem | Forms the procambial bundles |
|
2. Components |
epidermal cells, stomata and epidemic outgrowth | Simple permanent tissues – Parenchyma and Collenchyma | Xylem and Phloem |
| 3.Functions | Protection of plant body; absorption of water in roots; gas exchange for photosynthesis and respiration; transpiration in shoots | Gives mechanical support to the organs; prepares and stores food in leaf and stem |
Conducts is water and food: gives mechanical strength
|
Question 10.
Tabulate the Anatomical differences between stem and monocot stem
Answer:
|
Characters |
Dicot stem |
Monocot stem |
| 1. Hypodermis | Collenchymatous | Sclerenchymatous |
| 2. Ground tissue | Differentiated into cortex, endodermis, and peri cycle and pith | Not differentiated but it is a continuous mass of parenchyma |
| 3. Starch sheath | Present | Absent |
| 4. Medullary rays | Present | Absent |
| 5. Vascular bundles | a. Collateral and open b. Arranged in a ring c. Secondary growth occurs |
a. Collateral and closed b. Scattered in ground tissue c. Secondary growth usually does not occur |
Question 11.
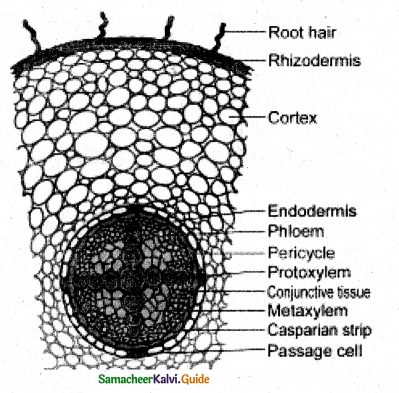
Explain the internal structure of Dicot root.
Answer:
The transverse section shows the following structure.
Piliferous layer or Epiblemma or Rhizodermis;
- Single-layer of parenchyma cells compactly arranged with out inter cellular space, devoid of circle and stomata (epidermal pores)
- Single called root hairs arise from the small cell known as trichoblast
Function: Protection & absorption
Cortex:
- Made of loosely arranged parenchyma cells with intercellular spaces.
- Starch grains are stored in, them leucoplasts occur in the cells.
Endodermis:
- Inner most layer of cortex – made up of single layer of barrel-shaped parenchyma cells.
- The radial and inner tangential walls have suberin and lignin thickening known as Casparian thickening.
- The cells opposite to protoxylem do not have Casparian thickening, known as Passage cells, which allow water to pass through but not the cells with Casparian thickening.
Stele:
All the tissue present inside endodermis comprise the stele, include pericycle & vascular bundle,
a) Pericycle:
Outer most layer of stele Single layer of parenchyma. The lateral roots originate from pericycle, so known to have endogenous origin.
Vascular bundle:
Made up of xylem and phloem.
Radial arrangement: In dicot root xylem and phloem are in different radii known as radial arrangement.
Exarch condition:
The protoxylem is pointing towards the periphery.
Tetrarch:
There are four protoxylem points, present this condition is known as tetrarch. Conjunctive tissue: The parenchyma tissue that separates xylem and phloem are known tissue.
Metaxylem – Vessels: are generally polygonal in cross-section.
Pith or Medulla: absent.
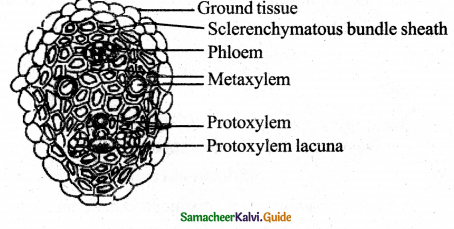
Question 12.
Explain the structure of the vascular bundle of Maize stem.
Answer:
- Vascular bundles are skull-shaped, numerous, bigger bundles towards the centre and numerous small bundles arranged in the periphery.
- Vascular bundles are scattered in the parenchymatous ground tissue. This condition is known as Atactostele.
- V – Bs Conjoint Collateral, Closed and Endarch in nature
- Pith or Medulla is absent.
Question 13.
Describe the Anatomy of Dicot stem.
Answer:
Epidermis:
A single layer of compactly arranged rectangular parenchymatous cells, with out intercellular space. Cuticle: on the outer walls check transpiration
Stomata: may be present here and their Chloroplasts: usually absent Multicellular hairs: occur in large numbers Function: Protective Cortex:
Lies below epidermis has 3 zones
1. Hypodermis:
Epidemial hay Made upof few layers of colknchyma cells liv- Cuticleing with thickenings at the successive tangential Epidenms avers giving mechanical inheiweeit
2. Chloresrchma:
A few layers below hypodermis with resin ducts in between
3. Parenchyma:
3rd zone, store food material.
Lndodermis or Starch Sheath:
inner most layer of cortex. barrel-shaped cells compactly managed without intercellular spaces.
Since starch grains are abundant in it. It is also a Medullary ray known as starch sheath, homologous to endodermis of root.
Stete: fonn a central ring inner to endodermis made up of Pericycle, VascuLar bundle & Pith.
Perlccle: A few layer of sclerenchyma outside the phloem, known as Hundk cap or Hard bast and also parenchynia cells between them constitute pericycic.
Vascular bundles:
Muscular bundles wedge-shaped arranged the form of a ring – (Eustelic)
Vascular bridle is made upon xylem. Phloem and cambium.
V – B is Conjoint, Collateral. Open and Endarch
Phloem: lies towards periphery.

Function : Conduction of organic food material
Cambium: brick-shaped thin-walled meristem responsible for secondary growth so. V – B is known as open V – B.

Function: Conduction of water and mineraLs from root to other parts.
Pith (Medulla): Central pith is present. It is parenchymatous.
Medullar ray: Pith extends between V – Bs as primary medullary ray.
FunctIon : Storage.
![]()
Question 14.
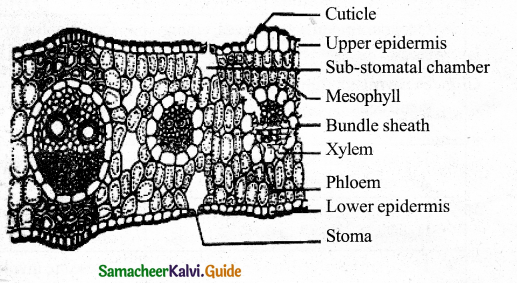
Explain the internal structure of monocot leaf. Epidermis:
Answer:
- A single layer of thin-walled cells with outer walls covered by thick cuticle.
- Stomata occur on both epidermis – stomata surrounded by dumbbell-shaped guard cells.
- Subsidiary cells: Surround guard cells.
- Bulliform cells : Occur on upper epidermis help for the rolling and un rolling of the leaf according to the weather change.
- Silica ceils: Some epidermal cells are filled with silica
Mesophyll:
- Grass, being isobilateral, mesophyll is not differentiated into palisade and spongy tissue, but compactly arranged cells with limited intercellular space.
Vascular Bundles:
- V.Bs differ in size – most of them are smaller, Large bundles occur at regular intervals
- Above and below large bundles sclerenchymatous patches occur – provide mechanical support, they are
- absent in small bundles.
- Bundle sheath – Each V.B is surrounded by a parenchymatous bundle sheath, generally contain starch grains.
- V.B has xylem upward and phloem towards the lower epidermis.
- V.Bs are Conjoint Collateral and Closed.
- In C4 grasses the bundle sheath cells are called Kranz sheath, involve in C4 cycle.
Question 15.
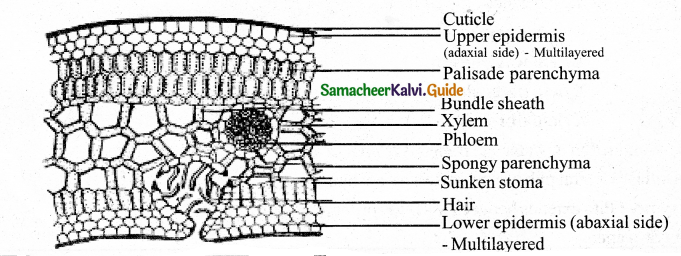
Draw the internal structure of Nerium leaf & Add a note on it’s special adaptive special features.
Answer:
- Multiseriate upper and lower Epidermis.
- Thick cuticle on the surface of upper epidermis
- Mesophyll is distinguished in to upper palisade and lower spongy parenchyma.
- Well developed vascular bundles with upper xylem and lower phloem (conjoint collateral closed V.B).
- Sunken stomata on the lower epidermis with trichomes, to reduce the rate of transpiration.
Question 16.
Difference between Stomata and Hydathodes.
Answer:
|
Stomata |
Hydathodes |
| 1. Occur in the epidermis of leaves, young stems | Occur at the tip or margin of leaves that are grown in moist shady place. |
| 2. Stomatal aperture is guarded by two guard cells | The aperture of hydathodes are surrounded by a ring of cuticularized cells |
| 3. The two guard cells are generally surrounded by subsidiary cell. | Subsidiary cells are absent |
| 4. Opening and closing of the stomatal aperture is regulated by guard cells. | Hydathodes pores remain always open. |
| 4. These are involved in transpiration and exchange of gases. | These are involved in guttation |
![]()






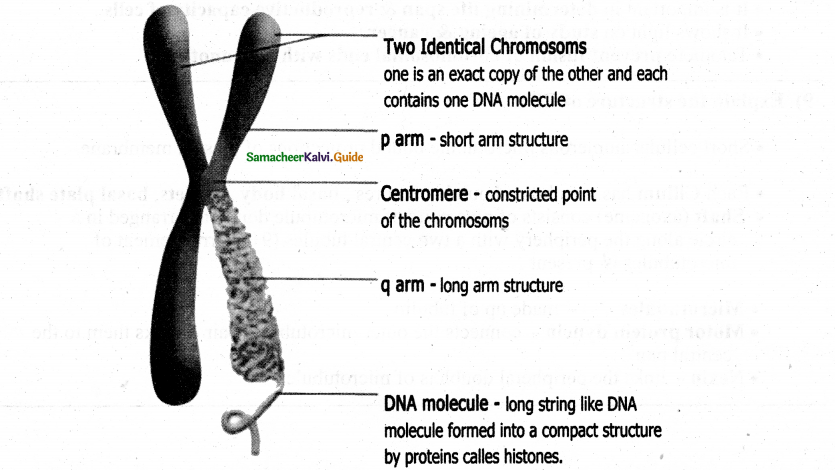
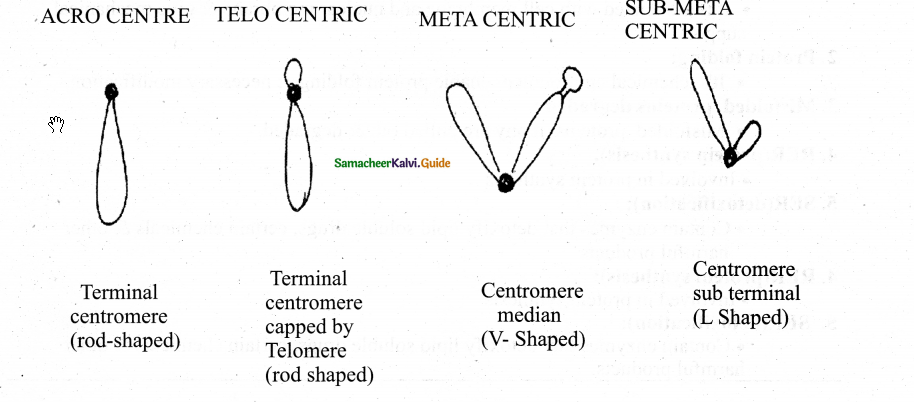
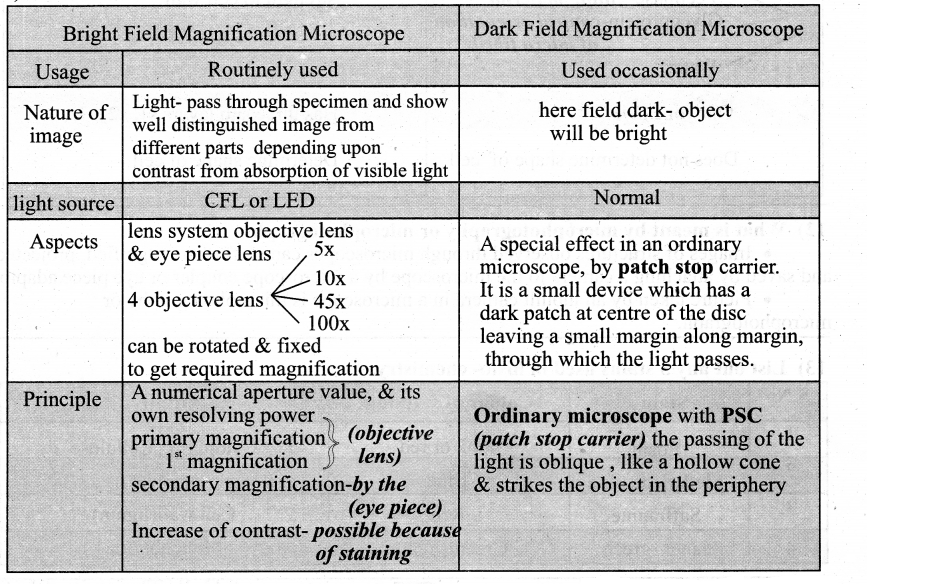
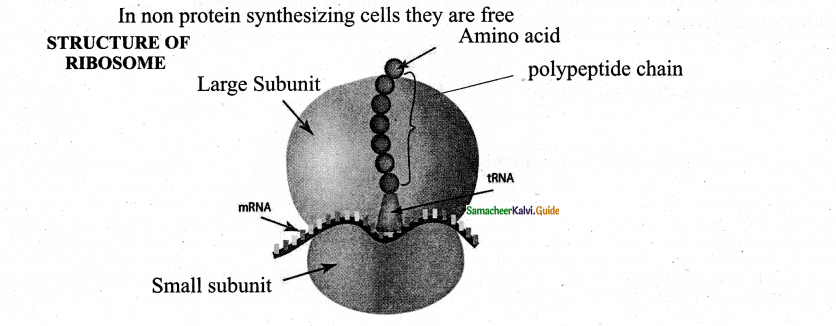
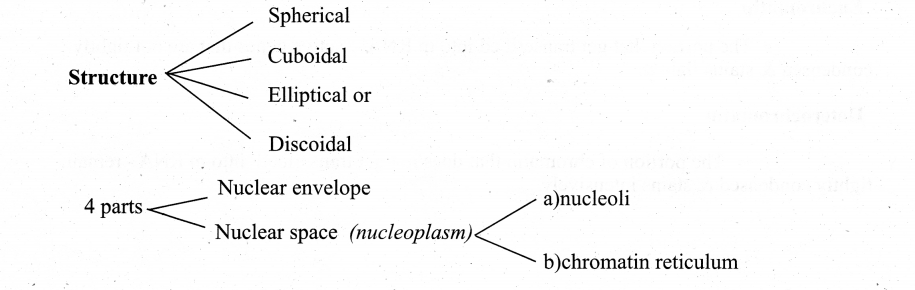 Nuclear envelope Nuclear space (nucleoplasm)
Nuclear envelope Nuclear space (nucleoplasm)