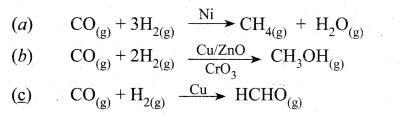
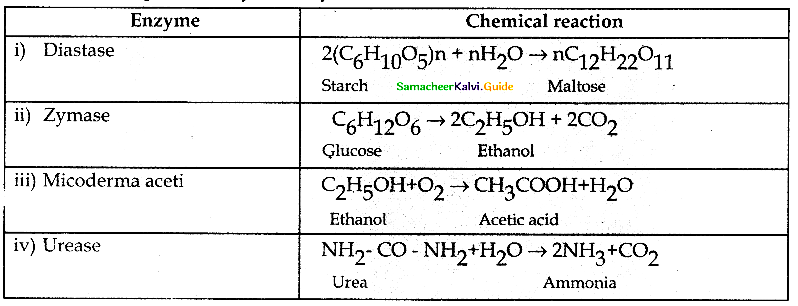
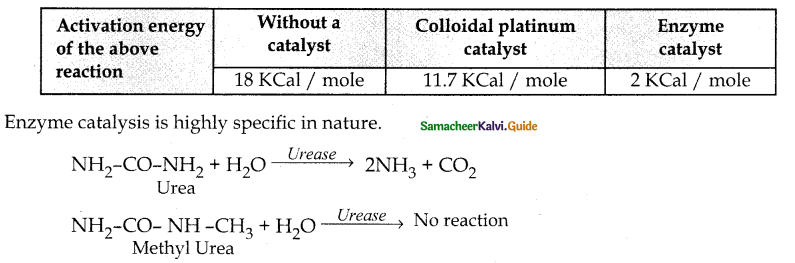
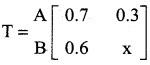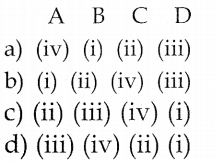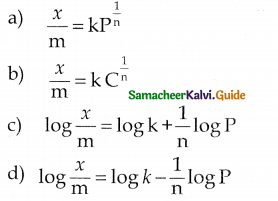Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Chemistry Guide Pdf Chapter 11 Hydroxy Compounds and Ethers Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 11 Hydroxy Compounds and Ethers
12th Chemistry Guide Hydroxy Compounds and Ethers Text Book Questions and Answers
Part – I Text Book Evaluation
I. Choose the correct answer
Question 1.
An alcohol (x) gives blue colour in victormayer’s test and 3.7g of X when treated with metallic sodium liberates 560 mL of hydrogen at 273 K and 1 atm pressure what will be the possible structure of X?
(a) CH3 CH (OH) CH2CH3
(b) CH3 – CH(OH) – CH3
(c) CH3 – C (OH) (CH3)2
(d) CH3 – CH2 – CH (OH) – CH2 – CH3
Answer:
(a) CH3 CH (OH) CH2CH3
Hint:
2R – OH + Na → 2RONa + 2H2 ↑ 2 moles of alcohol gives 1 mole of H2 which occupies
22.4L at 273K and 1 atm
number of moles of alcohol = \(\frac{2 \text { moles of } \mathrm{R}-\mathrm{OH}}{22.4 \mathrm{L} \text { of } \mathrm{H}_{2}}\) x 560 mL = 0.05 moles
number of moles = \(\frac{\text { mass }}{\text { molar mass }}\)
= molar mass = \(\frac{3.7}{0.05}\) = 74 g mol-1
General formula for
R – OH Cn H2n+1 – OH
n(12) + (2n+1) (1) + 16 +1 = 74
14n = 74 – 18
14n = 56
n = \(\frac { 56 }{ 4 }\) = 4
The 2° alcohol which contains 4 carbon is CHn CH(OH)CH2 CH3
![]()
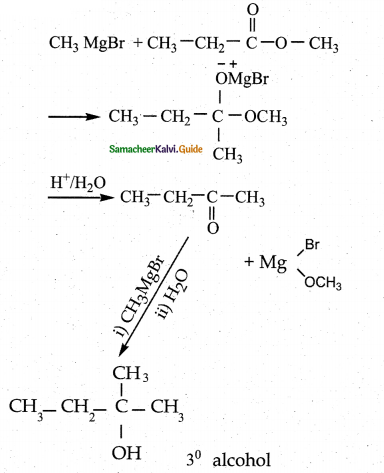
Question 2.
Which of the following compounds on reaction with methyl magnesium bromide will give tertiary alcohol.
(a) benzaldehyde
(b) propanoic acid
(c) methyl propanoate
(d) acetaldehyde
Answer:
(c) methyl propanoate
Solution:
Question 3.
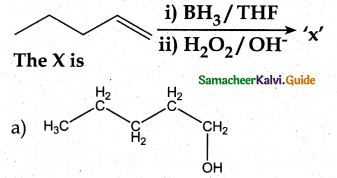
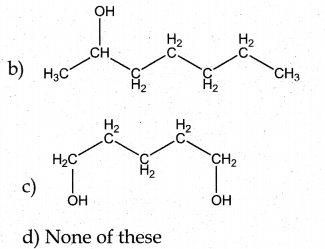
Answer:
a
Solution:
hydro boration – Anti markownikoff product
i.e CH3 – CH2 – CH – CH2 – CH2 – OH
![]()
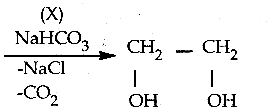
Question 4.
In the reaction sequence, Ethane
![]()
Ethan – 1, 2 – diol. A and X respectively are ………….
(a) Chioroethane and NaOH
(b) ethanol and H2SO4
(c) 2 – chloroethan – 1 – ol and NaHCO3
(d) ethanol and H2O
Answer:
(c) 2 – chloroethan – 1 – ol and NaHCO3\

Solution:
Question 5.
Which one of the following is the strongest acid ………..
(a) 2 – nitrophenol
(b) 4 – chlorophenol
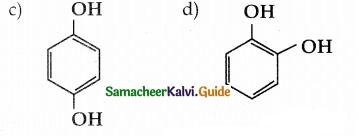
(c) 4 – nitrophenol
(d) 3 – nitrophenol
Answer:
(c) 4 – nitrophenol
![]()
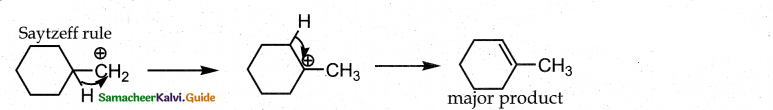
Question 6.
on treatment with Con. H2SO4, predominately gives ……………..
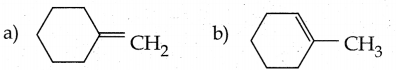
Answer:
b
Solution:
Question 7.
Carbolic acid is …………..
(a) Phenol
(b) Picric acid
(c) benzoic acid
(d) phenylacetic acid
Answer:
(a) Phenol
![]()
Question 8.
Which one of the following will react with phenol to give salicyladehyde after hydrolysis …………..
(a) Dichioro methane
(b) trichioroethane
(c) trichloro methane
(d) CO2
Answer:
(c) trichloro methane (Riemer Tiemann reaction)
Question 9.
![]()
(a) (CH3)3 CCH = CH2
(b) (CH3)2 C = C (CH3)2
(c) CH2 = C(CH3)CH2 – CH2 – CH3
(d) CH2 = C (CH3) – CH2 – CH2 – CH3
Answer:
(b) (CH3)2 C = C (CH3)2
Solution:
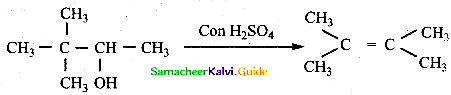
![]()
Question 10.
The correct IUPAC name of the compound,
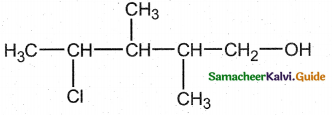
(a) 4 – chloro – 2, 3 – dimethyl pentan – 1 – ol
(b) 2, 3 – dimethyl – 4 – chloropentan – 1 – ol
(c) 2, 3, 4 – trimethyl – 4 – chiorobutan – 1 – ol
(d) 4 – chioro – 2, 3, 4 – trimethyl pentan – 1 – ol
Answer:
(a) 4 – chloro – 2, 3 – dimethyl pentan – 1 – ol
Question 11.
Assertion: Phenol is more acidic than ethanol
Reason: Phenoxide ion is resonance stabilized
(a) if both assertion and reason are true and reason is the correct explanation of assertion.
(b) if both assertion and reason are true but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false
(d) both assertion and reason are false.
Answer:
if both assertion and reason are true and reason is the correct explanation of assertion.
Question 12.
In the reaction Ethanol
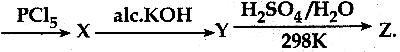
is ………………
(a) ethane
(b) ethoxyethane
(c) ethylbisuiphite
(d) ethanol
Answer:
(d) ethanol
Solution:
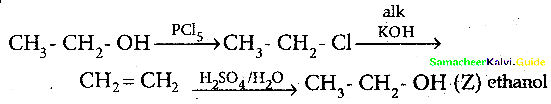
![]()
Question 13.
The reaction
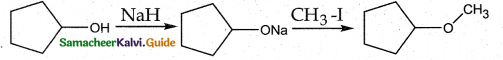
can be classified as
(a) dehydration
(b) Williams on alcohol synthesis
(c) Williamson ether synthesis
(d) dehydrogenation of alcohol
Answer:
(c) Williamson ether synthesis
Solution:
Cyclic alcohol → sodium cyclic alkoxide → Williamson ether synthesis
Question 14.
Isoprophylbcnzene on air oxidation in the presence of dilute acid gives …………
(a) C6H5COOH
(b) C6H5COCH3
(c) C6H5COC6H5
(d) C6H5 – OH
Answer:
(a) C6H5 – OH (phenol)
Question 15.
Assertion: Phenol is more reactive than benzene towards electrophilic substitution reaction
Reason: In the case of phenol. the intermediate arenium ion is more stabilized by resonance.
(a) if both assertion and reason are true and reason is the correct explanation of assertion.
(b) if both assertion and reason are true but reason is not the correct explanation of assertion.
(c) assertion is true but reason is false
(d) both assertion and reason are false,.
Answer:
(a) if both assertion and reason are true and reason is the correct explanation of assertion.
![]()
Question 16.
HO CH2 CH2 – OH on heating with periodic acid gives ………..
(a) methanoic acid
(b) Glyoxal
(c) methanol
(d) CO2
Answer:
(c) methanol
Question 17.
Which of the following compound can be used as artireeze in automobile radiators?
(a) methanol
(b) ethanol
(c) Neopentyl alcohol
(d) ethan -1, 2-diol
Answer:
(d) ethan -1, 2-diol
Question 18.
The reaction
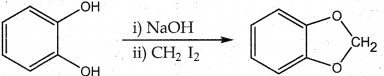
is an example of …………..
(a) Wurtz reaction
(b) cyclic reaction
(c) Williamson reaction
(d) Kolbe reactions
Answer:
(c) Kolbe reactions
![]()
Question 19.
One mole of an organic compound (A) with the formula C3H8O reacts completely with two moles of HI to form X and Y. When Y is boiled with aqueous alkali it forms Z. Z answers the iodoform test. The compound (A) is ……………
(a) propan – 2 – ol
(b) propan- 1- ol
(c) ethoxy ethane
(d) methoxy ethane
Answer:
(d) methoxy ethane
Solution:
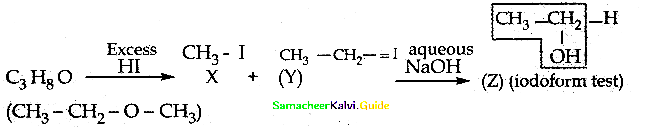
![]()
Question 20.
Among the following ethers which one will produce methyl alcohol on treatment with hot HI?
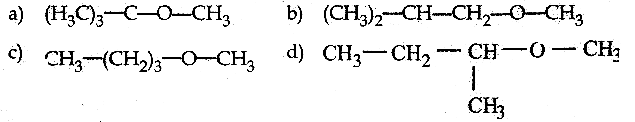
Answer:
a
Solution:
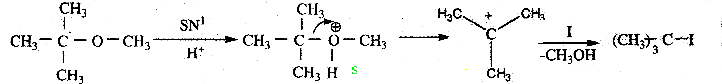
![]()
Question 21.
Williamson synthesis of preparing dimethyl ether is a / an
(a) SN1 reactions
(b) SN2 reaction
(c) electrophilic addition
(d) electrophilic substitution
Answer:
(b) SN2 reaction
Question 22.
On reacting with neutral ferric chloride, phenol gives
(a) red colour
(b) violet colour
(c) dark green colour
(d) no colouration
Answer:
(b) violet colour
II. Short Answer
Question 1.
IdentIfy the product (s) is/are formed when 1 – methoxy propane is heated with excess HI. Name the mechanism involved in the reaction.
Answer:
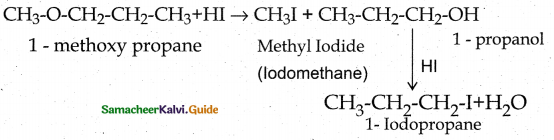
Ethers having primary alkyl group undergo \(\mathrm{S}_{\mathrm{N}}^{2}\) reaction
Question 2.
Draw the major product formed when 1 – ethoxyprop – 1 – ene is heated with one equivalent of HI
Answer:
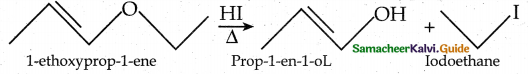
Question 3.
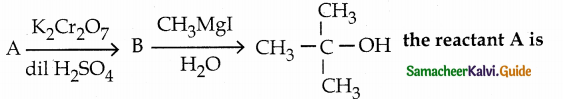
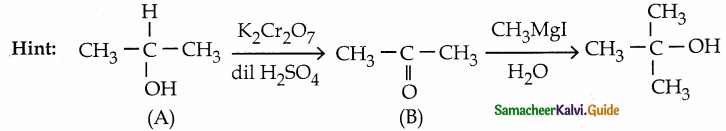
Suggest a suitable reagent to prepare secondary alcohol with an identical groups using a Grignard reagent.
Answer:
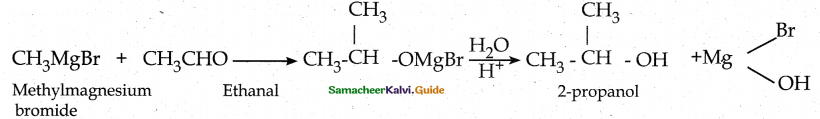
![]()
Question 4.
What is the major product obtained when two moles of ethyl magnesium bromide is treated with methyl benzoate followed by acid hydrolysis
Answer:
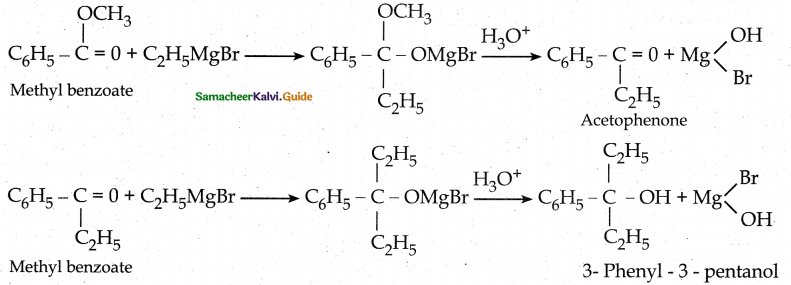
Question 5.
Predict the major product, when 2-methyl but – 2 – ene is converted into alcohol in each of the following methods.
- Acid-catalyzed hydration
- Hydroboration
- Hydroxylation using bayers reagent
Answer:
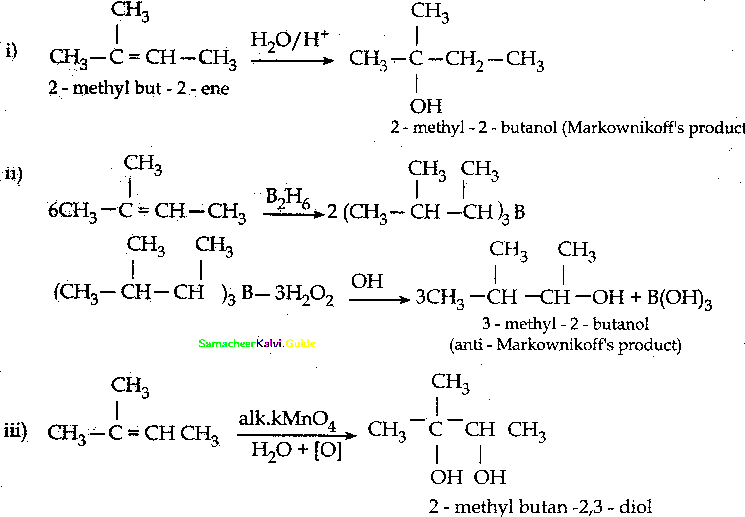
![]()
Question 6.
Arrange the following in the increasing order of their boiling point and give a reason for your ordering
- Butan – 2 – ol, Butan – 1 – SI, 2 – methylpropan – 2 – ol
- Propan – 1 – ol, propan – 1, 2, 3 – triol, propan – 1, 3 – diol, propan – 2 – ol
Answer:
1. Boiling points increases regularly as the molecular mass increases due to a corresponding increase in their Van der Waal’s force of attraction. Among isomeric alcohols, 2° – alcohols have lower boiling points than 1° – alcohols due to a corresponding decrease in the extent
of H-bonding because of steric hindrance. Thus the boiling point of Butan – 2 – ol is lower than that of Butan – 1 – ol. Overall increasing order of boiling points is, 2 – methyl propane – 2 – ol < Butan – 2 – ol < Butan – 1 – ol
2. 2°-alcohols have lower boiling points than 1° – alcohols due to a corresponding decrease in the extent of H – bonding because of steric hindrance. Therefore Propan – 1 – ol has higher boiling point than Propan – 2 – ol. The hydrogen group increases, boiling point also increases. Overall increasing order of boiling points is, propan – 2 – ol < Propan – 1 – ol < propan – 1, 3 – diol < propan -1, 2, 3 – triol
Question 7.
Can we use nucleophiles such as NH3, CH3O for the Nucleophilic substitution of alcohols
Answer:
1. Increasing order of nucleophilicity,
NH3 < – OH⊕ < CH3O⊖-
2. Higher electron density will increase the nucleophilicity.
3. Negatively charged species are almost always more nucleophiles than neutral species.
4. RO⊖ has an alkyl group attached, allowing a greater amount of polarizability. This means oxygen’s lone pairs will be more readily available to reach in RO⊖ than in OH⊖. Hence CH3O – is the better nucleophile for the nucleophilic substitution of alcohols. NH3 cannot act as nucleophiles for the nucleophilic substitution of alcohols.
![]()
Question 8.
Is it possible to oxidise t – butyl alcohol using acidified dichromate to form a carbonyic compound.
Answer:
3° – alcohols do not undergo oxidation reaction under normal conditions, but at elevated temperature, under strong oxidising agent cleavage of C – C bond takes place to give a mixture of carboxylic acid.
Yes, it is possible. t – butyl alcohol is readily oxidsing in acidic solution (K2Cr2O7 / H2SO4) to a mixture of a ketone and an acid each containing lesser number of carbon atoms than the original alcohol. The oxidation presumably occur via alkenes formed through dehydration of alcohols under acidic conditions.
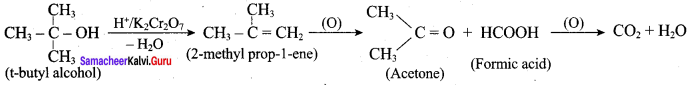
Question 9.
What happens when 1 – phenyl ethanol is treated with acidified KMnO4.
Answer:
1 – phenyl ethanol reacts with acidified KMnO4 to give Acetophenone.
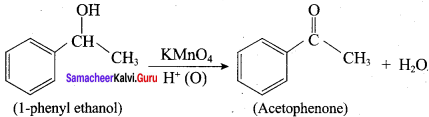
Question 10.
Write the mechanism of acid catalysed dehydration of ethanol to give ethene.
Answer:
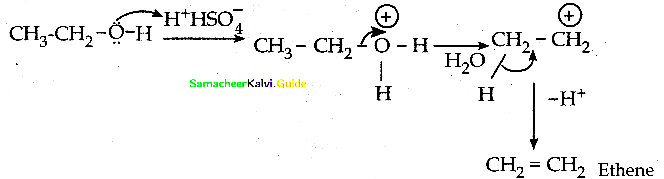
Question 11.
How is phenol prepared form
- chloro benzene
- isopropyl benzene
Answer:
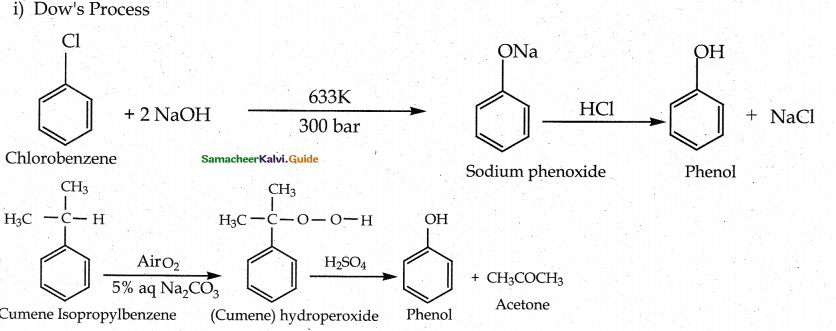
![]()
Question 12.
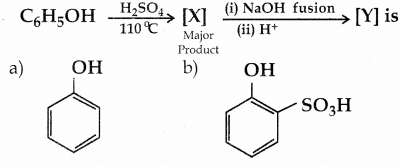
Explain Kolbe’s reaction
Answer:
Kolbe’s (or) Kolbe’s Schmitt reaction:
In this reaction, phenol is first converted into sodium phenoxide which is more reactive than phenol towards electrophilic substitution reaction with CO2. Treatment of sodium phenoxide with CO2 at 400K, 4 -7 bar pressure followed by acid hydrolysis gives salicylic acid.
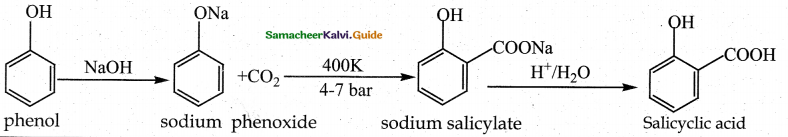
Question 13.
Writes the chemical equation for Williamson synthesis of 2 – ethoxy – 2 – methyl pentane starting from ethanol and 2 – methyl pentan – 2 – ol
Answer:
A tertiary alkoxide and primary alkyl halide easily undergo williamson ether synthesis
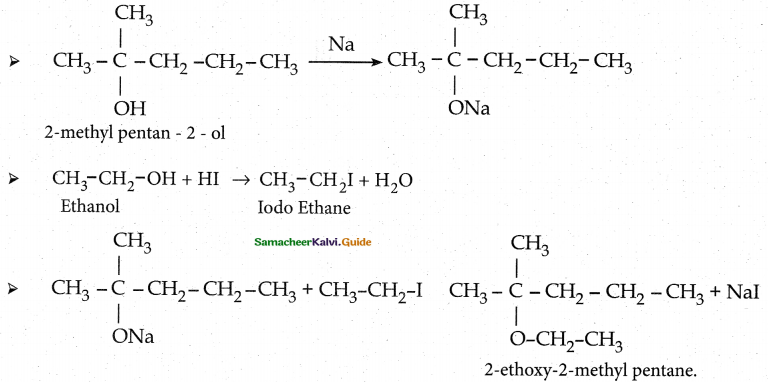
Question 14.
Write the structure of the aldehyde, carboxylic acid and ester that yield 4 – methylpent – 2 – en – 1 – ol.
Answer:
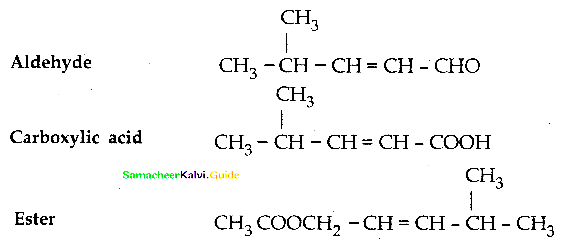
![]()
Question 15.
What is metamerism? Give the structure and IUPAC name of metamers of 2 – methoxy propane
Answer:
Metamerism:
It is a special type of isomerism in which molecules with same formula, same functional group, but different only in the nature of the alkyl group attached to oxygen.
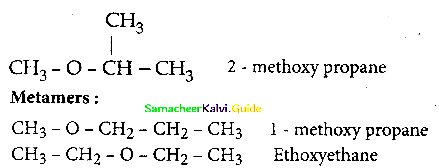
Question 16.
How are the following conversions effected
- benzyl chlorjde to benzyl alcohol
- benzyl alcohol to benzoic acid
Answer:
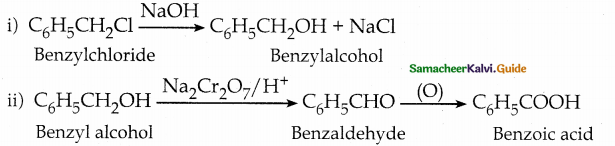
Question 17.
Complete the following reactions
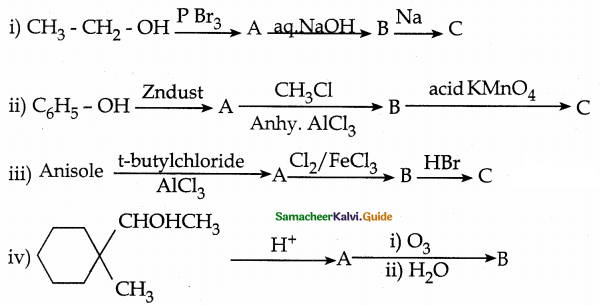
Answer:
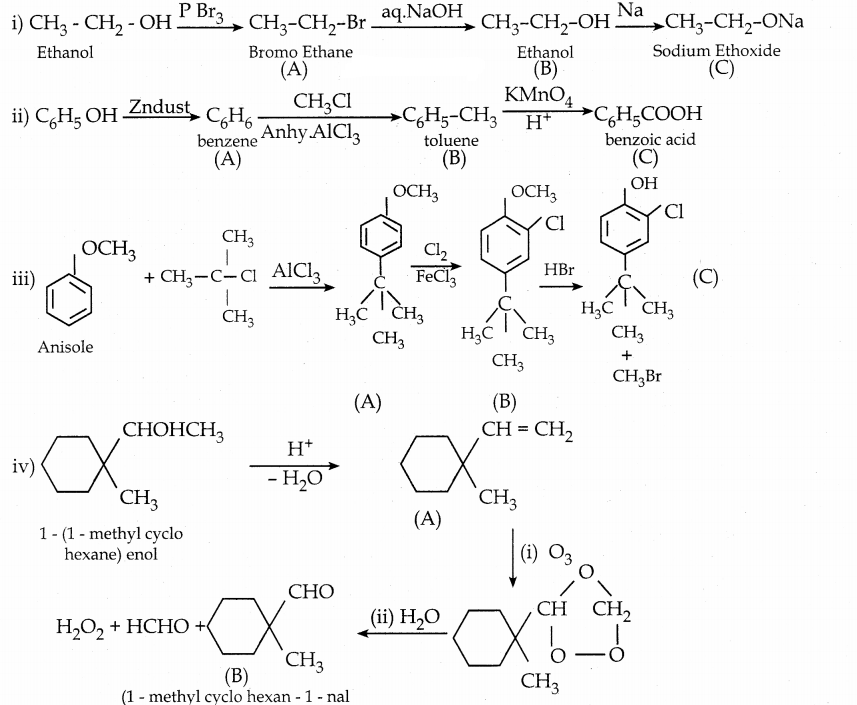
![]()
Question 18.
O.44g of a monohydric alcohol when added to methyl magnesium iodide in ether liberates at STP 112 cm3 of methane with PCC the same alcohol form a carbonyl compound that answers silver mirror test. Identify the compound.
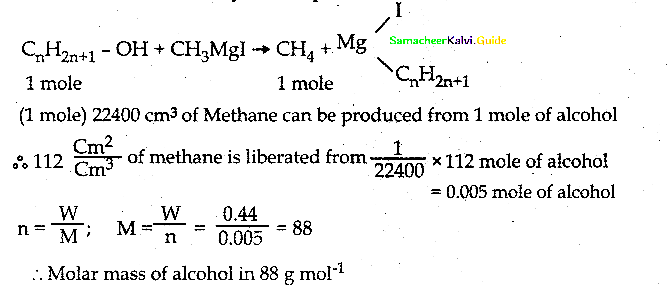
CnH2n+1+OH
⇒ n x 12+ (2n + 1) x 1 + 1 x 16 + 1 x 1 = 88
12n + 2n + 1 + 16 + 1 = 88
14n + 18 = 88
14n = 88 – 18
14n = 70
n = 70/14 = 5
Answer:
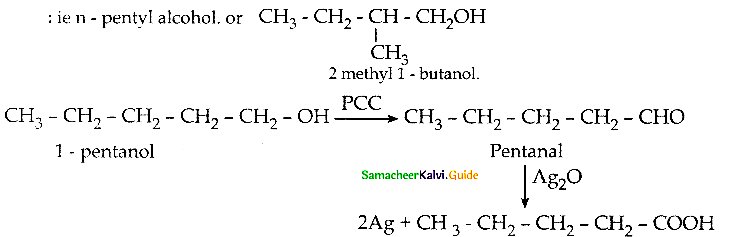
Question 19.
Complete the following reactions
Answer:
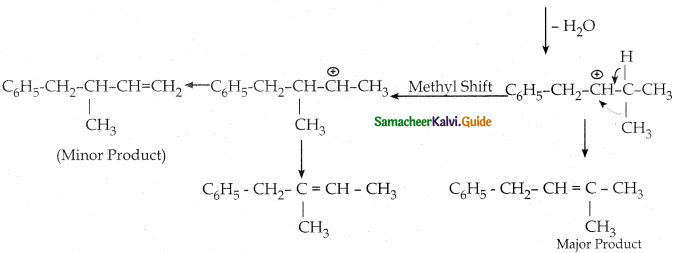
(According to Saytzeff s rule, during intramolecular dehydration, if there is a possibility to form C = C bond at different locations, the preferred location is the one that gives the more substituted alkene je, the stable alkene).
Question 20.
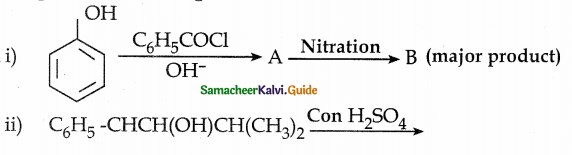
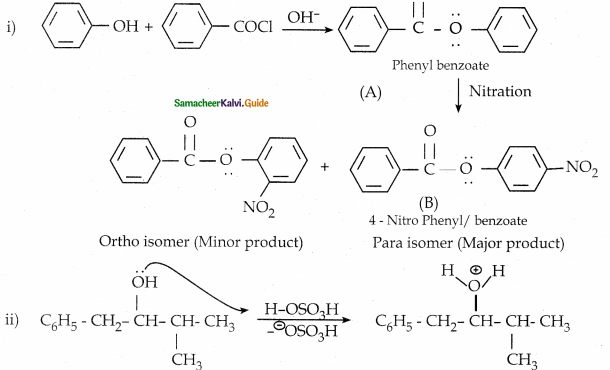
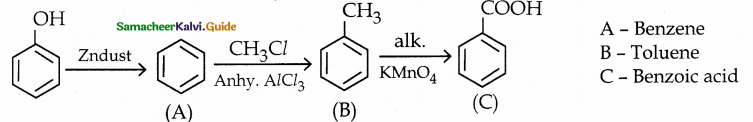
Phenol is distilled with Zn dust gives (A) followed by Friedel – crafts alkylation with propyl chloride to give a compound B, B on oxidation gives (C). Identify A,B and C.
Answer:
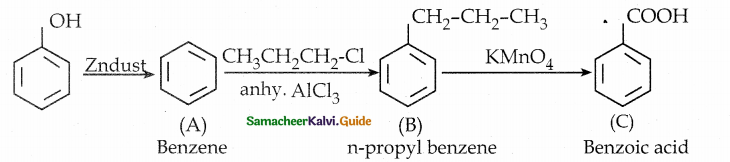
Note:
Carbon directly attached to the aromatic riñg is called benzylic carbon.
If there is hydrogen attached to benzylic carbon it will undergo oxidation.
Question 21.
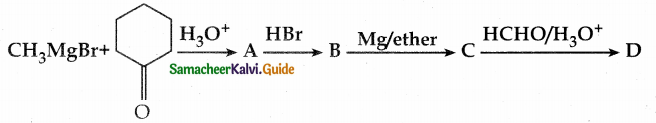
Identify A, B, C, D and write the complete equation.
Answer:
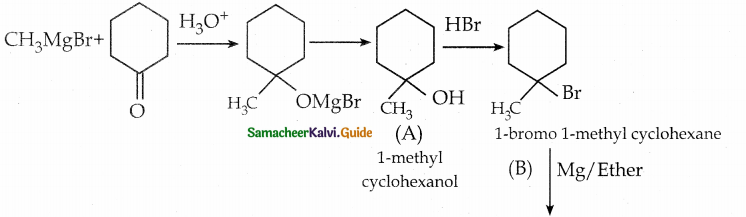
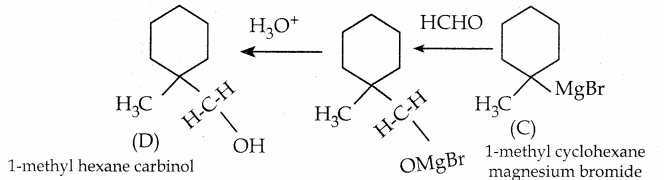
![]()
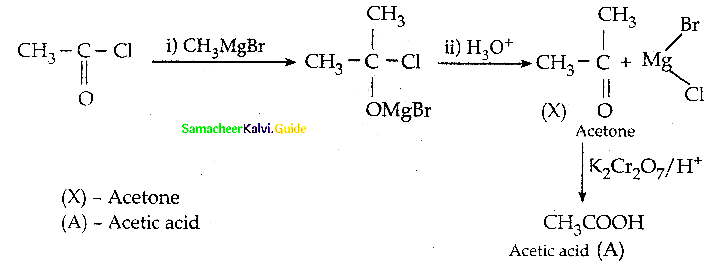
Question 22.
What will be the product for the following reaction
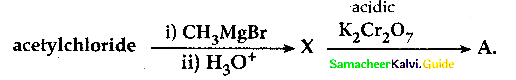
Answer:
Question 23.
How will you convert acetylene into n – butyl alcohol.
Answer:
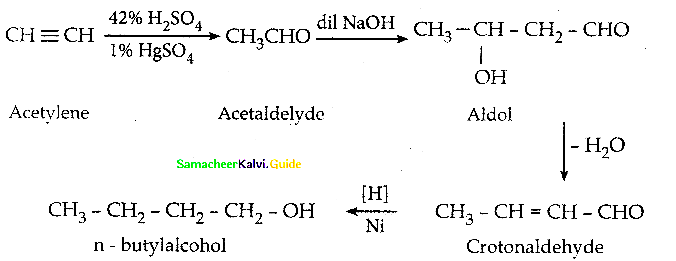
Question 24.
Predict the product A, B, X and Y in the following sequence of reaction
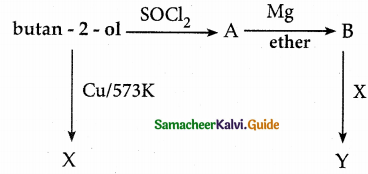
Answer:
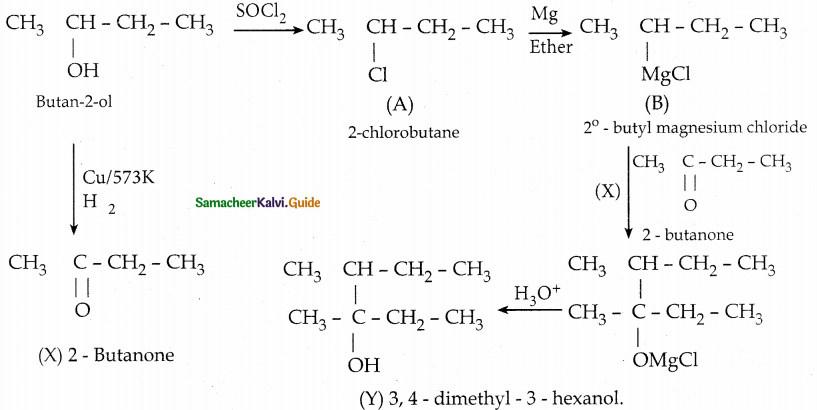
![]()
Question 25.
3,3 – dimethyl butane – 2 – ol on treatment with conc. H2SO4 to give tetramethyl ethylene as a major product. Suggest suitable mechanisms.
Answer:
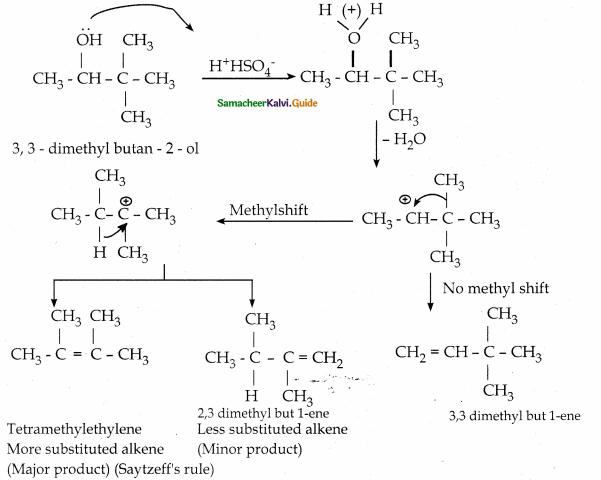
III. Evaluate yourself
Question 1.
Classify the following alcohols as 10, 20, and 30 and give their IUPAC Names.
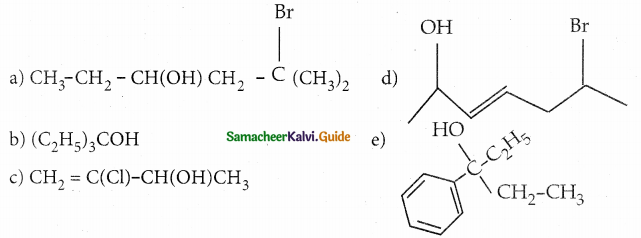
Answer:
| a 2° alcohol | 5- bromo -5- methyl -3 hexanol |
| b 3° alcohol | 3 – ethyl -3- pentanol |
| c 2° alcohol | 3- chiorobut -3- en – 1 – o! |
| d 2°alcohol | 6-bromohept-3-en-2-ol |
| e 3° alcohol | 3- phenyl -3 – pentanol |
![]()
Question 2.
Write all the possible isomers of alcohol having the molecular formula C15H12O and their IUPAC names.
Answer:
Eight isomers are possible for C15H12O. They are,
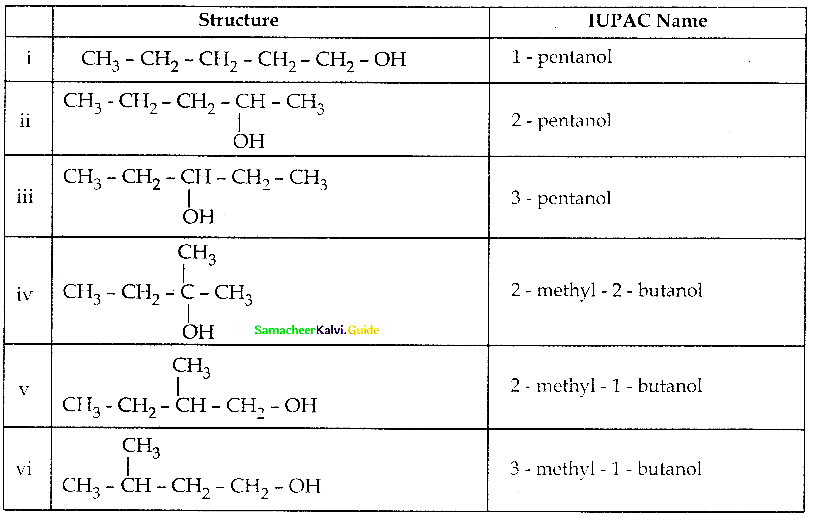
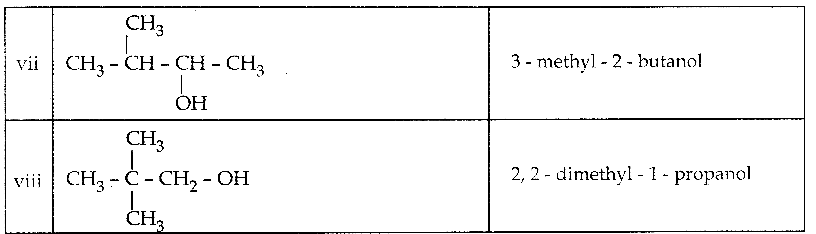
![]()
Question 3.
Suggest a suitable carbonyl compound for the preparation of pent – 2 – en – 1 ol using LiAlH4.
Answer:

Question 4.
2 – methylpropan – 2 – ene
![]()
Answer:
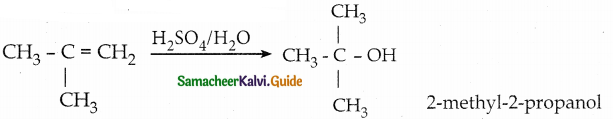
![]()
Question 5.
How will you prepare the following using a Grignard reagent?
- t – butyl alcohol
- allyl alcohol
Answer:
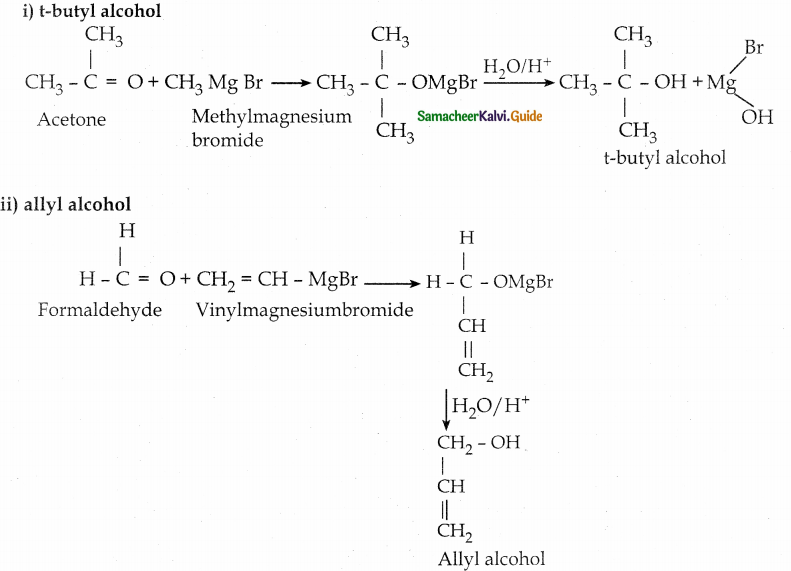
Question 6.
Identify the products in the following reactions. Write their IUPAC names and mention the mechanism involved in the reactions.

Answer:
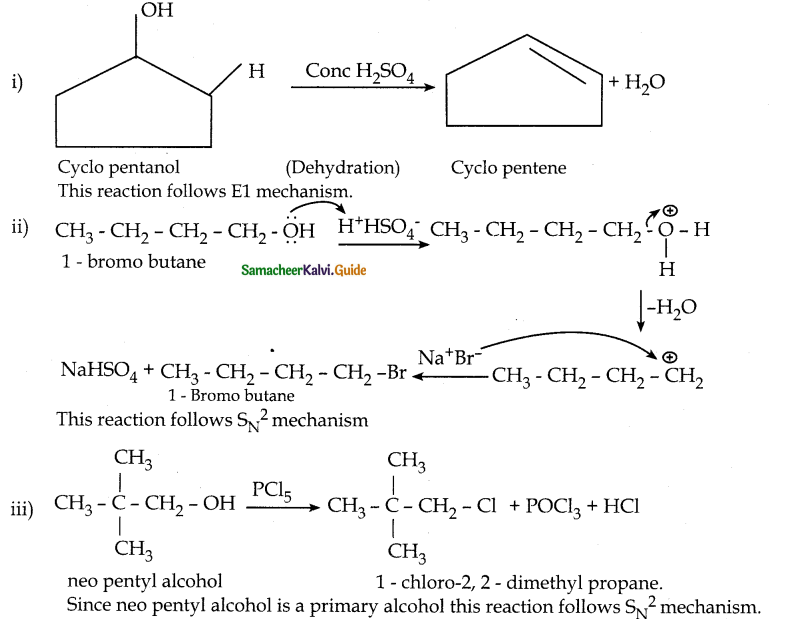
![]()
Question 7.
What is the major product obtained when 2, 3 – dimethyl pentan – 3 – ol is heated in the presence of H2SO4
Answer:
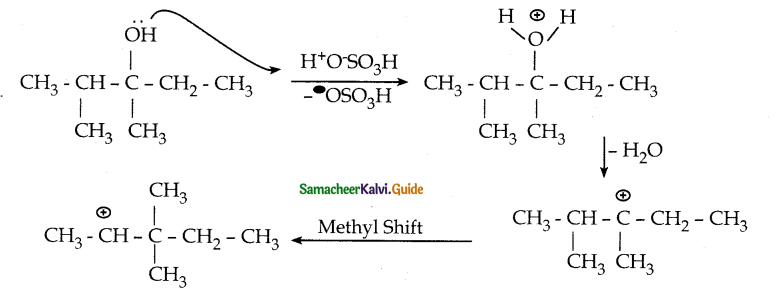
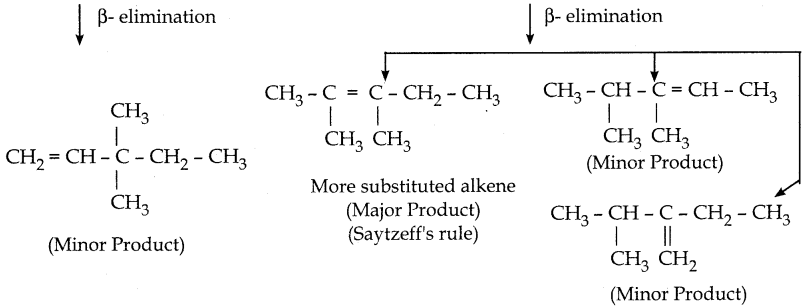
Question 8.
Which of the following set of reactants will give 1 – methoxy – 4 – nitrobenzene.
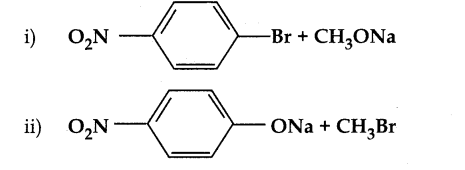
Answer:
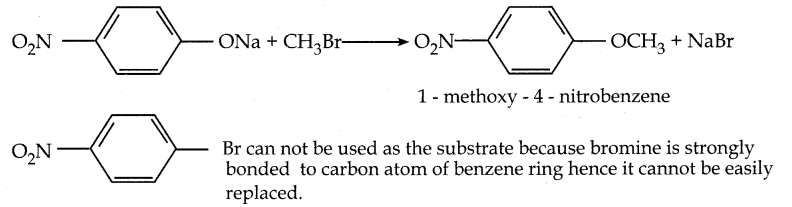
Question 9.
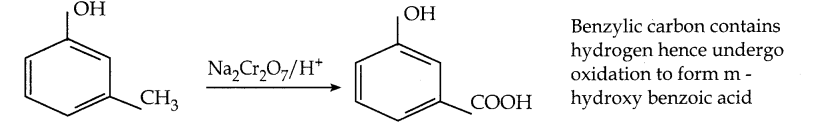
What happens when m – cresol is treated with an acidic solution of sodium dichromate?
Answer:
Question 10.
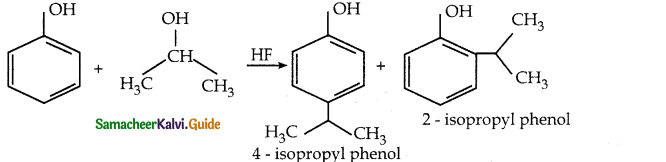
When phenol is treated with propan – 2 – ol in the presence of HF, Friedel – Craft reaction takes place. Identify the products.
Answer:
![]()
Question 11.
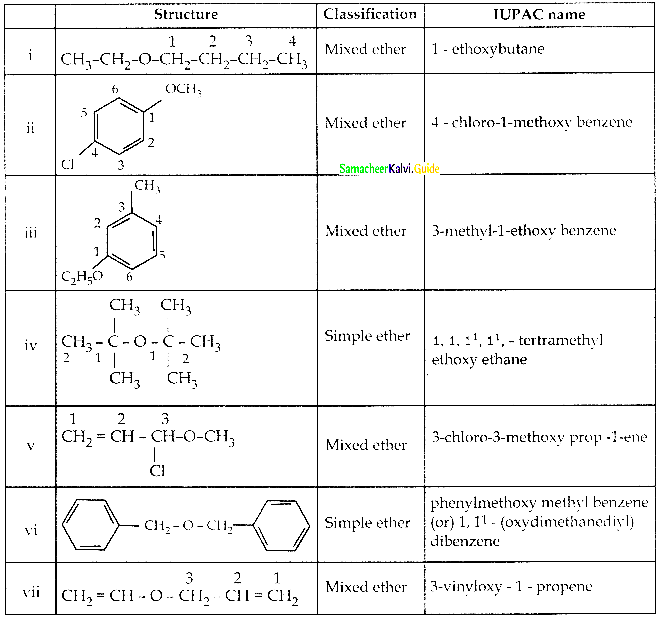
Given the IUPAC name for the following ethers and classify them as simple or mixed.
Answer:
Question 12.
1. Which of the following reaction will give 1 – methoxy – 4 – nitrobenzene.
- 4 – nitro – 1 – bromobenzene + sodium methoxide.
- 4 – nitrosodium phenoxide + bromomethane
Answer:
4-nitrosodium phenoxide + bromo methane → 1 – methoxy – 4 – nitrobenzene
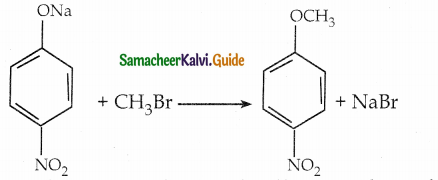
Reason: 4 – nitro -1- brornobnzene + sodium methoxide can not be used, because bromine is strongly bonded to carbon atom of hcnzcne ring and it can not be replaced easily.
Question 13.
Arrange the following compounds in the increasing order of their acid strength. propan – 1 – ol, 2, 4, 6 – trinitroptienol, 3 – nitrophenol, 3,5 – dinitrophenol, phenol, 4 – methyl phenol.
Answer:
Phenols are stronger acids than alcohols because the phenoxide ion left after the removal of proton is stabilized by resonance while the alkoxide ion left after the removal of a proton from alcohol is not stabilized. Thus propan – 1 – ol is much weaker acid than any phenol.
Thus propan- 1 – ol is a much weaker acid than any phenol. We know that electron-donating groups decrease the acidic character and stronger is the electron-donating group, weaker is the phenol.
Compare to propan – 1 – ol, 4 – methyl phenol is a stronger acidic character. But comparing phenol and 4-methyl phenol, phenol is stronger acidic. Since electron-withdrawing groups increase the acidic character of phenols and the effect is more pronounced at the para position than at the meta position.
Therefore 4 – nitrophenol is a stronger acid than 3 – nitro phenol. Further as the number of electron-withdrawing groups increases the acidic strength further increases. Therefore 2, 4, 6 – trinitrophenol is a stronger acid than 3, 5 – dinitrophenol. It may be noted here that although the two nitro groups in 3, 5 – dinitrophenol are at m – position with respect to OH group,
their combined effect is however greater than one nitro group at p – position. Therefore 3, 5 – dinitrophenol is a stronger acid than 4-nitro phenol. Thus, the overall increasing order of acid strength is. Propan – 1 – 01 < 4 – methyl phenol < phenol < 3 – nitrophenol < 3, 5 – dinitrophenol < 2, 4, 6 – trinitrophenol.
![]()
Question 14.
1 mole of HI is allowed to react with t – butyl methylether. Identify the product and write down the mechanism of the reaction.
Answer:
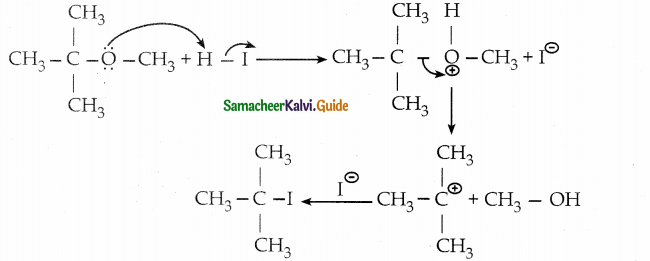
The reaction involves the protonation of oxygen which is followed by the SN1 mechanism.
The products are t-butvl iodide and methyl alcohol.
12th Chemistry Guide Hydroxy Compounds and Ethers Additional Questions and Answers
Part – II – Additional Questions
I. Choose the correct answer
Question 1.
In ethanol the – OH group is attached to hybridised carbon
a) sp
b) sp2
c) sp3
d) dsp2
Ans :
c) sp3
Question 2.
In Vinyl alcohol the -OH group is attached to hvbridised carbon
a) sp
b) sp2
c) sp3
d) dsp2
Ans :
b) sp2
![]()
Question 3.
Sorbitol is
a) monohydric alcohol
b) dihydric alcohol
c) trihydric alcohol
d) hexa hydric alcohol
Answer:
d) hexa hydric alcohol
Question 4.
The IUPAC name of sorbitol is
a) Ethan -1, 2 – diol
b) Propan – 1, 2, 3 – triol
c) Hexan – 1, 2, 3, 4, 5, 6 – hexol
d) Ethenol
Answer:
c) Hexan – 1, 2, 3,4, 5, 6 – hexol
Question 5.
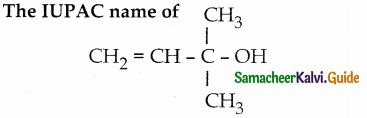
a) prop – 2 – en – 1 – ol
b) 2 – methyl but – 3 – en – 2 ol
c) 3 – methyl but – 1 – en – 3 – ol
d) 1,1 – di methyl prop – 2 – en – 1 – ol
Answer:
b) 2 – methyl but – 3 – en – 2 ol
Question 6.
The C – O – H bond angle in methanol is
a) 107°
b) 109°.5°
c) 108.9°
d) 104°
Answer:
c) 108.9°
![]()
Question 7.
CH3-CH2-a + NaOH → CH3-CH2-OH + NaCl This reaction follows mechanism
a) \(S_{N}^{1}[latex]
b) [latex]S_{N}^{2}\)
c) E1
d) E2
Answer:
b) \(S_{N}^{2}\)
Question 8.
Conversion of isobutyl chloride and isopropyl chloride into isobutyl alcohol and isopropyl alcohol respectively using dilute aquous NaOH follow the mechnisms

Answer:
c)
Reason : Isobutylchloride – Primary alkyl halide – \(S_{N}^{2}\) mechanism.
Iso propyl chloride – Secondary alkyl halide – Sjsj mechanism.
\(S_{N}^{1}\)
![]()
Question 9.
Addition of water across the double bond
of an unsymmetrical alkene follows
a) Saytzeffs rule
b) Markownikoff’s rule
c) anti Markownikoff’s rule
d) Popoff’s rule
Answer:
b) Markownikoff’s rule
Question 10.
Hydroboration of an alkene follows
a) Saytzeff’s rule
b) Markownikoff’s rule
c) anti Markownikoff’s rule
d) Popoff’s rule
Answer:
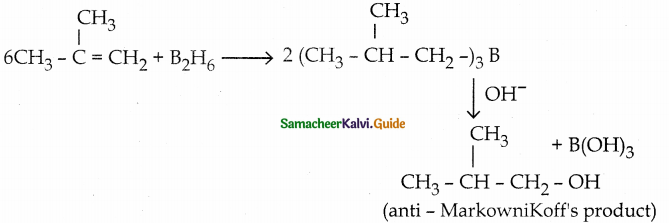
c) anti – Markownikoff’s rule
Question 11.
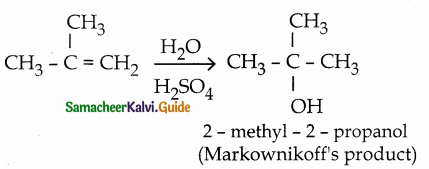
Addition of water to 2-methyl propene in
presence of cone, sulphuric acid gives
a) 2-propanol
b) 2 – methyl – 2 – propanol
c) 2 – methyl – 1 – propanol
d) 2 – butanol
Answer:
b) 2 – methyl – 2 – propanol
Question 12.
2 – methyl propene reacts with diborane followed by H2O2 in presence of NaOH gives
a) 2 – proponol
b) 2 – methyl – 2 – propanol
c) 2 – methyl – 1 – propanol
d) 2 – butanol
Answer:
c) 2 – methyl -1 – propanol
![]()
Question 13.
To prepare a primary alcohol a Grignard reagent must be reacted with
a) HCHO
b) RCHO
c) RCOR
d) RNH2
Answer:
a) HCHO
Question 14.
To prepare a secondary alcohol a
Grignard reagent must be reacted with
a) HCHO
b) RCHO
c) RCOR
d) RNH2
Answer:
b) RCHO
Question 15.
To prepare a tertiary alcohol a Grignard reagent must be reacted with
a) HCHO
b) RCHO
c) RCOR
d) RNH2
Ans:
b) RCOR
Question 16.
Phenyl magnesium bromide reacts with formaldehyde followed by hydrolysis gives
a) phenol
b) Benzyl alcohol
c) Benzaldehyde
d) Benzoic acid
Answer:
b) Benzyl alcohol

![]()
Question 17.
Name the product obtained when 1 mole of methyl magnesium bromide reacts with ethyl methanoate
a) propan -2- ol
b) propan – 1 – ol
c) ethanal
d) ethanol
Answer:
c) ethanal
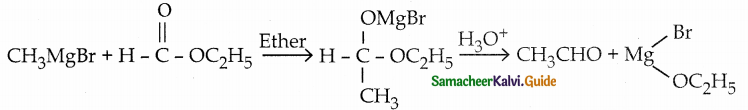
Question 18.
The reducing agent X is
a) Ramey Ni
b) NaBH4
c) LiA/H4
d) Na – Hg
Answer:
b) NaBH4
Question 19.
The best reducing agent to prepare unsaturated alcohols from unsaturated carbonyl compounds is
a) Raney Ni
b) NaBH4
c) LiA/H4
d) both (b) & (c)
Answer:
d) both (b) & (c)
Question 20.
Ethene is converted into ethylene glycol by treating with
a) acidified potassium dichromate
b) alkaline potassium permanganate
c) Chromyl chloride
d) nitric acid
Answer:
b) alkaline potassium permanganate
![]()
Question 21.
Cold dilute alkaline potassium permanganate is called as
a) Fenton’s reagent
b) Tollen’s reagent
c) Baeyer’s reagent
d) Schiff’s reagent
Answer:
c) Baeyer’s reagent
Question 22.
Glyceryl palmitate on alkaline hydrolysis gives
a) glycerol and soap
b) glycerol and sodium palmitate
c) glycerol and sodium hexadentate
d) all the above
Answer:
d) all the above
Question 23.
The preparation of glycerol and soap from oils and fats is known as
a) esterification
b) saponification
c) hydroboration
d) transesterification
Answer:
b) saponification
![]()
Question 24.
Lucas reagent is
a) dil HCl & ZnCl2
b) Cone. HCl & anhydrous ZnCl2
c) dil H2SO4 & AlCl3
d) Conc. H2SO4 & anhydrous AlCl3
Answer:
b) Cone. HCl & anhydrous ZnCl2
Question 25.
Which among the following will give immediate turbidity with Lucas reagent?
a) propan – 2 – ol
b) propan – 1 – ol
c) 2 – methyl propan – 2 – ol
d) 2 – methyl propan – 1 – ol
Answer:
c) 2 – methyl propan – 2 – ol
Hint: A teritary alcohol gives immediate turbidity
Question 26.
In Victor Meyer’s test, the alcohol which
gives blue colour is
a) propan – 2 – ol
b) propan – 1 – ol
c) 2 – methyl propan – 2 – ol
d) 2 – methyl propan – 1 – ol
Answer:
a) propan – 2 – ol
Hint: A secondary alcohol gives blue colour.
![]()
Question 27.
In the given equation
Answer:
c
Question 28.
Among butyl alcohols which one have the lowest boiling point?
a) n-butyl alcohol
b) isobutyl alcohol
c) sec-butyl alcohol
d) ter – butyl alcohol
Answer:
d) ter – butyl alcohol Hint: Increasing order of boiling point is 30 < 2° < 1°
Question 29.
Which among the alcohol has a higher boiling point?
a) n-butyl alcohol
b) n-propyl alcohol
c) ethyl alcohol
d) methyl alcohol
Answer:
a) n-butyl alcohol
Hint: As molecular weight of the alcohol increases, boiling point increases.
Question 30.
Which among the following is less soluble in water?
a) n-butyl alcohol
b) n-propyl alcohol
c) ethyl alcohol
d) methyl alcohol
Answer:
a) n-butyl alcohol Hint: As the molecular weight increases, the solubility of alcohol decreases.
![]()
Question 31.
Conversion of alcohols into alkyl halide is an example of
a) Nucleophilic addition
b) Nucleophilic substitution
c) Electrophilic addition
d) Electrophilic substitution
Answer:
b) Nucleophilic substitution
Question 32.
Conversion of 2 – methyl – 1 – propanol into 2 – methyl – 1 – bromopropane is …………. reaction
a) SN2
b) SN1
c) E2
d) E1
Answer:
a) SN2
Question 33.
Primary alcohols undergo dehydration by mechanism
a) SN2
b) SN1
c) E2
d) E1
Ans:
d) E1
Question 34.
2 – methyl – 2 – propanol when reacted with cone. H2SO4 gives 2 – methyl propene. This reaction follows mechanism
a) SN2
b) SN1
c) E2
d) E1
Answer:
d) E1
![]()
Question 35.
During intramolecular dehydration of 3, 3 – dimethyl – 2 – butanol the major product obtained is
a) 2, 3 – dimethyl but – 1 – ene
b) 2, 3 – dimethyl but – 2 – ene
c) 3, 3 – dimethyl but – 1 – ene
d) 3, 3 – dimethyl but – 2 – ene
Answer:
b) 2, 3 – dimethyl but – 2 – ene
Question 36.
The major product obtained when phenol reacts with con.H2SO4 at 280 K is
a) Salicyclic acid
b) picric acid
c) o-phenol sulphonic acid
d) p-phenol sulphonic acid
Answer:
c) o-phenol sulphonic acid
Question 37.
During intramolecular dehydration of alcohols say tzeff’s rule favours the formation of
a) unstable alkenes
b) less substituted alkenes
c) more substituted alkenes
d) none of the above
Answer:
c) more substituted alkenes
Question 38.
To stop the oxidation reaction of alcohols at the aldehyde / ketone stage…………..is used as an oxidising agent
a) Pottassium permanganate
b) Py ridinium chloro chromate (PCC)
c) Potassium di chromate
d) Sodium di chromate
Answer:
b) pyridinium chloro chromate (PCC)
![]()
Question 39.
In Swern oxidation of alcohols into aldehydes/ketones the oxidising agent used is
a) Pyridinium chlorochromate (PCC)
b) Dimethyl sulfoxide (DMSO)
c) Alkaline potassium permanganate (Baeyer’s reagent)
d) Ferrous sulphate / H2O2 (Fenton’s reagent)
Answer:
b) Dimethyl sulfoxide (DMSO)
Question 40.
To detoxify the alcohol produced in animals by the fermentaion of food, the oxidising agent used is
a) ADH
b) ADP
c) NAD
d) ATP
Answer:
c) NAD
Question 41.
The catalyst which catalyses the oxidation of toxic alcohols into non-toxic aldehyde in animals is
a) ADH
b) ADP
c) NAD
d) ATP
Answer:
a) ADH
![]()
Question 42.
When ethan-1, 2-diol is heated with anhydrous ZnCl2 under pressure it gives
a) Ethanol
b) Ethanal
c) Ethanoic acid
d) Ethene
Ans:
b) Ethanal
Question 43.
Glycerol can be oxidised to meso oxalic acid by
a) dii HNO3
b) HIO4
c) Bismuth nitrate
d) Fenton’s reagent
Answer:
c) Bismuth nitrate
Question 44.
The alcohol used in the manufacture of dynamite is
a) methanol
b) ethanol
c) ethv lene glycol
d) glycerol
Answer:
d) glycerol
Question 45.
The correct order of acidic nature of alcohols is
a) Ethanol < propan – 2 – ol < 2 – methyl propan – 2- ol
b) Propan – 2 – ol < Ethanol < 2 – methyl propan — 2 — ol
c) 2 – methyl propan – 2 – ol < Ethanol < propan – 2 – ol
d) 2 – methyl propan – 2 — ol Answer:
d) 2 – methyl propan – 2 – ol
![]()
Question 46.
1, 3 – dihydroxy benzene is commonly
known as
a) Cresol
b) Catechol
c) Resorcinol
d) Quinol
Answer:
c) Resorcinol
Question 47.
The JUPAC name of pyrogallol is
a) 1, 2, 3 — trthydroxy benzene
h) 1, 2, 4 — trihydroxy beneze
c) 1, 3, 5 — trihydroxv benzene
d) 1, 4, 5 — tri hydroxy benzene .
Answer:
a) 1, 2, 3 — trihydroxy benzene
Question 48.
Oricinol is
a) 1, 2 – dihydroxy benzene
h) 3 — methyl phenol :
c) 3, 5 — dihydroxy toluene
d) 3, 5 — dimethyl toluene
Answer:
c) 3, 5 – dihydroxy toluene
![]()
Question 49.
Which of the following reaction will give ether?
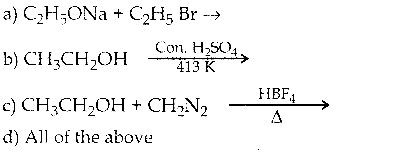
Ans:
d) All of the above
Question 50.
Phenol on oxidation with acidified K2Cr2O7gives
a) 1, 4 – dihydroxy benzene
b) 1,4- benzoquinone
c) cyclohexanol
d) cumene
Answer:
b) 1,4- benzoquinone
Question 51.
Which among the following is a simple ether?
a) 1 – methoxy propane
b) 2 – methoxy propane
c) methoxy methane
d) methoxv benzene
Answer:
c) methoxy methane
Question 52.
The oxygen atom of ether is
hybridised
a) sp
b) sp2
c) sp3
d) dsp2
Answer:
c) sp3
![]()
Question 53.
Williamson’s synthesis is an example of
a) nucleophilic addition
b) nucleophilic substitution
c) electrophilic addition
d) electrophilic substitution
Answer:
b) nucleophilic substitution
Question 54.
Which among the following is more reactive
towards ethers?
a) HF
b) HCl
c) HBr
d) HI
Answer:
d) HI
Question 55.
The products obtained when methoxy
ethane reacted with one mole of HI are
a) methanol & iodoethane
h) iodomethane & ethanol
c) iodomethane & jodo ethane
d) Methanol & ethanol
Answer:
b) iodomethane & ethanol
![]()
Question 56.
Which is used as a precursor to the synthesis of perfumes and insecticide pheromones?
a) Phenol
b) Phenoxy methane
c) methoxy benzene
d) Ethoxy benzene
Answer:
c) methoxy benzene
Question 57.
According to Lewis concept of acids and bases an ether is
a) acidic
b) basic
c) neutral
d) amphoteric
Answer:
b) basic
Question 58.
a) CH3COCH3
b) C2H5OH
c) CH3-CH(OH)CH3
d) CH3CHO
Answer:
c) CH3-CH(OH)CH3
Question 59.
![]()
a)Propan-1,3-diol
b) Ethan -1,2- diol
c) Propan -1,2,3- Triol
d) Ethanal
Answer:
b) Ethan -1,2- diol
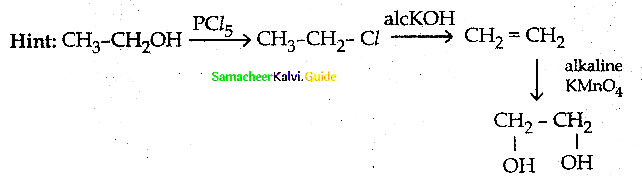
Question 60.
Which of the following has lowest boiling point?
a) phenol
b) O-nitro phenol
c) m-nitro phenol
d) p-nitro phenol
Answer:
b) O-nitro phenol
Hint: O – nitrophenol has intramolecular hydrogen bonding while others intermolecular hydrogen bonding.
![]()
II. Assertion and Reason
Question 1.
Assertion : Tertiary alcohols undergo dehydration more readily than primary alcohol.
Reason : Tertiary alcohols are less acidic than primary alcohol.
a) Both assertion and reason are true and reason is the correct explanation of assertion
b) Both assertion and reason are true but reason is not the correct explanation of assertion
c) Assertion is true but reason is false.
d) Both assertion and reason are false.
Answer:
b) Both assertion and reason are true but reason is not the correct explanation of assertion
![]()
Question 2.
Assertion (A) : Alcohols can act as Bronsted acids
Reason (R) : It is due to the presence of unshared electron pairs on oxygen which make them proton acceptors.
a) Both (A) and (R) are correct, R explain A.
b) Both (A) and (R) are correct, R does not explain A.
c) (A) is correct but R is correct.
d) (A) is wrong but R is correct.
Answer:
d) (A) is wrong but R is correct Correct Reason (A) : Alcohols can act as Bronsted bases.
Question 3.
Assertion (A) : Phenol is more acidic than aliphatic alcohols
Reason (R) : Alkyl substituted phenols show a decreased acidity due to the electron releasing +1 effect of alkyl group.
a) Both (A) and (R) are correct, R explain A.
b) Both (A) and (R) are correct, R does not explain A.
c) (A) is correct but R is correct.
d) (A) is wrong but R is correct.
Answer:
b) Both (A) and (R) are correct, R does not explain A.
Correct Reason (R) : The phenoxide ion is more stabilised by resonance than phenol.
Question 4.
Assertion (A): Orthonitro phenol is slightly soluble in water and more volatile whereas p – nitro phenol is more soluble in water and less volatile.
Reason (R) : Orthonitro phenol forms intramolecular hydrogen bonding and p-nitro phenol forms intermolecular hydrogen bonding.
a) Both (A) and (R) are correct, R explains A.
b) Both (A) and (R) are correct, R does not explain A.
c) (A) is correct but R is correct.
d) (A) is wrong but R is correct.
Answer:
a) Both (A) and (R) are correct, R explains A.
![]()
III. Pick out the correct statements
Question 1.
(i) The structure of – OH group attached to a sp3– hybridised carbon in alcohol is similar to the structure of – OH group attached to a hydrogen in water.
(ii) Due to lone pair – lone pair repulsion, the – C – O – H bond angle in methanol is reduced to 104° from the regular tetrahedral bond angle of 109.5°.
(iii) With Grignard reagent formaldehyde gives primary alcohol and other aldehydes give secondary alcohols.
(iv) Reaction of Grignard reagent with aldehydes and ketones to form alcohols is an example for nucleophilic substitution reaction.
a) (i) & (ii)
b) (ii) & (iii)
c) (i) & (iii)
d) (i) & (iv)
Answer:
c) (i) & (iii)
Correct statements : (ii) Due to lone pair – lone pair repulsion, the – C – O – H bond anlge in methanol is reduced to 108.9° from the regular tetrahedral bond angle of 109.5°.
(iv) Reaction of Grignard reagent with adlehydes and ketones to form alcohols is an example for nucleophilic addition reaction.
Question 2.
(i) Lower alcohols are waxy solids and the higher members are colourless liquids.
(ii) Due to the presence of intermolecular hydrogen bonding alcohols have higher boiling point than the corresponding alkanes, aldehydes, ethers etc.
(iii) Due to the formation of intermolecular hydrogen bonding with water lower alcohols are highly soluble in water.
(iv) Among isomeric alcohols primary alcohols have lower boiling point and tertiary alcohols have higher boiling points.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statements : (i) Lower alcohols are colourless liquids and the higher members are waxy solids.
(iv) Among isomeric alcohols primary alcohols have higher boiling point and the tertiary alcohols have lower boiling points.
![]()
Question 3.
(i) In Swern Oxidation DMSO, oxalyl chloride and triethylamine are used to convert alcohols into aldehydes / ketones.
(ii) Vapours of primary alcohol passed over heated copper at 573 K undergoes dehydration. .
(iii) Methanol reacts with ethanoic acid in presence of an acid to form ethylethanoate.
(iv) Vapours of tertiary alcohols react with heated copper at 573 K to give alkenes.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statements : (ii) Vapours of primary alcohol passed over heated copper at 573 K undergoes dehydrogenation.
(iii) Methanol reacts with ethanoic acid in presence of an acid to form methyl ethanoate. (or)
Ethanol reacts with ethanoic acid in presence of an acid to form ethyl ethanoate.
![]()
Question 4.
(i) Glycerol contains two primary alcoholic group and one secondry alcoholic group.
(ii) Oxidation of glycerol with cone. HNO3 gives mainly formic acid.
(iii) Glycerol is used in making printing inks, and stamp pad ink.
(iv) Glycerol is used as a preservative for biological specimens
a) (i) & (ii)
b) (ii) & (iii)
c) (i) & (iii)
d) (i) & (iv)
Answer:
c) (i) & (iii)
Correct statements : (ii) Oxidation of glycerol with cone. HNO3 gives mainly glyceric acid
(iii) Ethanol is used as a preservative for biological specimens
![]()
IV. Pick out the incorrect statements
Question 1.
(i) The overall reaction of hydroboration is hydration of an alkene.
(ii) Hydroboration reaction occurs according to Markownikoff’s rule.
(iii) Raney Ni does not reduce the Carbon – Carbon double bond present in the Carbonyl compound to form unsaturated alcohols.
(iv) When two or more functional groups are present in a molecule sodium borohydride to used as a reducing agent to reduce the more reactive group
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statements : (ii) Hydroboration reaction occurs against Markownikoff’s rule.
(iii) Lithium aluminium hydride does not reduce the Carbon – Carbon double bond present in the Carbonyl compound to form unsaturated alchols.
Question 2.
(i) Ethanol forms a turbidity with Lucas reagent within 10 minutes.
(ii) 2 – Methyl propan – 2 – ol forms immediate turbidity with Lucas reagent due to the formation of insoluble 2 – chloro – 2 – methyl propane.
(iii) Ehanol forms red colour in Victor Meyer’s test.
(iv) 2 – 2 – dimethyl propan – 1 – ol gives a colourless solution in victor Meyer’s test,
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statements : (i) Ethanol forms no tubridity with lucas reagent at room temperature.
(iv) 2,2 – dimethyl propan -1 – ol gives red colour in victor mayer’s test.
![]()
Question 3.
(i) Alcohols undergo nucleophilic substitution reaction with hydrohaloacids to form alkyl halides.
(ii) Alkyl halide formation from primary alchols follow E2 mechanism.
(iii) Alkyl halide formation from tertiary alcohols follow SN2 mechanism.
(iv) The conversion of methanol into chloromethane with thionyl chloride in the presence of pyridine follows SN2 mechanism.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statements : (ii) Alkyl halide formation from primary alcohols follow \(\mathrm{S}_{\mathrm{N}}^{2}\) mechanism.
(iii) Alkyl halide formation from tertiary alcohols follow \(\mathrm{S}_{\mathrm{N}}^{1}\) mechanism.
Question 4.
(i) In phenol the carbon bearing the – OH group is sp2 hybridised.
(ii) Unlike alcohols phenol reactswith sodium hydroxide to form sodium phenoxide.
(iii) In substituted phenols, the electron with drawing groups decreases the acidic nature of phenol.
(iv) Alkyl substituted phenols show increased acidity due to electron releasing +1 effect of alkvl group.
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
c) (iii) & (iv)
Correct statements: (iii) In substituted phenols, the electron withdrawing groups increases the acidic
nature of phenol.
(iv) Alkyl substituted phenols show a decreased acidity due to electron releasing + I effect of alkyl
group.
V. Match the Following
Question 1.
| Oxidising agent |
Oxidation product of glycerol |
| i) dil HNO3 | a meso oxalic acid |
| ii) Conc. HNO3 | b formaldehyde & formic acid |
| iii) Bismuth nitrate | c oxalic acid |
| iv) Fenton’s reagent | d glyceric acid & tartronic acid |
| v) HIO4 | e glyceric acid |
| vi) Acidified KMnO4 | f glycerose |
Answer:
i) d glyceric acid & tartronic acid
ii) e glyceric acid
iii) a meso oxalic acid
iv) f glycerose
v) b formaldehyde & formic acid
vi) c oxalic acid
![]()
Question 2.
| Example | Type of Phenol |
| i) Orcinol | a Monohydric pheno |
| ii) Catechol | b Trihydric phenol |
| iii) Cresol | c Dihydric phenol |
| iv) Phioroglucinol | d Substituted phenol |
Answer:
i) d Substituted phenol
ii) c Dihydric phenol
iii) a Monohydric phenol
iv) b Trihydric phenol
Question 3.
| Compound | Uses |
| i) Phenol | a Antifreeze |
| ii) Glycerol | b Perfumery |
| iii Glycol | c Carbolic soaps |
| iv Anisole | d Refrigerant |
| v) Diethyl ether | e Cordite |
Answer:
i) c Carbolic soaps
ii) e Cordite
iii) a Antifreeze
iv) b Perfumery
v) d Refrigerant
![]()
Question 4.
| Type of Alcohol | Example |
| i 1° alcohol | a Glycerol |
| ii 2° alcohol | b Sorbitol |
| iii 3° alcohol | c Ethylene glycol |
| iv Dihydric alcohol | d Isopropyl alcohol |
| v Trihydric alcohol | e Neo pentyl alcohol |
| vi Polyhydric alcohol | f 2-phenyl propan-2-ol |
Answer:
i) e Neo pentyl alcohol
ii) d Isopropyl alcohol
iii) f 2-phenyl propan-2-ol
iv) c Ethylene glycol
v) a Glycerol
vi) b Sorbitol
VI. Two Marks Questions
Question 1.
Illustrate Markownikoff’s rule in the addition of water to an alkene
Answer:
Markownikoff’s rule states that the negative part of the adding molecules gets attached with the carbon containing least number of hydrogen atoms across the double bond.
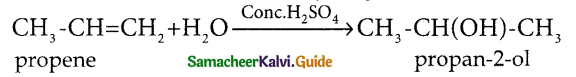
![]()
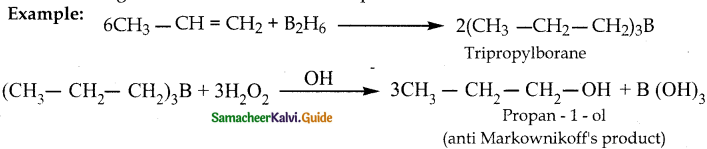
Question 2.
Write a note on hydroboration reaction
Answer:
Diborane reacts with an alkene to form trialkyl borane which on treatment with H2O2 in presence of NaOH gives an alcohol.
The overall reaction is hydration of an alkene.
This reaction gives an anti MarkowniKoff’s product
Question 3.
Convert crotonaldehyde into crotylalcohol
Answer:
LiAlH4 does not reduce C = C bond present in the carbonyl compound.
Hence it is the best reagent to prepare unsaturated alcohols.
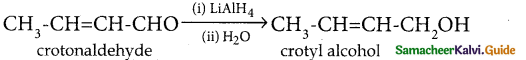
Question 4.
An organic compound (A) – C3H8O3 used as sweetening agent, which on oxidation with Fenton’s reagent gives a mixture of compounds B and C. Identify A, B & C. Write possible reactions.
Answer:
An organic compound (A) – C3H8O3 is glycerol.
Oxidation of glycerol with Br2/H2O (or) NiaOBr (or) Fenton’s reagent (FeSO4 + H2O2) gives a mixture of glyceraldehvde and dihydroxv acetone ( This mixture is named as glvcerose)
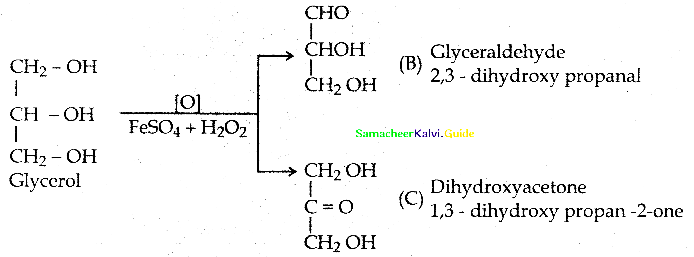
![]()
Question 5.
What is saponification?
Answer:
The alkaline hydroysis of naturally occurring oils and fats into glycerol and soap is called saponification.
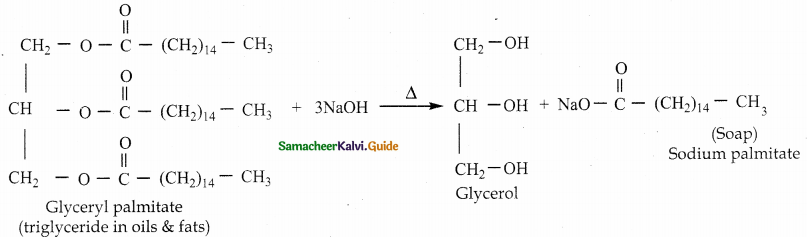
Question 6.
What is Saytzeff’s rule
Answer:
During intramolecular dehydration of an alcohol if there is a possibility to form a carbon- carbon double bond at different locations, the preferred location is the one that gives the more substituted alkene ie., the stable alkene.
Question 7.
Convert propan -1 – ol to propanal.
Answer:
To stop the oxidation reaction of alcohols at the aldehyde / ketone stage, pyridinium chloro chromate (PCC) is used as an oxidising agent.

Question 8.
Write a note on Swern Oxidation
Answer:
In Swern oxidation dimethvl sulfoxide (DMSO) is used as the oxidising agent.
It converts alcohols to ketones / aldehydes.
Alcohol is treated with DMSO and oxah I chloride followed by the addition of triethylarnine.
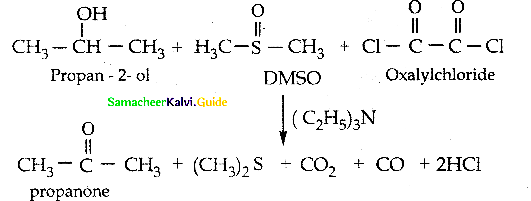
![]()
Question 9.
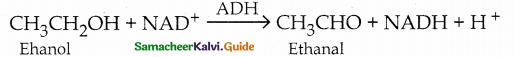
Write note on biological oxidation that occurs in animals.
Answer:
- The fermentation of the food consumed by an animal produces alcohol.
- To detoxify the alcohol, liver produces an enzyme called alcohol dehydrogenase (ADH)
- Nicotinamide adenine dinucleotide (NAD) present in animals acts as an oxidising agent.
- ADH catalyses the oxidation of toxic alcohols into non-toxic aldehyde.
Question 10.
What happens when ethylene glycol is reacted with PI3 ?
Answer:
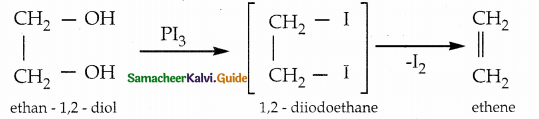
Question 11.
What happens when ethylene glycol is heated with conc. HNO3 and conc. H2SO4?
Answer:
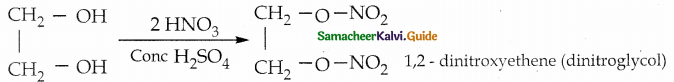
Question 12.
How is nitroglycerine prepared
Answer:
Glycerol reacts with nitric acid in the presence of cone. H2SO4 to form nitroglycerine (TNG)
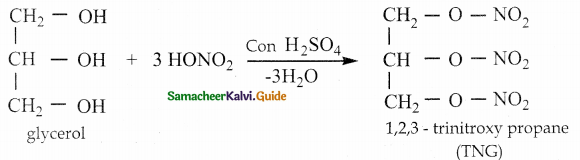
![]()
Question 13.
Write about the dehydration of glycerol
Answer:
When glycerol is heated with dehydrating agents such as H2SO4, KHSO4 etc., it undergoes dehvration to form acrolein.
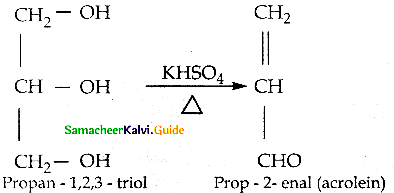
Question 14.
Convert aniline into phenol
Answer:
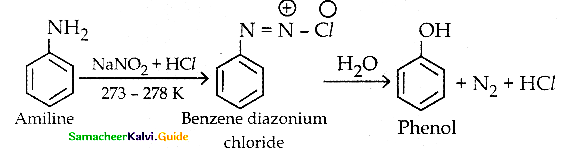
Question 15.
What is Schotten – Baumann reaction
Answer:
Benzovlation of phenol with benzoyl chloride in presence of NaOH is known as Schotten Baumann reaction.
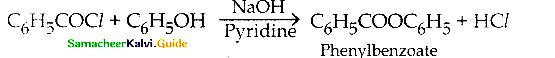
Question 16.
Write a note on williamson ether synthesis?
Answer:
Methyl iodide undergoes nucleophilic substitution by phenoxide ion to form anisole
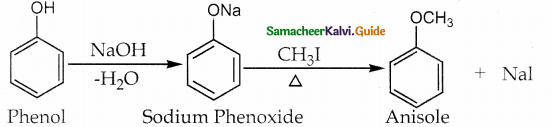
![]()
Question 17.
Convert phenol into (i) 1, 4 – benzoquionone (ii) cyclo hexanol
Answer:
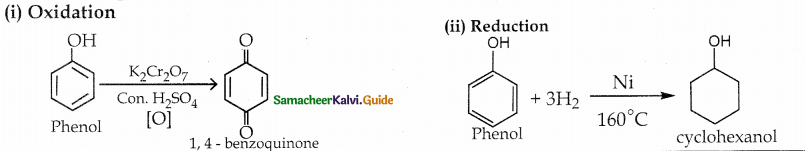
Question 18.
How is picric acid prepared?
Answer:
Phenol undergoes nitration with cone HNO3 and cone H2SO4 at 298 K to form 2, 4/ 6 – trinitrophenol known as picric acid
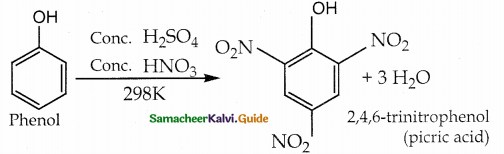
Question 19.
Differentiate phenol and ethanol
Answer:
|
Reaction |
Phenol |
Ethanol |
| With benzene diazonium chloride | forms a red orange dye | No reaction |
| With neutral ferric chloride | forms a purple coloration | does not form purple colouration |
| WithNaOH | gives sodium phenoxide | does not react |
Question 20.
Give four uses or diethyl ether.
Answer:
- Diethyl ether is used as a surgical anaesthetic agent in surgery.
- It is a good solvent for organic reactions and extraction.
- It is used as a volatile starting fluid for diesel and gasoline engine.
- It is used as a refrigerant.
Question 21.
Write the mechanism of intermolecular dehydration of alcohols?
Answer:
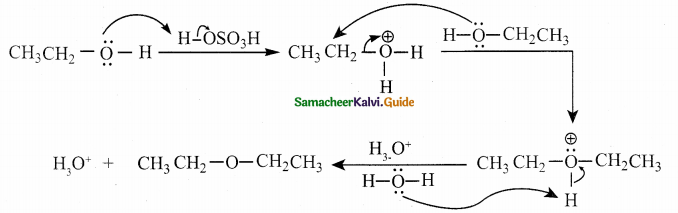
![]()
Question 22.
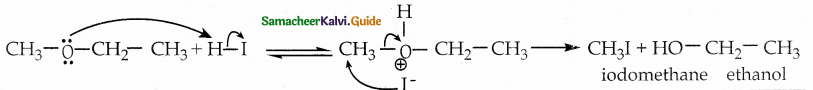
Write the mechanism of nucleophilic substitution of ethers
Answer:
Mechanism:
Ethers having primary alkyl group undergo SN2 reaction while tertiary alkyl ether undergo SN1 reaction.Protonation of ether is followed by the attack of halide ion.The halide ion preferentially attacks the less sterically hindered of the two alkyl groups which are attached to etherial oxygen.
When excess HBr or HI is used, the alcohol formed will further react with HBr or HI to form alkyl halides.
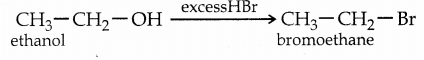
Question 23.
What is autooxidation of ethers?
Answer:
- Ethers stored in the presence of atmospheric oxygen slowly oxidise to form hydroperoxides and dialkyl peroxides.
- These are explosive in nature.
- This spontaneous oxidation by atmospheric oxygen is called auto oxidation
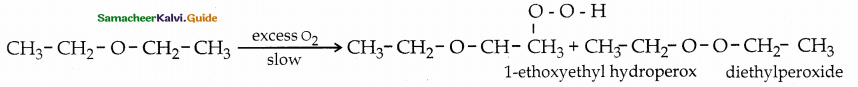
Question 24.
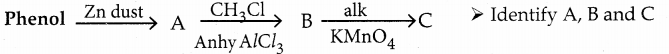
Answer:
![]()
Question 25.
Complete the reaction
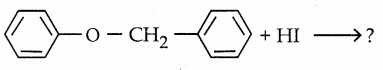
Answer:
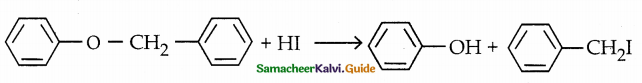
The bond of oxygen with phenyl ring is strong, hence it can not be broken and iodo benzene is not formed.
VII. Three Mark Questions
Question 1.
Explain the structure of the functional group of alcohol
Answer:
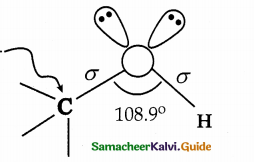
- The structure of – O – H group attached to a SP3 hybridised carbon is similar to the structure of – O – H group attached to a hydrogen in water, ie., ‘V! shaped.
- In such alcohols, one of the sp3 hybridised orbital of oxygen linearly overlap with the sp3 hybridised orbital of carbon to form a C – O, ‘σ’ bond.
- Another SP3 hybridised orbital linearly overlap with IS orbital of hydrogen to form a O-H, a’ bond.
- Remaining two sp3 hybridized orbitals of oxygen are occupied by two lone pairs of electrons.
- Due to lone pair – lone pair repulsion, the C – O – H bond angle in methanol is reduced to 108.9C from the regular tetrahedral bond angle of 109.5°.
Question 2.
Convert acetaldehyde into crotyl alc0hol
Answer:
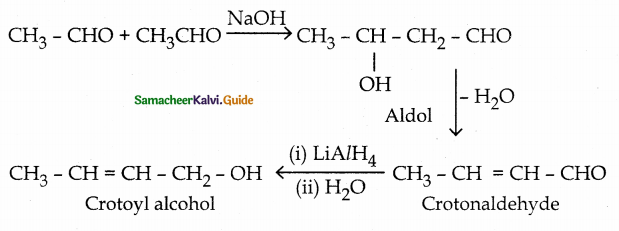
LiAlH4 selectively reduces the carbonyl group leaving the C = C bond.
![]()
Question 3.
Explain Lucas test of differentiating three types of alcohols
Answer:
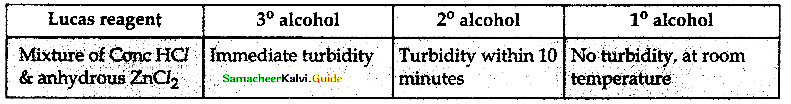
The alkyl chloride formed in this reaction is insoluble in water, hence it appears as turbidity.
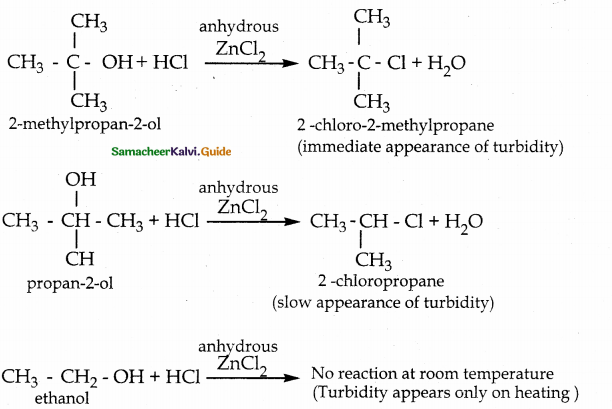
![]()
Question 4.
Write a note on catalytic dehydrogenation of three types of alcohols.
Answer:
Catalytic dehydrogenation :
When the vapours of a primary or a secondary alcohol are passed over heated copper at 573K, dehydrogenation takes place to form aldehyde or ketone.
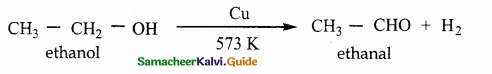
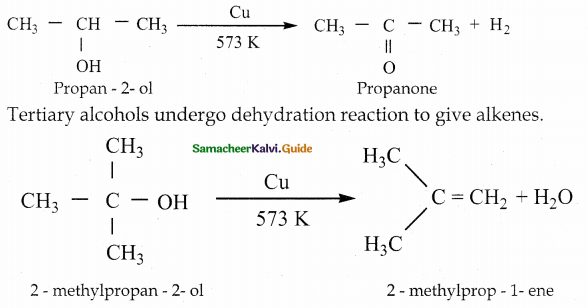
Question 5.
Write about the oxidation of ethylene glycol
Oxidation of glycol
Answer:
On oxidation, glycol gives a variety of products depending on the nature of oxidizing agent and
other reaction conditions.
i) When nitric acid (or) alkaline potassium permanganate is used as the oxidizing agent, the following products are obtained.
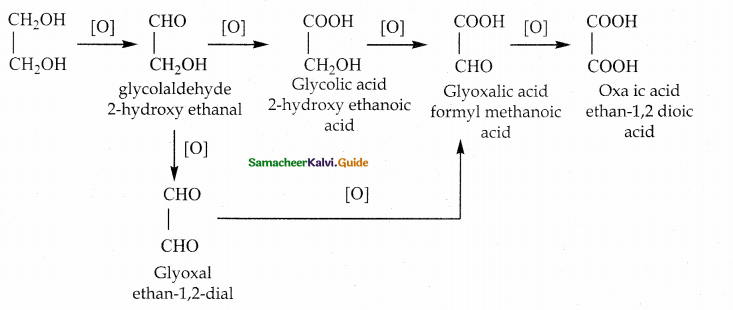
ii) Oxidation of glycol with periodic acid :
Ethylene glycol on treatment with periodic acid gives formaldehyde. This reaction is selective for vicinal 1,2 – diols and it proceeds through a cyclic periodate ester intermediate.
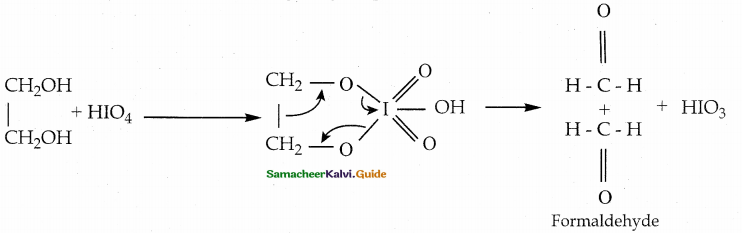
Question 6.
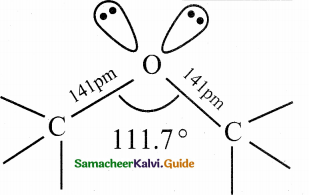
Why is C-O-C bond angle in ether slightly greater than the tetrahedral bond angle.
Answer:
- Structure of ethereal oxygen attached to two alkyl groups is similar to the structure of – O – H group of alcohol.
- Oxygen atom is sp3 hybridised.
- Two sp3 hybridised orbitals of oxygen linearly overlap with two sp3 hybrid orbitals of the two carbon atoms attached directly to the oxygen forming two C – O ‘o ‘ bonds.
- C-O-C bond angle is slightly greater than the tetrahedral bond angle due to the repulsive interaction between the two bulkier alkyl group.
![]()
Question 7.
For the preparation of mixed ether having primary and tertiary alkyl group, primary alkyl halide and alkoxide are used. Why?
Answer:
Primary alkyl halides are more susceptible for SN2 reaction.
Hence for the preparation of mixed ether having primary and tertiary alkyl group, primary alkyl halide
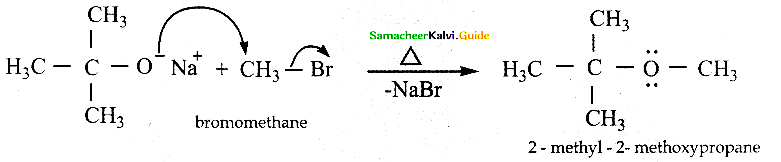
On the other hand, if tertiary’ alkyl halide and primary alkoxide are used elimination reaction dominates and succeeds over substitution reaction to form an alkene.

Question 8.
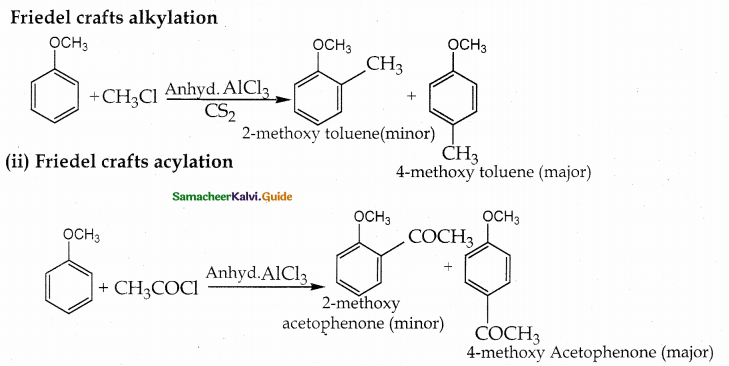
How do you convert anisole into (i) Methoxy toluene (ii) methoxy acetophenone
Answer:
Question 9.
375 mg of an alcohol reacts with required amount of methyl magnesium bromide and releases 140 mL of methane gas at STP. What is the formula of the alcohol?
Solution:
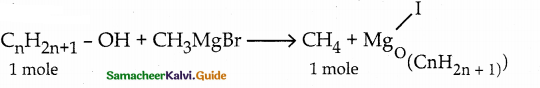
(1 mole) 22,400 mL of methane can be produced from 1 mole of alcohol.
140 ml of methane is liberated from 1/22400 x 140 mole of alcohol = 6.25 x 10-3 mole of alcohol
w = 375 mg = 375 x 10-3 g; n = 6.25 x 10-3
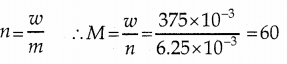
Molar mass of alcohol is 60 gmol-1
CnH2n+1 -OH=>nxl2 + (2n + 1)xl + lxi6+1xl=60
12n + 2n + 1 + 16 + 1 = 60 14n + 18 = 60
14n = 60 – 18
Hence the formula of the alcohol is C3H7OH .
n = 42/14 ⇒ n = 3
![]()
Question 10.
How will you convert methanol into ethanol?
Answer:
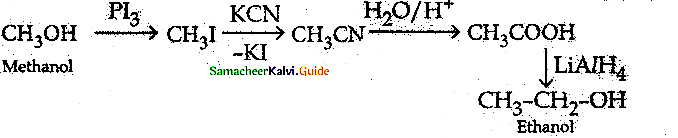
VIII. Five Mark Questions
Question 1.
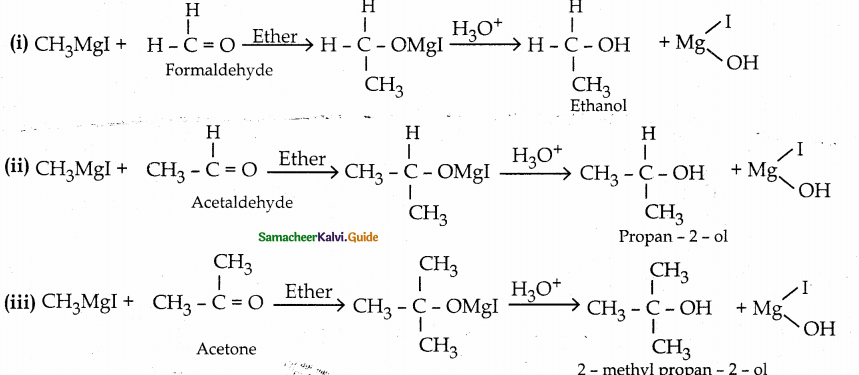
Convert methyl magnesium iodide into
(i) ethanol
(ii) propan – 2 – ol
(iii) 2 – methyl propan – 2 – ol
Answer:
Question 2.
Bring about the following conversion using Grignard reagents
i) Methanal into phenyl methanol
ii) Ethanal into butan – 2 – ol
iii) Propanone into 2 – methyl hexan – 2 -ol
iv) Ethyl methanoate into propan – 2 – ol
v) Formaldehyde into propan-l-ol
Answer:
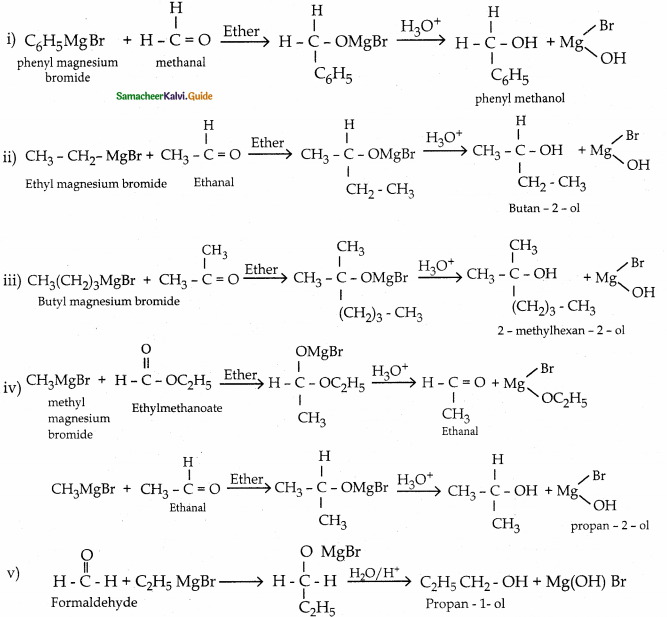
![]()
Question 3.
A ether (A) C5H12O when heated with excess of hot concentration HI, produced two alkyl halides, which on hydrolysis forms compound (B) and (C). Oxidation of (B) gives an acid (D) where as oxidation of (C) gives ketone (E). Identify A, B, C, D and E and write the chemical equation.
Answer:
A ether (A) C5H12O when heated with excess of hot concentration HI, produced two alkyl halides.
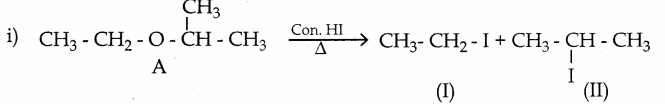
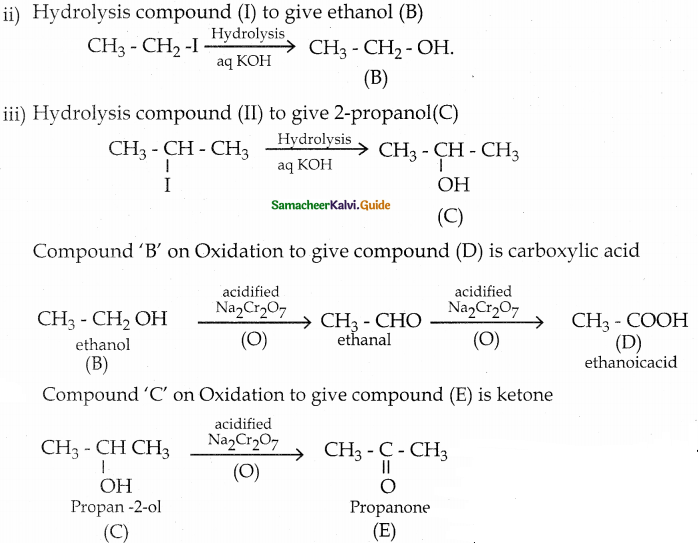
| Compound | Name |
| A | 2- ethoxy propane |
| B | ethanol |
| C | 2- propanol |
| D | Acetic acid |
| E | Propanone |
Question 4.
Explain E1mechanism involved in the dehydration of tertiary butyl alcohol
Answer:
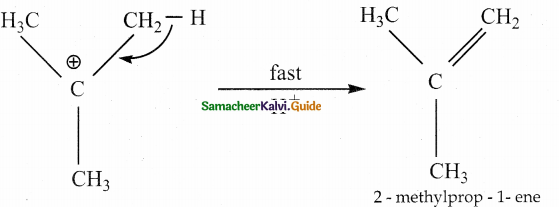
Tertiary alcohols undergo dehydration by E1 mechanism. It involves the formation of a carbocation.
Protonation of alcohol
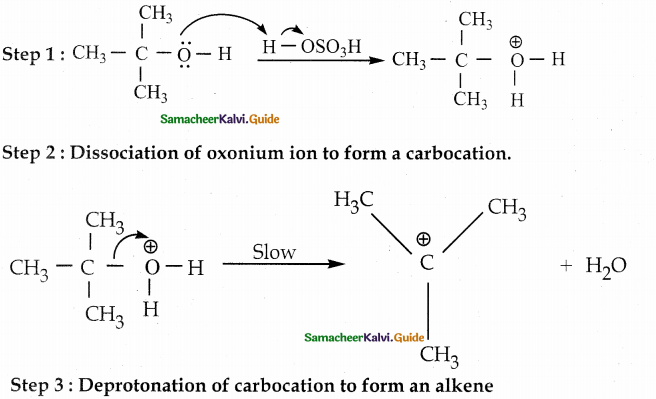
![]()
Question 5.
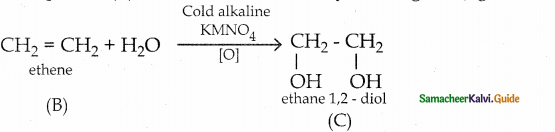
An organic compound C2H6O (A) heated with Cone. H2SO4 at 443K to give and unsaturated hydrocarbon C2H4 (B), which on treatment with Baeyer’s reagent to give compound C2H6O2 (C). Which is used as antifreeze in automobile radiator. Compound (C) distilled with cone. H2SO4 to give cyclic compound (C4H8O2) (D). Compound (A) is heated with cone H2SO4 at 413K to give compound C4H10O (E). Identify compounds (A) to (E) and write equations Compound (A) is Ethanol heated with Cone. H2SO4 at 443K to give compound (B) is Ethene
Answer:

Compound (B) on treatment with Baeyer’s reagent to give compound (C) is Ethylene glycol
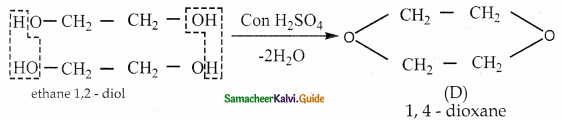
Compound ( C) distilled with cone. H2SO4 to give cyclic compound (D) 1,4- dioxene
Compound (A) is heated with cone. H2SO4 at 413K to give compound (E) is diethylether
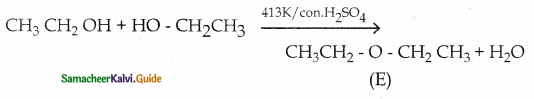
| Compound | Name |
| A | Ethanol |
| B | Ethene |
| C | Ethane 1,2-diol |
| D | 1,4 dióxane |
| E | Diethylether |
![]()
Question 6.
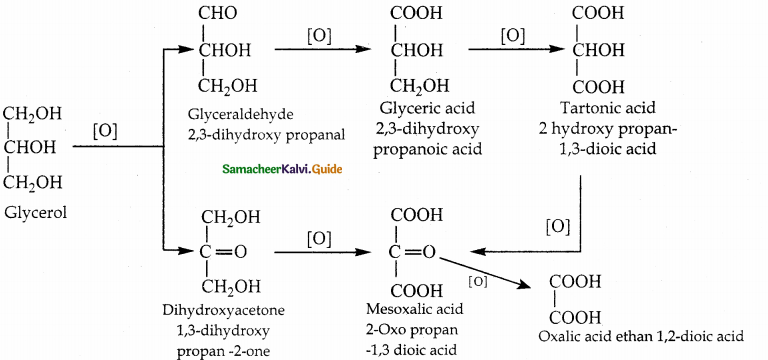
Explain the oxidation of glycerol
Answer:
| Oxidising agent | Product formed |
| i) dil HNO3 | glyceric acid & tartronic acid |
| ii) Cone HNO3 | glyceric acid |
| iii) bismuth nitrate | meso oxalic acid |
| iv) BD/H2O (or) NaOBr (or) Fenton’s reagent (FeSO4 + H2O2) |
a mixture of glyceraldehyde and dihydroxyacetone knew as glycerose |
| v) HIO4 (or) Lead tetraacetate (LTA) | formaldehyde & formic acid? |
| vi) acidified KMnO4 | Oxalic acid |
Question 7.
Write a note on (i) Riemer Tiemann reaction (ii) Phthalein reaction (iii) coupling reaction
Answer:
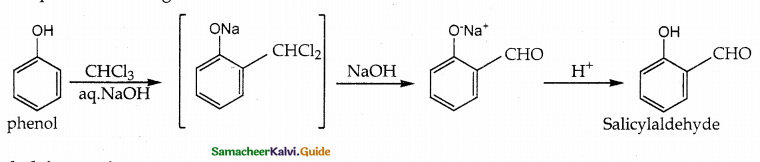
i) Riemer Tiemann Reaction:
On treating phenol with CHCl3 /NaOH, a -CHO group is introduced at ortho position This reaction proceeds through the formation of substituted benzal chloride intermediate.
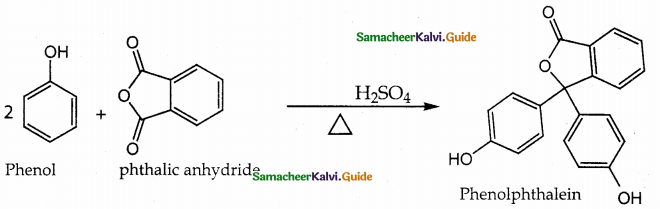
(ii) Phthalein reaction:
On heating phenol with phthalic anhydride in presence of con.H2SO4 phenolphthalein is obtained.
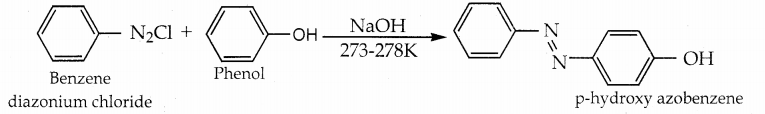
iii) Coupling reaction:
Phenol couples with benzene diazonium chloride in an alkaline solution to form p-hydroxy azobenzene (a red-orange dye).
![]()
Question 8.
Write any three methods of preparing ethers
Answer:
(i) Intermolecular dehydration of alcohol
Ethanol undergoes intermolecular dehydration with conc. H2SO4 at 413 K to form diethyl ether. Conc. H2SO4
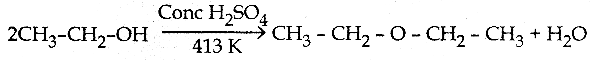
(ii) Williamson ether synthesis
When an alkyl halide is heated with an alcoholic solution of sodium alkoxide, the corresponding ether is obtained. This involves SN2 mechanism.
![]()
(iii) Methylation of alcohol
Methyl ethers can be prepared by treating alcohol with diazomethane in presence of catalyst fluoroboric acid
![]()
Question 9.
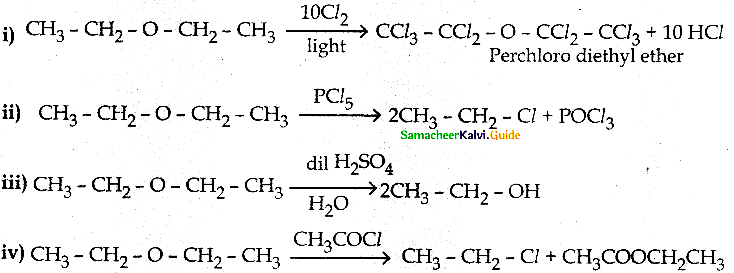
How does diethylether react with the following
(i) Cl2/light
(ii) PCl5
(iii) dilH2SO4
(iv) CH3COCl/anhy.ZnCl2
Answer:
Question 10.
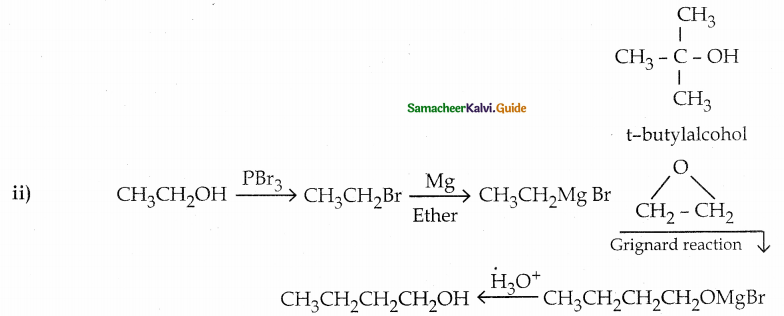
Convert
(i) methanol into tertiary butyl alcohol
(ii) ethanol into 1- butanol
Answer:
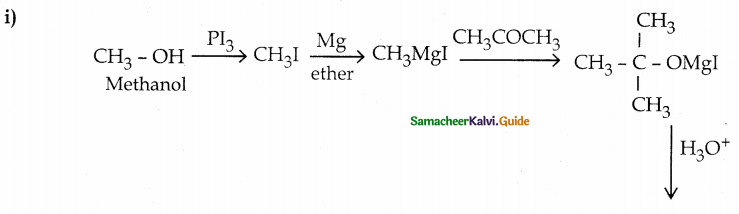
![]()
Question 11.
Complete the reaction
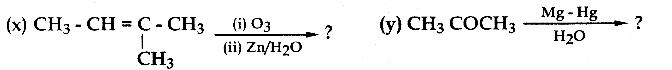
Answer:
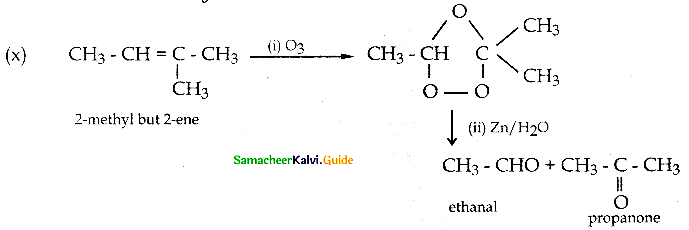
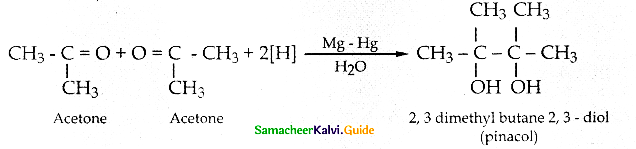
(y) Reduction to pinacols: Ketones, on reduction with magnesium amalgam and water, are reduced to symmetrical diols known as pinacol.
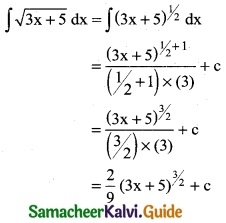

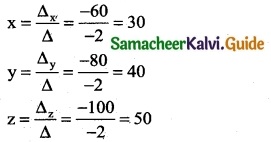
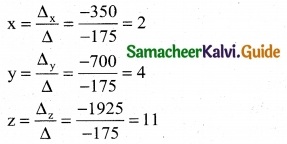



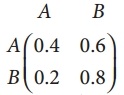 is a transition probability matrix, then at equilibriuium A is equal to
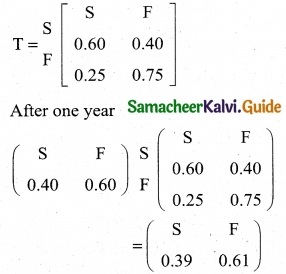
is a transition probability matrix, then at equilibriuium A is equal to

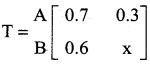 is a transition probability matrix, then the value of x is
is a transition probability matrix, then the value of x is