Tamilnadu State Board New Syllabus Samacheer Kalvi 12th Chemistry Guide Pdf Chapter 8 Ionic Equilibrium Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 12th Chemistry Solutions Chapter 8 Ionic Equilibrium
12th Chemistry Guide Ionic Equilibrium Text Book Questions and Answers
Part – I Text Book Evaluation
I. Choose the correct answer
Question 1.
Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.24 x 10-4 mol L-1 solubility product of Ag2C2O4 is ………………
(a) 2.42 x 10-8 mol3 L-3
(b) 2.66 x 10-12 12 mol3 L-3
(c) 45 x 10-11 mol3 L-3
(d) 5.619 x 10-12 mol3 L-3
Answer:
(d) 5.619 x 10-12 mol3 L-3
Solution:
Ag2C2O4 2Ag+ + C2 O42-
[Ag+] = 2.24 x 10-4 mol L-1
[C2 O42-] = \(\frac{2.24 \times 10^{-4}}{2}\) mol L-1
= 1.12 x 10-4 mol L-1
Ksp = [Ag+]2 [C2O42-]
=(2.24 x 10-4 mol L-1)2 (1.12 x 10-4 mol L-1)
=5.619 x 10-12 mol3 L-3
![]()
Question 2.
Following solutions were prepared by mixing different volumes of NaOH of HCl different concentrations.
(i) 60 mL \(\frac { M }{ 10 }\) HCI + 40 mL \(\frac { M }{ 10 }\) NaOH
(ii) 55 mL \(\frac { M }{ 10 }\) HCl + 45 mL \(\frac { M }{ 10 }\) NaOH
(iii) 75 mL \(\frac { M }{ 5 }\) HCI +25 mL \(\frac { M }{ 5 }\) MNaOH
(iv) 100 mL \(\frac { M }{ 10 }\) HCI+ 100 mL \(\frac { M }{ 10 }\) NaOH
pH of which one of them wilt be equal to 1?
(a) (iv)
(b) (i)
(c) (ii)
(d) (iii)
Answer:
(d) (iii) 75 mL \(\frac { M }{ 5 }\) HCI + 25 mL \(\frac { M }{ 5 }\) NaOH
No of moles of HCl = 0.2 x 75 x 10-3 = 15 x 10-3
No of moles of NaOH = 0.2 x 25 x 10-3 = 5 x 1o-3
No of moles of HCl after mixing = 15 x 10-3 – 5 x 10-3
∴ Concentration of HCl
\(\frac{\text { No of moles of }}{\mathrm{HCl} \text { Vol inlitre }}\)
for (iii) solution, pH of 0.1 M HCI = – 1og10 (0.1) = 1.
Question 3.
The solubility of BaSO4 in water is 2.42 x 10-3 gL-1 at 298K. The value of its solubility product
(Ksp) will be …………………..
(Given molar mass of BaSO4 = 233g mol-1)
(a) 1.08 x 10-14 mol2L2
(b) 1.08 x 10-12 mol2L2
(c) 1.08 x 10-10 mol2 L2
(d) 1.08 x 10-8 mol2L-2
Answer:
(c) 1.08 x 10-10 mol2 L2
Solution:
BaSO4 \(\rightleftharpoons\) Ba2+ + SO42-
Ksp = (s) (s)
Ksp = (s)2
= (2.42 x 10-3g L-1)2
= \(\left(\frac{2.42 \times 10^{-3} \mathrm{~g} \mathrm{~L}^{-1}}{233 \mathrm{~g} \mathrm{~mol}^{-1}}\right)^{2}\)
= (0.01038 x 1o-3)2
= (1.038 x 10-5)2
= 1.077 x 10-10
= 1.08 x 10-10 mol2 L2
![]()
Question 4.
pH of a saturated solution of Ca(OH)2 is 9. The Solubility product (Ksp) of Ca(OH)2
(a) 0.5 x 10-15
(b) 0.25 x 10-10
(c) 0.125 x 10-15
(d) 0.5 x 10-10
Answer:
(a) 0.5 x 10-15
Solution:
Ca(OH)2 \(\rightleftharpoons\) Ca2+ + 2OH–
Given that pH = 9
pOH = 14 – 9 = 5
[p0K = – 1og10[OH–]]
[OH–] = 10-pOH
[OH–] =10-5M
Ksp = [Ca2+] [OH–]2
= \(\frac{10^{-5}}{2} \times\left(10^{-5}\right)^{2}\) = 0.5 x 10-15
Question 5.
Conjugate base for bronsted acids H2O and HF are ………………
(a) OH– and H2FH+, respectively
(b) H3O+ and F–, respectively
(c) OH– and F–, respectively
(d) H3O+ and H2F+, respectively
Answer:
(c) OH– and F–, respectively
Solution:
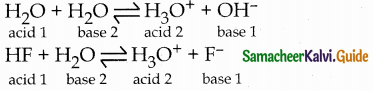
∴ Conjugate bases are OH– and F– respectively
Question 6.
Which will make basic buffer?
(a) 50 mL of 0.1M NaOH + 25mL of 01M CH3COOH
(b) 100 mL of 0.1M CH3COOH + 100 mL of 0.1M NH4OH
(c) 100 mL of 0.1M HCI + 200 mL of 0.1M NH4OH
(d) 100 mL of 0.1M HCI + 100 mL of O.1 M NaOH
Answer:
(c) 100 mL of 0.1M HCI + 200 mL of 0.1M NH4OH
Solution:
Basic buffer is the solution which has weak base and its salt

![]()
Question 7.
Which of the following fluro – compounds is most likely to behave as a Lewis base?
(a) BF3
(b) PF3
(c) CF4
(d) SiF4
Answer:
(b) PF3
Solution:
BF3 → electron deficient → Lewis acid
PF3 → electron rich → Lewis base
CF4 → neutral → neither lewis acid nor base
SiF4 → neutral → neither lewis acid nor base
Question 8.
Which of these is not likely to act as lewis base?
(a) BF3
(b) PF3
(c) CO
(d) F–
Answer:
(a) BF3
Solution:
BF3 → electron deficient → Lewis acid
PF3 → electron rich → Lewis base
CO → having lone pair of electron → Lewis base
F– → unshared pair of electron → lewis base
![]()
Question 9.
What is the decreasing order of strength of bases?
OH–, NH2–, H – C ≡ C– and CH3 – CH2–
(a) OH– > NH2– > H – C ≡ C > CH3 – CH2–
(b) NH2– > OH– > CH3 – CH2– > H – C ≡ C–
(c) CH3 – CH2–, > NH2– > H – C ≡ C– > OH–
(d) OH– > H – C ≡ C– > CH3 – CH2– > NH2–
Answer:
(c) CH3 – CH2–, > NH2– > H – C ≡ C– > OH–
Solution:
Acid strength decreases in the order
HOH > CH ≡ CH > NH3 > CH3CH3
Its conjucate bases arc in the reverse order
CH3 – CH2– > NH2– > H – C ≡ C– > OH–
Question 10.
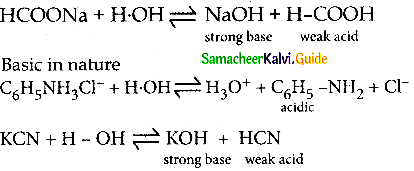
The aqueous solutions of sodium formate, anilinium chloride and potassium cyanide are respectively
(a) acidic, acidic, basic
(b) basic, acidic, basic
(c) basic, neutral, basic
(d) none of these
Answer:
(b) basic, acidic, basic
Solution:
Question 11.
The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5NH) in a 0.10M aqueous pyridine solution (Kb for C5H5N = 1.7 x 10-9) iS ……………..
(a) 0.006%
(b) 0.013%
(c) 0.77%
(d) 1.6%
Answer:
(b) 0.013%
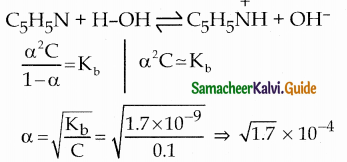
Percentage of dissociation
= \(\sqrt { 1.7 }\) x 10-4 x 100 = 1.3 x 10-2 = 0.013%
![]()
Question 12.
Equal volumes of three acid solutions of pH 1,2 and 3 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
(a) 37 x 10-2
(b) 10-6
(c) 0.111
(d) none of these
Answer:
(a) 3.7 x 10-2
pH = – log10 [H+]
[H+] = 10-pH
Let the volume be x mL
V1M1 + V2M2 + V3M3 = VM
x mL of 10-1M + x mL of 10-2M + x mL of 10-3 M
= 3 x mL of [H+]
= 3 x mL of [H+]
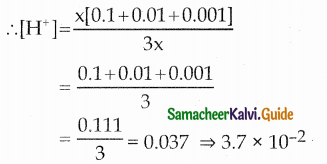
= 0.037 = 3.7 x 10-2
Question 13.
The solubility of AgCl (s) with solubility product 1.6 x 10-10 in 0. 1 M NaCl solution would be ………….
(a) 1.26 x 10-5 M
(b) 1.6 x 10-9 M
(c) 1.6 x 10-11 M
(d) Zero
Answer:
(b) 1.6 x 10-9 M
AgCl (s) \(\rightleftharpoons\) Ag+(aq) + Cl–(aq)
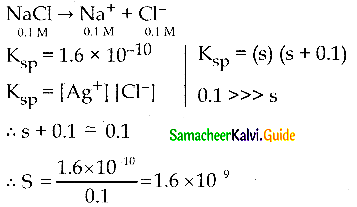
![]()
Question 14.
If the solubility product of lead iodide is 3.2 x 10-8, its solubility will be …………..
(a) 2 x 10-3M
(b) 4 x 10-4 M
(c) 1.6 x 10-5 M
(d) 1.8 x 10-5 M
Answer:
(a) 2 x 10-3M
Solution:
PbI2 (s) \(\rightleftharpoons\) Pb2+ (aq) + 2I– (aq)
Ksp = (s) (2s)2
3.2 x 10-8 = 4s3
S = \(\left(\frac{3.2 \times 10^{-8}}{4}\right)^{1 / 3}\)
= (8 x 10-9)1/3 = 2 x 10-3MNaOH
Question 15.
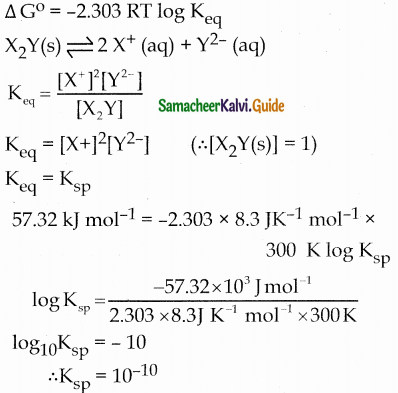
Using Gibb’s free energy change, ∆G0 = 57.34 KJ mol-1, for the reaction, X2Y(s) \(\rightleftharpoons\) 2X+ + Y2-(aq), calculate the solubility product of X2Y in water at 300K (R = 8.3 J K-1 Mol-1)
(a) 10-10
(b) 10-12
(c) 10-14
(d) can not be calculated from the given data
Answer:
(a) 10-10
Solution:
Question 16.
MY and NY3, are insoluble salts and have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true with regard to MY and NY3?
(a) The salts MY and NY3 are more soluble in O.5 M KY than in pure water
(b) The addition of the salt of KY to the suspension of MY and NY3 will have no effect on
(c) The molar solubities of MY and NY3 in water are identical
(d) The molar solubility of MY in water is less than that of NY3
Answer:
(d) The molar solubility of MY in water is less than that of NY3
Solution:
Addition of salt KY (having a common ion Y–) decreases the solubility of MY and NY3 due to common ion effect. Option (a) and (b) are wrong.
For salt MY, MY \(\rightleftharpoons\) M+ + Y–
Ksp = (s) (s)
6.2 x 10-13 = s2
therefore \(\mathrm{s}=\sqrt{6.2 \times 10^{-13}} \simeq 10^{-7}\)
for salt NY3,
NY3 ⇌ N3+ + 3Y–
Ksp = S (3S)3
Ksp = 27s4
\(S=\left(\frac{6.2 \times 10^{-13}}{27}\right)^{1 / 4} \mathrm{~s} \simeq 10^{-4}\)
The molar solubility of MY in water is less than of NY3
![]()
Question 17.
What is the pH of the resulting solution when equal volumes of 0.1M NaOH and 0.01M HCl are mixed?
(a) 2.0
(b) 3
(c) 7.0
(d) 12.65
Answer:
(d) 12.65
Solution:
x ml of 0.1 m NaOH + x ml of 0.01 M HCI
No. of moles of NaOH = 0.1 x x x 10-3 = 0.l x x 10-3
No. of moles of HCl = 0.01 x x x 10-3 = 0.01 x x 10-3
No. of moles of NaOH after mixing = 0.1 x x 10-3 – 0.01x x 10-3
= 0.09x x 10-3
Concentration of NaOH = \(\frac{0.09 x \times 10^{-3}}{2 x \times 10^{-3}}=0.045\)
[OH–] = 0.045
pOH = – log (4.5 x 10-2)
= 2 – log 4.5
= 2 – 0.65 = 1.35
pH = 14 – 1.35 = 12.65
Question 18.
The dissociation constant of a weak acid is 1 x 10-3 . In order to prepare a buffer solution with a pH =4, the [Acid] / [Salt] ratio should be ………………..
(a) 4:3
(b) 3:4
(c) 10:1
(d) 1:10
Answer:
(d) 1:10
Solution:
Ka = 1 x 10-3 ; pH = 4
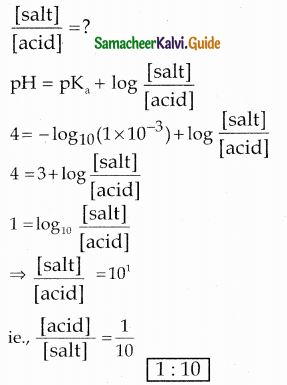
![]()
Question 19.
The pH of 10-5 M KOH solution will be …………..
(a) 9
(b) 5
(c) 19
(d) none of these
Answer:
(a) 9
Solution:
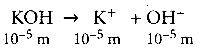
[OH–] = 10-5M.
pH = 14 – pOH .
pH = 14 – ( – log [OH–])
= 14 + log [OH–]
= 14 + log 10-5
= 14 – 5 = 9
Question 20.
H2PO4– the conjugate base of …………….
(a) PO43-
(b) P2O5
(c) H3PO4
(d) HPO42-
Answer:
(c) H3PO4
Solution:
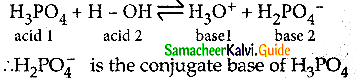
Question 21.
Which of the following can act as lowery – Bronsted acid well as base?
(a) HCl
(b) SO42-
(c) HPO42-
(d) Br–
Answer:
(c) HPO42-
Solution:
HPO42- can have the ability to accept a proton to form HPO4–
It can also have the ability to donate a proton to form PO4-3.
![]()
Question 22.
The pH of an aqueous solution is Zero. The solution is ……………..
(a) slightly acidic
(b) strongly acidic
(c) neutral
(d) basic
Answer:
(b) strongly acidic
Solution:
pH = – log10[H+]
[H+] =10-pH
= 100 = 1
[H+] = 1 M
The, solution is strongly acidic
Question 23.
The hydrogen ion concentration of a buffer solution consisting of a weak acid and its salts is given by ………………
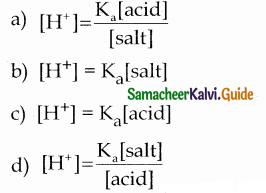
Answer:
a
Solution:
According to Henderson equation
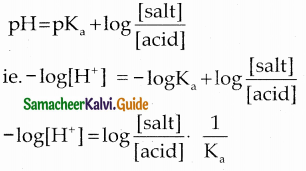
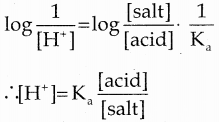
Question 24.
Which of the following relation is correct for degree of hydrolysis of ammonium acetate?
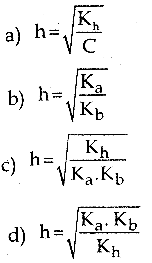
Answer:
c) h = \(\sqrt{\frac{K_{h}}{K_{a} \cdot K_{b}}}\)
Solution:
h = \(\sqrt{\frac{K_{h}}{K_{a} \cdot K_{b}}}\)
![]()
Question 25.
Dissociation constant of NH4OH is 1.8 x 10-5 the hydrolysis constant of NH4Cl would be …………….
(a) 1.8 x 10-19
(b) 5.55 x 10-10
(c) 5.55 x 10-5
(d) 1.80 x 10-5
Answer:
(b) 5.55 x 1010
Solution:
\(\mathrm{K}_{\mathrm{h}}=\frac{\mathrm{K}_{\mathrm{W}}}{\mathrm{K}_{\mathrm{b}}}=\frac{1 \times 10^{-14}}{1.8 \times 10^{-5}}\)
= 0.55 x 10-9
= 5.5 x 10-10
II. Answer the following questions
Question 1.
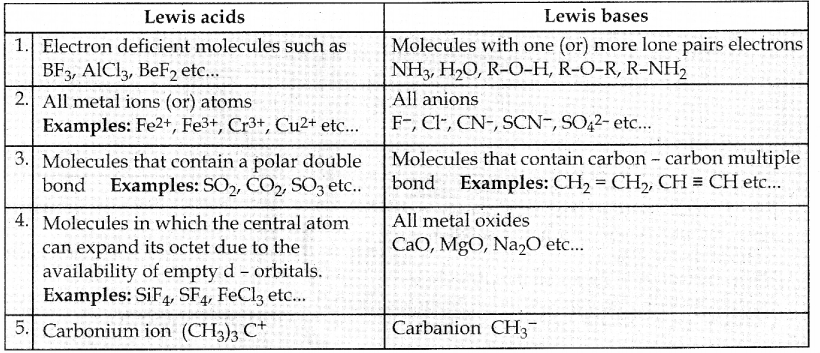
What are lewis acids and bases? Give two example for each.
Answer:
| Lewis acids: | Lewis bases: |
| 1. Electron pair acceptors | Electron pair donors |
| 2. EX: BF3 , AlCl3 | EX: NH3 ,H2O |
Question 2.
Discuss the Lowry – Bronsted concept of acids and bases.
Answer:
- Lowry Bronsted acids – proton donors
- Lowry Bronsted bases – proton acceptors
- An acid1 after donating a proton becomes a base1 (conjugate base)
- A base2 after accepting a proton becomes an acid2 (conjugate acid)
- In general Lowry – Bronsted acid — base reaction is
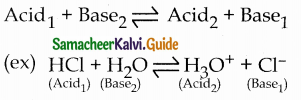
- In the above reaction
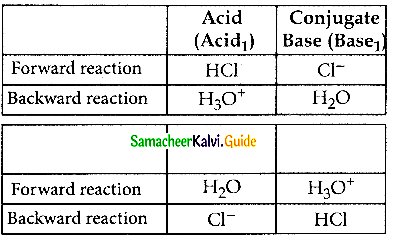
- A conjugate acid – base pair differs by a proton.
Limitation : BF3, AlCl3 etc., that do not donate protons are known to behave as acid s.
![]()
Question 3.
Indentify the conjugate acid base pair for the following reaction in aqueous solution.
i) HS– (aq) + HF \(\rightleftharpoons\) F– (aq) + H2S (aq)
ii) HPO42- + SO32- \(\rightleftharpoons\) PO43- + HSO3–
iii) NH4+ + CO32- \(\rightleftharpoons\) NH3 + HCO3–
Answer:
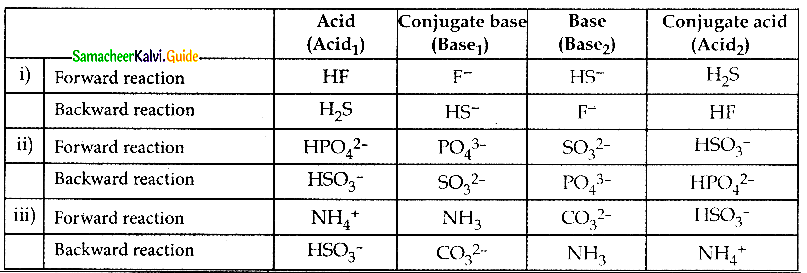
Question 4.
Account for the acidic nature of HClO4. In terms of Bronsted – Lowry theory, identify its conjugate base.
Answer:
HClO4 \(\rightleftharpoons\) H+ + ClO4–
1. According to Lowry – Bronsted concept, a strong acid has weak conjugate base and a weak acid has a strong conjugate base.
2. Let us consider the stabilities of the conjugate bases ClO4– , ClO3–, CIO2– and ClO– formed from these acid HClO4, HClO3, HCIO2, HOCI respectively.
These anions are stabilized to greater extent, it has lesser attraction for proton and therefore, will behave as weak base. Consequently, the corresponding acid will be strongest because weak conjugate base has strong acid and strong conjugate base has weak acid.
3. The charge stabilization mercases in the order, ClO– < ClO2– < ClO3– < ClO4– .
This means ClO4– will have maximum stability and therefore will have a minimum attraction for W. Thus CIO4– will be weakest base and its conjugate acid HCIO4 is the strongest acid.
4. CIO4– is the conjugate base of the acid HClO4.
Question 5.
When aqueous ammonia is added to CuSO4 solution, the solution turns deep blue due to the formation of tetrammine copper (II) complex, [Cu(H2O)6]2+(aq) + 4NH3 (aq) \(\rightleftharpoons\) [Cu(NH3)4]2+ (aq), among H2O and NH3 which is stronger Lewis base.
Answer:
- [Cu(H2O)6]2+(aq) + 4NH3 (aq) \(\rightleftharpoons\) [Cu(NH3)4]2+ (aq) + H2
- Nitrogen less electronegative than oxygen and donates its lone pair of electrons readily. Hence NH3 is a stronger Lewis base.
- If a better Lewis base (ligand) is available, a Lewis acid (central metal ion) will react (Ligand exchange reaction)
- In this reaction, H2O is exchanged with NH3.
- The Lewis acid Cu2+ exchanges the Lewis base H with better Lewis base N-H3 to form [Cu(NH3)4]2+
- Hence NH3 is a stronger Lewis base than H2O in this reaction.
Question 6.
The concentration of hydroxide ion in a water sample is found to be 2.5 x 10-6 M. Identify the nature of the solution.
Answer:
The concentration of OH ion in a water sample is found to be 2.5 x 10 M
pOH = – log10 [OH– ]
pOH = – 1og10 [2.5 x 10-6]
= – log10 [2.5] – log10 [10-6]
= – 0.3979 – ( – 6)
= – 0.3979 + 6
pOH = 5.6
We know that,
pH + pOH = 14
pH + 5.6 = 14
pH = 14 – 5.6
pH = 8.4
pH = 8.4, shows the nature of the solution is basic.
![]()
Question 7.
A lab assistant prepared a solution by adding a calculated quantity of HCl gas 25°C to get a solution with [H3O+] = 4 x 105 M. Is the solution neutral (or) acidic (or) basic.
Answer:
[H3O+] = 4 x 10-5M
pH = – log10 [H3O+]
pH = – 1og10[4 x 105]
pH = – log10 [4] – log10 [10-5]
pH = – 0.6020 – ( – 5) = – 0.6020 + 5
pH = 4.398
Therefore, the solution is acidic.
Since pH is less than 7, the solution is acidic
Question 8.
Calculate the pH of 0.04 M HNO3 solution.
Answer:
Concentration of HNO3 = 0.04M
[H3O+] = 0.04 mol dm-3
pH = – 1og[H3O+]
= – log (0.04)
= – log(4 x 10-2)
= 2 – log4 = 2 – 0.6021
= 1.3979
= 1.40
Question 9.
Define solubility product.
Answer:
Solubility product:
It is defined as the product of the molar concentration of the constituent ions, each raised to the power of its stoichiometric coefficient in a balanced equilibrium equation.
![]()
Question 10.
Define ionic product of water. Give its value at room temperature.
Answer:
1. The product of the concentration of H+ and OH– ions in water at a particular temperature is known as ionic product.
2. The ionic product of water at room temperature (25°C) is,
Kw = [H+] [OH+] (or)
Kw= [H3O+] [OH+]
Kw =(1 x 10-7) (1 x 10-7)
Kw= 1 x 10-14 mol2 dm-6
Question 11.
Explain common ion effect with an example.
Answer:
Common ion Effect:
When a salt of a weak acid is added to the acid itself, the dissociation of the weak acid is suppressed further. Acetic acid is a weak acid. It is not completely dissociated in aqueous solution and hence the following equilibrium exists.
CH3COOH (aq) \(\rightleftharpoons\) H+(aq)+ CH3COO– (aq)
However, the added salt, sodium acetate, completely dissociates to produce Na+ and CH3COO ion.
CH3COONa (aq) → Na+ (aq) + CH3COO (aq) Hence, the overall concentration ofCH3COO is increased, and the acid dissociation equilibrium is disturbed.
We know from Le chatelier’s priñciple that when a stress is applied to a system at equilibrium, the system adjusts itself to nullify the effect produced by that stress. So, in order to maintain the equilibrium, the excess CH3COO– ions combines with H ions to produce much more unionized CH3COOH i.e.,
the equilibrium will shift towards the left. In other words, the dissociation of CH3COOH is suppressed. Thus, the dissociation of a weak acid (CH3COOH) is suppressed in the presence of a salt (CH3COONa) containing an ion common to the weak electrolyte. It is called the common ion effect.
![]()
Question 12.
Derive an expression for Ostwald’s dilution law.
Answer:
- Ostwald’s dilution law relates the dissociation constant of the weak acid (Ka) with its degree of dissociation (a) and the concentration ( C)
- Ostwald’s dilution law states that, when dilution increases, the degree of dissociation of weak electrolyte also increases
- Degree of dissociation
\(\alpha=\frac{\text { Number of moles dissociated }}{\text { Total number of moles }}\) - Consider the dissociation of a weak acid (CH3COOH)
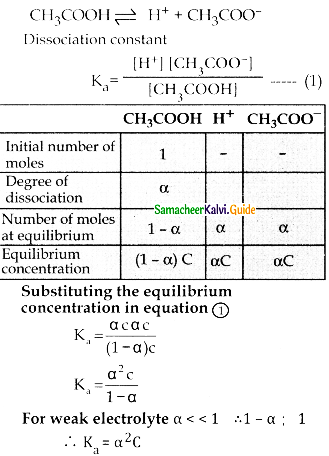
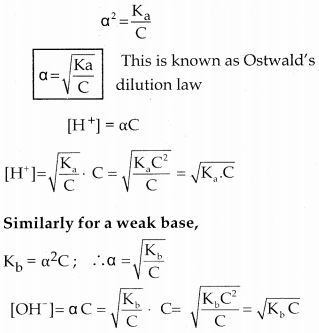
Question 13.
Define pH.
Answer:
pH of a solution is defined as the negative logarithm of base 10 of the molar concentration of the hydronium ions present in the solution.
pH = – log10 [H3O+] (or) pH = – log10 [H+]
Question 14.
Calculate the pH of 1.5 x 10-3 M solution of Ba(OH)2
Answer:
Acidity of Ba(OH)2 in 2
∴ Normality = Molarity x acidity
= 15 x 10-3 x 2
= 3 x 10-3
So, [OH–] = Normality = 3 x 10-3
pOH = – log [OH–]
= -log3 x 10-3
= -log3 – log10-3
= log 3 +3 log 10
= 3 – log3
= 3 – 0.4771
pOH = 2.5229
pH+ pOH = 14
pH = 14 – pOH
=14 – 2.5229
= 11.4771
pH = 11.48
![]()
Question 15.
50 ml of 0.05 M HNO3 is added to 50 ml of 0.025 M KOH. Calculate the pH of the resultant solution.
Solution:
Number of millinioles VrnI x Molarity
Millimoles of HNO3 = 50 x 0.05 = 2.5
Millimoles of KOH = 50 x 0.025 = 1.25
After mixing millimoles of
HNO3 remaining = 2.5 – 1.25 = 1.25
Total volume = 50 + 50 = 100 ml
Molarity = \(\frac{\text { Number of millimoles }}{\mathrm{Vml}}\)
= 1.25/100
Molarity = 1.25 x 10-2
HNO3 is mono basic.
∴ Normality Molarity x basicity
= 1.25 x 10-2 x 1
= 1.25 x 10-2
∴[H3O+] = 1.25 x 10-2
pH = – log[H3O+]
= – log 1.25 x 10-2
= – log 1.25 – log 10-2
= – log 1.25 + 2 log 10
=2-log 1.25 = 2-0.0969
pH = 1.9031
Question 16.
The Ka value for HCN is 10-9. What is the pH of 0.4 M HCN solution?
Answer:
Ka =10-9
c = O.4M
pH = – log10 [H3O+]
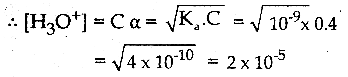
∴ pH = – log(2 x 10-5)
= – log 2 – log (10-5)
= – 0.3010 + 5
pH = 4.699
![]()
Question17.
Calculate the extent of hydrolysis and the pH of 0.1 M ammonium acetate Given that.
Ka = Kb = 1.8 x 10-5
Solution:
Ka = Kb = 1.8 x 10-5
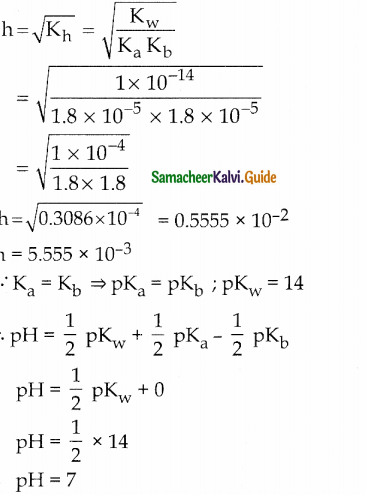
Question 18.
Derive an expression for the hydrolysis constant and degree of hydrolysis of salt of strong acid and weak base.
Answer:
Consider the reaction between the strong acid HCl and the weak base NH4OH to form the salt NH4Cl and water.
\(\mathrm{HCl}_{(\mathrm{aq})}+\mathrm{NH}_{4} \mathrm{OH}_{(\mathrm{aq})} \rightleftharpoons \mathrm{NH}_{4} \mathrm{Cl}_{(\mathrm{aq})}+\mathrm{H}_{2} \mathrm{O}_{(\mathrm{l})}\)
In aqueous solution NH4C1 is completely dissociated as follows:
\(\mathrm{NH}_{4} \mathrm{Cl}_{(\mathrm{aq})} \rightarrow \mathrm{NH}_{4}{ }^{+}{ }_{(\mathrm{aq})}+\mathrm{Cl}^{-}{ }_{(\mathrm{aq})}\)
NH4+ is the strong conjugate acid of the weak base NH4OH.
Hence it has a tendency to react with 0H from water to form unionised NH4OH.
\(\mathrm{NH}_{4(\mathrm{aq})}^{+}+\mathrm{H}_{2} \mathrm{O}_{(l)} \rightleftharpoons \mathrm{NH}_{4} \mathrm{OH}_{(\mathrm{aq})}+\mathrm{H}^{+}(\mathrm{aq})\)
No such tendency is shown by Cl– the weak conjugate base of the strong acid HCI.
In the above reaction 11+ ion is formed.
∴ [H+] > [OH–] and the solution is acidic and the pH is less than 7.
Equilibrium constant (hydrolysis constant) for the above equilibrium is
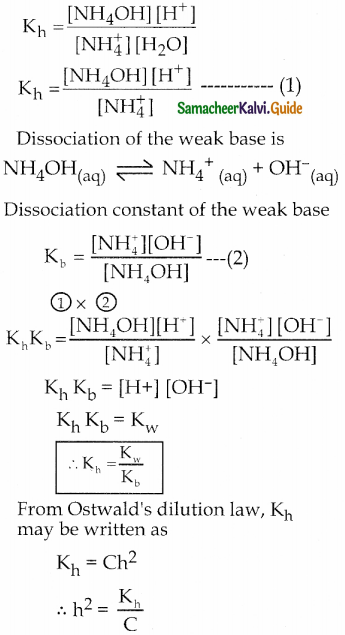
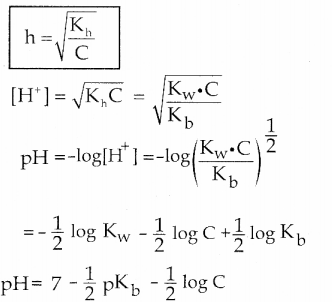
![]()
Question 19.
Solubility product of Ag2CrO4 is 1 x 10-12. What is the solubility of Ag2CrO4 in 0.01 M AgNO3 solution?
Answer:
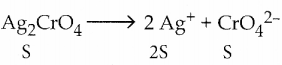
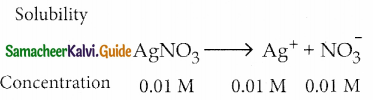
[Ag+] = 2 S + 0.01
0.01 > > 2 S, 2 S can be neglected.
∵ [Ag+] = 0.01 = 1 x 10-2
[CrO4–] = S; Ksp= 1 x 10-12
∴ Ksp = [Ag+]2 [CrO42-]
1 x 10-12 = (1 10-2)2 x s
∴ S = \(\frac{1 \times 10^{-12}}{1 \times 10^{-4}}\) ⇒ S = 1 x 10-8M
Question 20.
Write the expression for the solubility product of Ca3(PO4)2
Answer:
Ca3(PO4)2 (s) \(\rightleftharpoons\) 3Ca2+ (3s) + 2PO43- (2s)
Solubility of Ca3(PO4)2 is,
Ksp = [Ca2+]3 . [PO43-]2
Ksp = (3s)3 . (2s)2
Ksp= 27 s3 . 4s2
Ksp = 108s5.
Question 21.
A saturated solution, prepared by dissolving CaF2(s) in water, has [Ca2+] = 3.3 x 10-4 M. What is the Kspof CaF2?
Answer:
CaF2 (s) \(\rightleftharpoons\) Ca2+(aq) + 2F–(aq)
[F–] = 2 [Ca2+] = 2 x 33 x 10-4 M
= 6.6 x 10-4 M
= [Ca2+] [F–]2
= (3.3 x 10-4) (6.6 x 10-4)2
= 1.44 x 10-10
Question 22.
Ksp of AgCl is 1.8 x 10-10. Calculate molar solubility in 1 M AgNO3
Answer:
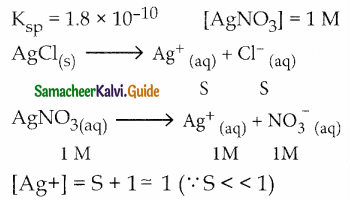
[Cl–] = S
Ksp = [Ag+] [Cl–]
1.8 x 10-10 = (1) (S)
S = 1.8 x 10-10M
![]()
Question 23.
A particular saturated solution of silver chromate Ag2CrO4 has [Ag+] = 5 x 10-5 and [CrO4]2- = 4.4 x 10 M. What is the value of for Ag2CrO4?
Answer:
Ag2CrO4 (s) \(\rightleftharpoons\) 2Ag+(aq) + CrO42-(aq)
Ksp = [Ag+]2 [CrO42-]
= (5 x 10-5)2 (4.4 x 10-4)
= 1.1 x 10-12
Question 24.
Write the expression for the solubility product of Hg2CI2.
Answer:
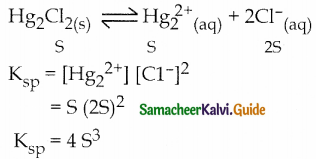
Question 25.
Ksp of Ag2CrO4 is 1.1 x 10-12 What is the solubility of Ag2CrO4 in 0.1M K2CrO4.
Answer:
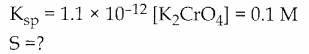
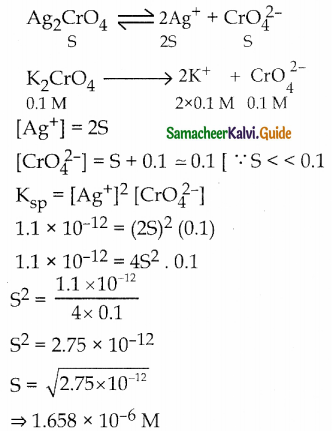
![]()
Question 26.
Will a precipitate be formed when 0.150 L of 0.1 M Pb(NO3)2 and 0.100 L of 0.2 M NaCl are mixed? (PbCI2) = 1.2 x 10-5.
Answer:
Total volume = 0.150 + 0.100 = 0.250L
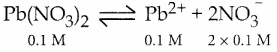
Number of moles of Pb2 = V x M
= 0.150 x 0.1
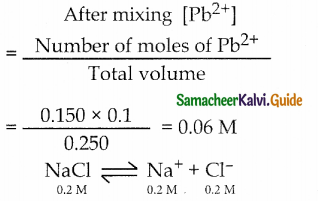
Number of moles of Cl– = V x M = 0.100 x 0.2
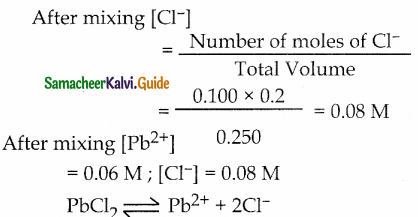
Ionic Product = [Pb2+] [Cl–]2
= (0,06) (0.08)2
= 3.84 x 10-4
Given Ksp= 1.2 x 10-5
Ionic product > Ksp
∴ Precipitation of PbCl2 will occur
Question 27.
of Al(OH)3 is 1 x 10-15 M. At what pH does 1.0 x 10-13 M AI3+ precipitate on the addition of buffer of NH4CI and NH4OH solution?
Answer:
Al(OH)3 Al3+ (aq) + 3OH– (aq)
Ksp = [Al3+] [OH–]3
Al(OH)3 precipitates when
[Al3+] [OH–]3 > Ksp
(1 x 10-3)[OH–]3 > Ksp
[OH–]3 > 1 x 10-12
[OH–] > 1 x 10-4M
[OH–] = l x 10-4 M
pOH = – 1og10[OH–] = – log (1 x 10-4) = 4
pH = 14 – 4 = 10
Thus, Al (OH)3 precipitates at a pH of 10
III. Evaluate Yourself
Question 1.
Classify the following as acid (or) base using Arrhenius concept
- HNO3
- Ba(OH)2
- H3PO4
- CH3COOH
Answer:
1. HNO3:
Nitric acid, dissociates to give hydrogen ions in water.
HNO3 is acid.
2. Ba(OH)2:
Barium hydroxide dissociates to give hydroxyl ions in water.
Ba(OH)2 is base.
3. H3PO4:
Orthophosphoric acid dissociates to give hydrogen ions in water.
H3PO4 is acid.
4. CH3COOH:
Acetic acid dissociates to give hydrogen ions in water.
CH3COOH is acid.
![]()
Question 2.
Write a balanced equation for the dissociation of the following in water and identify the conjugate acid-base pairs.
i) NH4
ii) H2SO4
iii) CH3COOH.
Evaluate.
Answer:
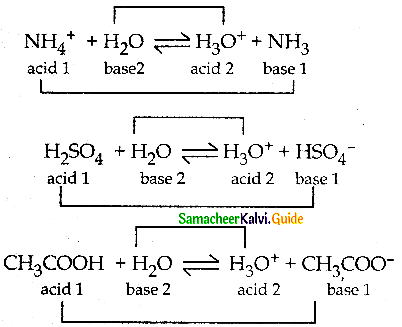
Question 3.
Identify the Lewis acid and the Lewis base in the following reactions.
(i). CaO + CO2 → CaCO3
ii) CH3 – O – CH3 – AlCl3 →
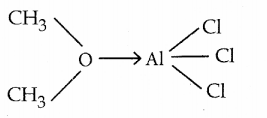
Answer:
(i). CaO + CO2 → CaCO3
CaO – Lewis base – All metals oxides are Lewis bases
CO2 – Lewis acid – CO2 contains a polar double bond.
ii)
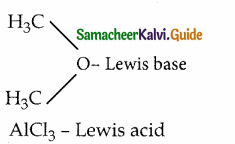
Question 4.
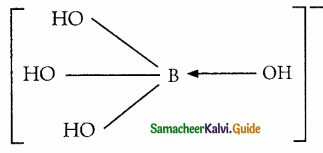
H3BO3 accepts hydroxide ion from water as shown below
Answer:
H3BO3 (aq) + H2O(l) = B(OH)4– + H+
Predict the nature of H3BO3 using Lewis concept.
![]()
Question 5.
At a particular temperature, the Kw of a neutral solution was equal to 4 x 10-14. Calculate the concentration of [H3O+] and [OH–].
Answer:
Given solution is neutral
[H3O+] = [OH–]
Let [H3O+] = x ; then [OH–] = x
Kw = [H3O+] [OH–]
4 x 10-14 = x . x
x2 = 4 x 10-14
X = \(\sqrt{4 \times 10^{-14}}=2 \times 10^{-7}\)
Question 6.
a) Calculate pH of 10-8 M H2SO4
b) Calculate the concentration of hydrogen ion in moles per litre of a solution whose pH is 5.4
c) Calculate the pH of an aqueous solution obtained by mixing 50 ml of 0.2 M HCI with 50 ml 0.1 M NaOH
Answer:
a.
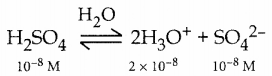
In this case the concentration of H2SO4 is very low and hence [H3O] from water cannot be neglected
∴ [H3O+] = 2 x 10-8 (from H2SO4) + 10-7 (from water)
= 10-8(2+ 10)
= 12 x 10-8 = 1.2 x 10-7
pH = – log10[H3O+]
= – log10( 1.2 x 10-7)
= 7 – log101.2
= 7 – 0.0791 = 6.9209
b. pH of the solution = 5.4
[H3O+] = antilog of (- pH)
= antilog of (- 5.4)
= antilog of (-6 + 0.6) = \(\overline{6} .6\)
= 3.981 x 10-6
i.e., 3.98 x 10-6 mol dm-3
c. No. of moles of HCl = 0.2 x 50 x 10-3 = 10 x 10-3
No. of moles of NaOH =0.1 x 50 x 10-3 = 5 x 10-3
No. of moles of HCl after mixing = 10 x 10-3 – 5 x 10-3
= 5 x 10-3
after mixing total volume = 100 mL
∴ Concentration of HCl in moles per litre
\(=\frac{5 \cdot 10^{-3} \mathrm{~mole}}{100 \cdot 10^{-3} \mathrm{~L}} ;\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=5 \times 10^{-2} \mathrm{M}\)
[H3O+] = 5 x 10-2 M
pH = – log ( 5 x 10-2)
= 2 – log 5
= 2 – 0.6990
= 1.30
![]()
Question 7.
Kb for NH4OH is 1.8 x 10-5 Calculate the percentage of ionisation of 0.06 M ammonium hydroxide solution.
Answer:
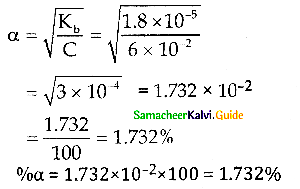
Question 8.
1. Explain the buffer action in a basic buffer containing equimolar ammonium hydroxide and ammonium chloride.
2. Calculate the pH of a buffer solution consisting of 0.4M CH3COOH and 0.4 M CH3COONa. What is the change in the pH after adding 0.01 mol of HCI to 500m1 of the above buffer solution.
Assume that the addition of HCI causes negligible change In the volume. Given: (K = 1.8 x 105).
Answer:
1. Dissociation of buffer components
NH4OH (aq) \(\rightleftharpoons\) NH4+ (aq) + OH– (aq)
NH4CI → NH4+ + Cl–
Addition of OH–
The added H+ ions are neutralized by NH4OH and there is no appreciable decrease in pH.
NH4OH(aq) + H+ \(\rightleftharpoons\) NH4+(aq) + H2O (1)
Addition of
NH4– (aq) + OH– (aq) → NH4OH (aq)
The added OH ions react with NH4 to produce unionized NH4OH. Since NH4OH is a weak base, there is no appreciable increase in pH.
2. pH of buffer
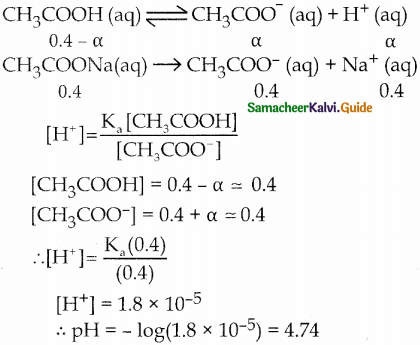
Addition of 0.01 mol HCI to 500 ml of buffer
Added [H+]
= \(\frac{0.01 \mathrm{~mol}}{500 \mathrm{~mL}}=\frac{0.01 \mathrm{~mol}}{\frac{1}{2} \mathrm{~L}}\)
= 0.02
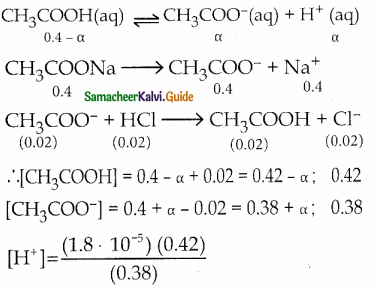
∴ pH = – log (1.99 x 10-5)
= 5 – log 1.99
= 5 – 0.30
= 4.70
![]()
Question 9.
1. How can you prepare a buffer solution of pH9. You are provided with 0.1 M NH4OH solution and ammonium chloride crystals. (Given: pKb for NH4OH is 4.7 at 25°C)
2. What volume of 0.6 M sodium formate solution is required to prepare a buffer solution of pH 4.0 by mixing it with 100 ml of 0.8 M formic acid. (Given: pKa for formic acid is 3.75.)
Answer:
1. We know that pH + pOH = 14
9 + pOH = 14
= pOH = 14 – 9 = 5
[NH4Cl] = 0.1 M x 1.995
= 0. 1995 M
=0.2 M
Amount of NH4CI required to prepare 1 litre 0.2 M solution = Strength of NH4CI x molar
mass of NH4CI
= 0.2 x 535
= 10.70 g
10.70 g ammonium chloride is dissolved in water and the solution is made up to one litre to get 0.2 M solution. On mixing equal volume of the given NH4OH solution and the prepared NH4CI solution will give a buffer solution with the required pH value (pH = 9).
2.

[Sodium formate] = number of moles of HCOONa
= 0.6 x V x 10-3
[formic acid] = number of moles of HCOOH
= 0.8 x 100 x 10-3
[formic acid] = number of moles of HCOOH
= 0.8 x 100 x 10-3
= 80 x 10-3
4 = 3.75 + log \(\frac { 0.6V }{ 80 }\)
0.25 = log \(\frac { 0.6V }{ 80 }\)
antilog of 0.25 = \(\frac { 0.6V }{ 80 }\)
0.6V = 1.778 x 80
= 1.78 x 80
= 142.4
V = \(\frac { 142.4 mL }{ 0.6 }\) = 237.33 mL
![]()
Question 10.
Calculate the
i) hydrolysis constant
ii) degree of hydrolysis and
iii) pH of 0.05M sodium carbonate solution pKa for HCO3– is 10.26.
Answer:
Sodium carbonate is a salt of weak acid, H2CO3 and a strong base, NaOH and hence the solution is alkaline due to hydrolysis.
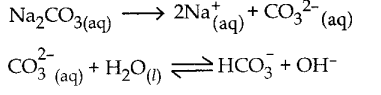
Given
Kw = 1 x 10-14
c = 0.05 M
PKa = 10.26
Ka = – log Ka
Ka = antilog of (- pKa)
Ka = antilog of (- 10.26)
Ka = 5.49 x 10-11
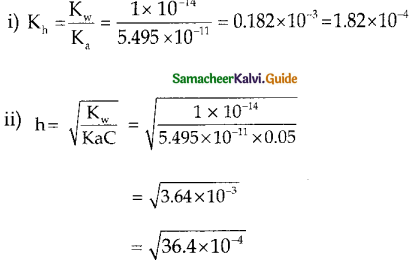
h = 6.034 x 10-2
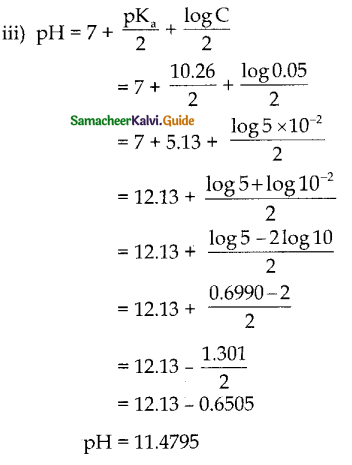
12th Chemistry Guide Ionic Equilibrium Additional Questions and Answers
I. Choose the best answer
Question 1.
The Latin word acidus means
a) bitter
b) sour
c) sweet
d) salty
Answer:
b) sour
Question 2.
According to Arrhenius concept, an acid is
a) hydrogen ion donor
b) hydrogen ion acceptor
c) hydroxyl ion donor
d) electron donor
Answer:
a) hydrogen ion donor
![]()
Question 3.
According to Arrhenius concept, a base is
a) hydrogen ion donor
b) hydroxyl ion acceptor
c) hydroxyl ion donor
d) electron acceptor
Answer:
c) hydroxyl ion donor
Question 4.
Arrhenius theory does not explain the behaviour of acids and bases in
a) aqueous solvents
b) non-aqueous solvents
c) water
d) None of the above
Answer:
b) non-aqueous solvents
Question 5.
According to Lowry-Bronsted theory, an acid is
a) proton donor
b) proton acceptor
c) hydroxyl ion donor
d) electron donor
Answer:
a) proton donor
![]()
Question 6.
According to Lowry-Bronsted theory, a base is
a) proton donor
b) proton acceptor
c) hydroxyl ion acceptor
d) electron donor
Answer:
b) proton acceptor
Question 7.
According to Lowry-Bronsted theory, an equilibrium exists between an acid and its
a) base
b) conjugate acid
c) conjugate base
d) water
Answer:
c) conjugate base
Question 8.
A conjugate acid-base pair differs only by
a) an electron
b) a proton
c) a hydroxyl ion
d) none of the above
Answer:
b) a proton
Question 9.
Lowry-Bronsted theory could not explain the acidic behaviour of
a) BF3
b) AlCl3
c) SO2
d) all the above
Answer:
d) all the above
![]()
Question 10.
According to Lewis concept an acid is
a) proton donor
b) proton acceptor
c) electron pair acceptor
d) electron-pair donor
Answer:
c) electron pair acceptor
Question 11.
According to Lewis concept a base is
a) hydroxyl ion donor
b) hydroxyl ion acceptor
c) electron pair acceptor
d) electron-pair donor
Answer:
d) electron-pair donor
Question 12.
In a coordination complex the central metal ion acts as
a) Arrhenius acid
b) Lewis base
c) Lowry-Bronsted acid
d) Lewis acid
Answer:
d) Lewis acid
Question 13.
Which of the following is true for acidic solutions?
a) [H3O+] > [OH–]
b) [H3O+] < [OH–]
c) [H3O+] = [OH–]
d) [H3O+] < [OH–]
Answer:
a) [H3O+] > [OH–]
Question 14.
Electron deficient molecules can act as
a) Arrhenius base
b) Lewis base
c) Lowry-Bronsted base
d) Lewis acid
Answer:
d) Lewis add
![]()
Question 15.
Molecules with one or more lone pairs of electrons can act as
a) Arrhenius acid
b) Lewis base
c) Lowry-Bronsted acid
d) Lewis acid
Answer:
b) Lewis base
Question 16.
Which among the following is a Lewis base?
a) BF3
b) SO3
C) SF4
d) CaO
Answer:
d) CaO
Question 17.
Which among the following is not a Lewis base?
a) MgO
b) CO2
c) H2O
d) Na2O
Answer:
b) CO2
Question 18.
Which among the following is a Lewis acid?
a) NH3
b) CaO
c) RNH2
d) FeCl3
Answer:
d) FeCl3
![]()
Question 19.
Which among the following is not a Lewis acid?
a) SiF4
b) CH2=CH2
c) BeF2
d) Fe3+
Answer:
b) CH2=CH2
Question 20.
The conjugate acid of H2O is
a) HCl
b) OH–
c) H3O+
d) HSO4–
Answer:
c) H3O+
Question 21.
The conjugate base of H2O is
a) HCl
b) OH–
c) H3O+
d) HSO4–
Answer:
b) OH–
Question 22.
Which of the following can act both as Bronsted acid and Bronsted base?
a)Cl–
b) H3O+
c) HCO3–
d) CO32-
Answer:
c) HCO3–
![]()
Question 23.
In which of the following cases, the sparingly soluble salt solution is unsaturated?
a) Ionic product > solubility product (Ksp)
b) Ionic product < solubility product (Ksp)
c) Ionic product = solubility product (KcD)
d) Both (a) and (b)
Answer:
b) Ionic product > solubility product (Ksp)
Question 24.
Which of the following is the strongest conjugate base?
a) Cl–
b) SO42-
c) CH3COO–
d) NO3–
Answer:
c) CH3COO–
Reason: The conjugate base of a weak acid is a stronger base.
Question 25.
Among the following the Ka value for a strongest acid is
a) 1.8 x 10-4
b) 1.8 x 10-5
c) 1.8 x 105
d) 1.8 x 104
Answer:
c) 1.8 x 105
Question 26.
Which among the following is the strongest base?
a) HSO4–
b) H2O
C) F–
d) H–
Answer:
d) H–
H2 is the weakest acid and its conjugate base
H– is the strongest base.
![]()
Question 27.
Which of the following salts do not undergo salt hydrolysis?
a) Sodium acetate
b) Ammonium acetate
c) Ammonium chloride
d) Sodium nitrate
Answer:
d) Sodium nitrate
Question 28.
If the hydrogen ion concentration of the solution is 10-5M, its hydroxyl ion concentration is
a) 10-5 M
b) 10-9M
c) 10-14M
d) 10-7M
Ans:
b) 10-9M
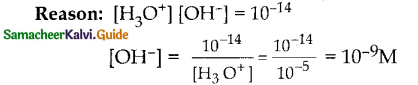
Question 29.
If the hydrogen ion concentration of a solution is 10~SM, its pOH is
a) 5
b) 9
c) 14
d) 7
Answer:
b) 9 Reason: pH = -log[H30+] = -log 10-5 = 5
pH + pOH = 14; pOH = 14 – pH = 14 – 5 = 9
![]()
Question 30.
If the pH of a solution is 9, the solution is
a) acidic
b) neutral
c) basic
d) strongly acidic
Answer:
c) basic
Reason:.pH < 7; pOH > 7 = Acidic
pH > 7; pOH < 7 = Basic
Question 31.
If the pH of a solution is zero the solution is
a) acidic
b) neutral
c) basic
d) strongly acidic
Answer:
d) strongly addic
![]()
Question 32.
The pOH of IN HCI is
a) 0
b) 1
c) 7
d) 14
Answer:
d) 14
Reason: pH = -log[H3O+] = -log 1 = 0
pOH = 14 – pH = 14 – 0 = 14
Question 33.
As [H3O+] of a solution increases, its pH
a) increases
b) deposes
c) remains the same
d) becomes
Answer:
b) decreases
![]()
Question 34.
The concentration of a solution of acetic acid changes from 10-4 M to 10-2 M, its degree of dissociation
a) increases
b) decreases
c) remains the same
d) becomes zero
Answer:
b) decreases
Reason: As dilution increases (Concentration decreases) degree of dissociation increases. Here concentration increases. Hence degree of dissociation decreases.
Question 35.
Which of the following represents Ostwald’s dilution law for a binary electrolyte whose degree of disscociation is a and concentration C
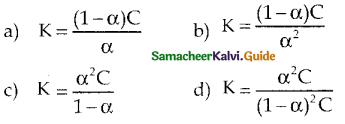
Answer:
c
Question 36.
NH4OH is a weak base because
a) It has a low vapour pressure
b) It is completely ionised
c) it is only partially ionised
d) It has low density
Answer:
c) it is only partially ionised
Question 37.
The concentration of acetic acid changes from 10-2M to 10-4 M its degree of dissociation
a) increases
b) decreases
c) remains the same
d) becomes zero
Answer:
a) increases
Question 38.
For a weak acid the hydrogen ion concentration is given as
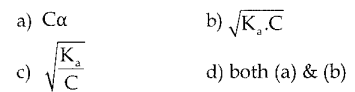
Answer:
d) both (a) & (b)
![]()
Question 39.
When ammonium chloride is added to ammonium hydroxide, the degree of dissociation of ammonium hydroxide
a) increases
b) decreases
c) remains the same
d) becomes zero
Answer:
b) decreases
Reason: Common ion effect
Question 40.
The relationship between the solubility product (Ksp) and molar solubility (S) for Ag2 (OO4) is
a) Ksp = S3
b) Ksp = S2
c) Ksp = 4S3
d) Ksp = 3S2
Answer:
c) Ksp = 4S3
Question 41.
The decrease in degree of dissociation of HF on addition of NaF is known as
a) buffer action
b) neutralization
c) common ion effect
d) hydrolysis
Answer:
c) common ion effect
![]()
Question 42.
Which is not a buffer solution?
a) CH3COOH + CH3COONa
b) HCl + NaCl
c) NH4OH + NH4Cl
d) H2CO3+NaHCO3
Answer:
b) HCl + NaCl
Reason: A strong acid and its salt is not a buffer solution. A weak acid or base and its salt is a buffer solution.
Question 43.
Which among the following does not undergo hydrolysis?
a) NH4Cl
b) CH3COONa
c) NaCl
d) CH3COONH4
Answer:
c) NaCl
Reason: NaCt is a salt of strong acid and strong base.
Question 44.
Hydrolysis of CH3COONa gives
a) acidic solution
b) basic solution
c) neutral solution
d) No solution
Answer:
b) basic solution
Reason: CH3COONa is a salt of weak acid and strong base. Hence CH3COO– undergoes hydrolysis giving a basic solution.
Question 45.
The pH of the solution resulting from the hydrolysis of NH4Cl is
a) 7
b) greater than 7
c) less than 7
d) 14
Answer:
c) less than 7
Reason: NH4Cl is a salt of strong acid and weak base. NH4C+ undergoes hydrolysis giving an acidic solution. Hence pH is less than 7.
![]()
Question 46.
For the hydrolysis of salt of weak acid and weak base which among the following is true?
a) Kh Ka = Kw
b) Kh Kb = kw
c) Ka Kb Kh = Kw
d) Kh= \(\frac{K_{1} K_{b}}{K_{w}}\)
Answer:
c) Ka Kb Kh = Kw
Question 47.
The pH of the solution obtained from the hydrolysis of salt of strong acid and weak base
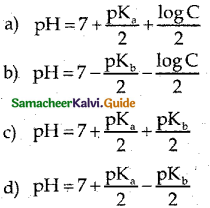
Answer:
b
Question 48.
\(X_{m} Y_{n(s)} \stackrel{H_{2} O}{\rightleftharpoons} m X_{(a q)}^{n+}+n Y_{(a q)}^{m-}\)
The solubility product of the salt XmYn is given as
a) Ksp = [Xm+]n[Yn-]m
b) Ksp = m[Xn+] n[Ym-]
c) Ksp = [Xn+]m [Ym-]n
d) Ksp =[XmYn]m+n
Answer:
c) Ksp = [Xn+]m [Ym-]n
Question 49.
The aqueous solution sdium acetate, ammonium chloflde, sodi maitrate are respectively. PTA -61
a) Neutral,acidic,basic
b) acidic, basic, neutral
c) basic, acidic, neutral
d) basic, aidk, basic
Answer:
d) basic, acidic, basic
![]()
Question 50.
The relationship between the solubility product and molar solubility of Al2(SO4)3 is
a) S2
b) 4S3
c) 108S5
d) 27S5
Answer:
c) 108S5
Reason:
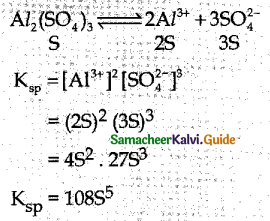
II. Pick out the correct statements
Question 1.
i) Ka measures the strength of an acid
ii) Larger the pKa value, stronger is the acid.
iii) Weak acids undergo complete ionisation
iv) Acids with Ka value greater than 10 are considered as strong acids.
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (iv)
d) (i) & (iv)
Answer:
d) (i) & (iv)
Correct statement:
ii) Larger the pKa value, weaker is the acid, (or) Lower the pKa value, stronger is the acid.
iii) Weak acids undergo partial ionisation, (or) strong acids undergo complete ionisation.
Question 2.
i) Degree of dissociation
\(=\frac{\text { Number of moles dissociated }}{\text { Total number of moles }}\)
ii) dilution law is applicable to strong acids.
iii) Strong acids undergo complete dissociation
iv) In a weak acid when dilution increases by 100 times the dissociation increases by 10 times.
a) (i) & (ii) b) (ii) & (iii)
c) (ii) & (iv) d) (i) & (iv)
Answer:
c) (ii) & (iv)
Correct statement:
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (iv)
d) (iii) & (iv)
Answer:
b) (i) & (iii)
Correct statement:
ii) Ostwald’s dilution law is applicable to strong acids.
iv) In a weak acid when dilution increases by 100 times the dissociation increases by 10 times.
![]()
Question 3.
i) A buffer solution is a mixture of a weak acid and its conjugate base
ii) Common ion effect is based on Le Chatelier’s principle.
iii) Blood contains a buffer solution of HCl and NaCl
iv) For a buffer solution
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
\(\mathrm{pH}=\mathrm{pK}_{\mathrm{a}}+\log \frac{[\mathrm{acid}]}{[\mathrm{salt}]}\)
Answer:
a) (i) & (ii)
Correct statement:
iii) Blood contains a buffer solution of H2CO3 and NaHCO3
iv) For a buffer solution
pH = pKa + log\(\frac{[\text { acid }]}{[\text { salt }]}\)
Question 4.
i) The solution obtained from the hydrolysis of CH3COONa is acidic
ii) The solution obtained from the hydrolysis of NaCl is neutral
iii) The nature of the solution obtained from the hydrolysis of a salt of weak acid and weak base depends on the strength of acid or base.
iv) The solution obtained from the hydrolysis of NH4C/ is basic
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
b) (ii) & (iii)
Correct statement:
i) The solution obtained from the hydrolysis of CH3COONa is basic
iv) The solution obtained from the hydrolysis of NH4Cl is acidic
III. Pic out the correct statements
Question 1.
For the equilibrium
\(\mathrm{HClO}_{4}+\mathrm{H}_{2} \mathrm{O} \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{ClO}_{4}^{-}\)
Which are the incorrect statements?
i) HClO4 is the conjugate acid of H2O
ii) H2O is the conjugate base of H3O+
iii) H3O+ is the conjugate base of H2O
iv) \(\mathrm{ClO}_{4}^{-}\) is the conjugate base of HClO4
a) (i) & (ii)
b) (i) & (iii)
c) (ii) & (iv)
d) (iii) & (iv)
Answer:
b) (i) & (iii)
Correct statement:
i) HClO4 is the conjugate acid of \(\mathrm{ClO}_{4}^{-}\)
iii) H3O+ is the conjugate acid of H2O
![]()
Question 2.
i) All metal ions are Lewis acids.
ii) All metal oxides are Lewis acids.
iii) All anions are Lewis bases
iv) Molecules that contain polar double bond
are Lewis bases
a) (i) & (ii)
b) (ii) & (iii)
c) (ii) & (ìv)
d) (i) & (iv)
Answer:
c) (ii) & (iv)
Correct statement:
ii) All metal oxides are Lewis bases.
iv) Molecules that contain polar double bond are Lewis acids.
Question 3.
i) The conjugate base of OH– is O2-
ii) The conjugate acid of \(\mathbf{N H}_{2}^{-}\) is NH3
iii) The conjugate acid of H2SO4 is \(\mathbf{H S O}_{4}^{-}\)
iv) The conjugate base of H2O is H3O+
a) (i) & (ii)
b) (ii) & (iii)
c) (iii) & (iv)
d) (i) & (iv)
Answer:
c) (iii) & (iv)
Correct statement:
iii) The conjugate acid of H2SO4 is \(\mathbf{H S O}_{4}^{-}\)
iv) The conjugate base of H2O is H3O+
![]()
Question 4.
i) If the hydrogen ion concentration of a solution is 10-9 M, it is basic
ii) 1f the pOH of a solution is 14, it is strongly basic.
iii)As pH of a solution increases, its pOH also increases.
iv) At 25°C the sum of pH and pOH of a solution
is 14.
a) (i) & (ii) b) (ii) & (iii) c) (iii) & (iv)
d) (j) & (iv) Ans: b) (ii) & (iii)
Correct statement:
ii) If the pOH of a solution is 14 it is strongly acidic. (or) 1f the pH of a solution is 14, it is strongly basic
iii) As pH of a solution increases, its pOH decreases. (or) As pH of a solution decreases, its pOH increases.
IV. Assertion and reason
Question 1.
Assertion (A): Ionic product of water Kw increases with increase in temperature
Reason (R): Dissociation of water is an exothermic reaction.
a) Both A & R are correct, R explains A
b) Both A & R are correct, R does not explain A.
c) A is correct, but R is wrong.
d) A is wrong, but R is correct.
Answer:
c) A is correct, but R is wrong.
Correct R: Dissociation of water is an endothermic reaction.
![]()
Question 2.
Assertion (A): Carbanion is a Lewis base
Reason (R): Carbanion can donate a pair of electron.
a) Both A & R are correct, R explains A
b) Both A & R are correct, R does not explain A.
c) A is correct, but R is wrong.
d) A is wrong, but R is correct.
Answer:
a) Both A& R are correct, R explains A Correct statement:
Question 3.
Assertion (A): An aqueous solution of IICI is acidic
Reason (R): An aqueous solution of HCl
contains less H30* than OH- ions.
a) Both A & R are correct, R explains A
b) Both A & R are correct, R does not explain A.
c) A is correct, but R is wrong.
d) A is wrong, but R is correct.
Answer:
c) A is correct, but R is wrong.
Correct R: An aqueous solution of HCl contains more H3O+ than 0H ions.
![]()
Question 4.
Assertion (A): When sodium acetate is added to acetic acid, the dissociation of acetic acid increases.
Reason (R): The addition of CH3COO– ion shifts the equilibrium of dissociation of acetic acid to the left.
a) Both A & R are correct, R explains A
b) Both A & R are correct, R does not explain A.
c) A is correct, but R is wrong.
d) A is wrong, but R is correct.
Answer:
d) A is wrong, but R is correct.
Correct A: When sodium acetate is added to acetic acid, the dissociation of acetic acid decreases.
V. Match the following:
Question 1.
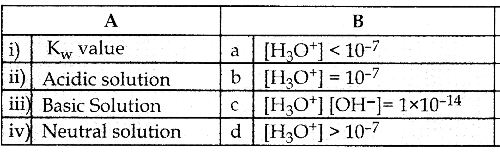
Answer:
i) c
ii) d
iii) a
iv) b
Question 2.
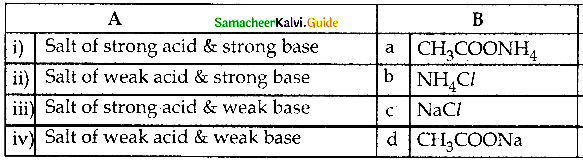
Answer:
i) c
ii) d
iii) b
iv) a
VII. Two Mark Questions
Question 1.
What are Arrhenius acids and base? Give examples.
Answer:
| Arrhenius acids | Arrhenius bases |
| 1. Hydrogen ion donor in water | Hydroxyl ion donor in water |
| Ex. HCl, H2SO4 | Ex. NaOH, Ca(OH)2 |
![]()
Question 2.
What are the limitations of Arrhenius concept of acids and bases?
Answer:
- Does not explain the behaviour of acids and bases in non-aqueous solvents such as acetone, THF etc.,
- Does not account for the basic nature of the substances like ammonia which do not contain a hydroxyl group.
Question 3.
How does BF3 act as Lewis acid?
Answer:
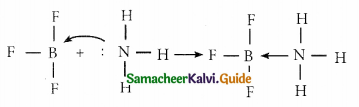
Boron has a vacant 2p-orbital to accept the lone pair of electrons donated by ammonia to form a new coordinate covalent bond. Hence BF3 acts as a Lewis acid.
Question 4.
Arrange HCl, HCOOH and CH3COOH in their increasing order of acid strength if their Ka values at 25°C are 2 x 106, 1.8 x 10-4 and 1.8 x 10-5 respectively.
Answer:
- As Ka value increases, the acid strength increases.
- Increasing order of Ka value is 1.8 x 10-5 < 1.8 x 10-4 < 2 x 106
- The increasing order of acid strength is CH3COOH < HCOOH < HCl.
Question 5.
What is neutralization?
Answer:
The reaction in which an acid reacts with a base to form salt and water is called neutralization.
Question 6.
What do yo mean by salt hydrolysis?
Answer:
Salt hydrolysis is the reaction of the cation or the anion or both the ions of the salt with water to produce either acidic or basic solution.
![]()
Question 7.
What is Buffer index (β) ?
Answer:
It is defined as the number of gram equivalents of acid or base added to 1 litre of the buffer solution to change its pFl by unity.
\(\beta=\frac{\mathrm{dB}}{\mathrm{d}(\mathrm{pH})}\)
dB = number of gram equivalents of acid/base added to one litre of buffer solution. d(pH) = The change in the pH after the addition of acid / base.
Question 8.
How solubility product is determined by molar solubility?
Answer:
Solubility product can be calculated from the molar solubility i.e,, the maximum number of moles of solute that can be dissolved in one litre of the solution.
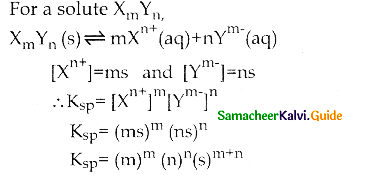
Question 9.
Define pOH
Answer:
pOH of a solution is defined as the negative logarithm of base 10 of the molar concentration of hydroxyl ions present in the solution. pOH = -log10[OH–]
Question 10.
Write the pH value of the following substances.
(a) Vinegar
(b) Black coffee
(c) Baking soda
(d) Soapy water
Answer:
| Substance | pH |
| Vinegar | 2 |
| Black coffee | 5 |
| Baking soda | 9 |
| soapy water | 12. |
VII.Three Mark Questions
Question 1.
Derive the relation between pH and pOH
Answer:
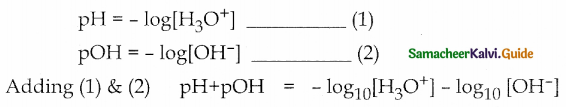
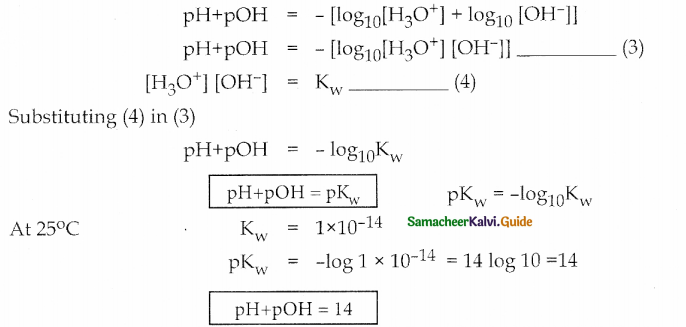
![]()
Question 2.
What are buffer solutions? Explain their types with examples.
Answer:
Solutions which resist drastic changes in its pH upon the addition of small amount of acids or bases are called buffer solutions.
A buffer solution consists of a mixture of a weak acid and its conjugate base (salt) or a weak base and its conjugate acid (salt)
Types:
Acidic buffer: Solution of acetic acid and sodium, acetate
Basic buffer: Solution of ammonium hydroxide and ammonium chloride.
Question 3.
What are conjugate acid-base pairs? Give example.
Acid1 + Base2 ⇌ Acid2 + Base1
The species that remains after the donation of
a proton is a base (Base}) and is called the conjugate base of the Bronsted acid (Acid}). In other words, chemical species that differ only by a proton are called conjugate acid – base pairs.
Conjugate acid – base pair
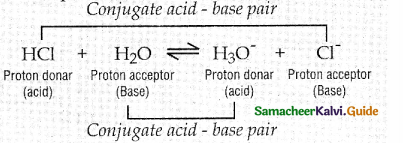
VIII. Five Mark Questions.
Question 1.
Write a note on Lewis’s concepts of acids and bases ?
Answer:
![]()
Question 2.
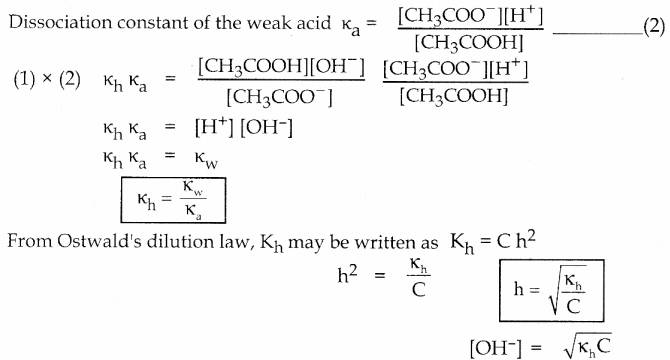
Derive an expression for the hydrolysis constant and degree of hydrolysis of salt of weak acid and strong base. Answer:
Consider the reaction between the weak acid CH3COOH and the strong base NaOH to form the salt CH3COONa and water.
\(\mathrm{CH}_{3} \mathrm{COOH}_{(\mathrm{aqq}}+\mathrm{NaOH}_{(\mathrm{aq})} \rightleftharpoons \mathrm{CH}_{3} \mathrm{COONa}_{(\mathrm{aq})}+\mathrm{H}_{2} \mathrm{O}_{(\mathrm{I})}\)
In aqueous solution CH3COONa is completely dissociated as follows,
\(\mathrm{CH}_{3} \mathrm{COONa}_{(\mathrm{aq})} \longrightarrow \mathrm{CH}_{3} \mathrm{COO}_{(\mathrm{aq})}+\mathrm{Na}_{(l)}^{+}\)
CH3 COO– is the strong conjugate base of the weak acid CH3COOH
\(\mathrm{CH}_{3} \mathrm{COO}_{\text {(aq) }}^{-}+\mathrm{H}_{2} \mathrm{O}_{(l)} \rightleftharpoons \mathrm{CH}_{3} \mathrm{COOH}_{(\mathrm{aq})}+\mathrm{OH}_{\text {(ач) }}^{-}\)
No such tendency is shown by Na+, the weak conjugate acid of the strong base NaOH.
In the above reaction OH– ion is formed.
[OH–] > [H+] and the solution is basic and the pH is greater than 7.
Equilibrium constant (hydrolysis constant) for the above
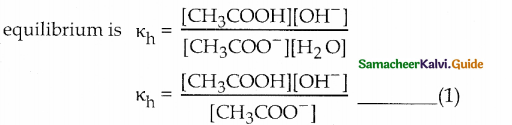
Dissociation of the weak acid is CH3COOH(aq) \(\mathrm{CH}_{3} \mathrm{COOH}_{(\mathrm{aq})} \rightleftharpoons \mathrm{CH}_{3} \mathrm{COO}^{-}{ }_{(\mathrm{aq})}+\mathrm{H}^{+}{ }_{(a q)}\)
Dissociation constant of the weak acid
Question 3.
Explain buffer action of acidic buffer?
Answer:
- The chemical reaction which is responsible to resist the pH change of a buffer solution is called its buffer action.
- To resist changes in its pH on the addition of an acid or base, the buffer solution should contain both acidic as well as basic components.
- These components neutralise the effect of added acid or base.
- At the same time, these components should not consume each other.
- Consider an acidic buffer containing CH3COOH and CH3COONa.
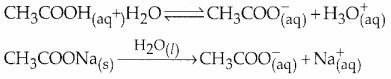
When an acid is added to this buffer, the added H+ ions are consumed by the conjugate base CH3COO– to form undissociated CH3COOH. This keeps the H+ concentration and the pH unchanged.
\(\mathrm{CH}_{3} \mathrm{COO}_{(a \mathrm{q})}^{-}+\mathrm{H}_{(\mathrm{aq})}^{+} \longrightarrow \mathrm{CH}_{3} \mathrm{COOH}_{(\mathrm{ay}}\)
When a base is added to this buffer, the added OH- ions are consumed by H30+. To maintain the equilibrium more CH3COOH is dissociated keeping the H+ Concentration and the pH unchanged
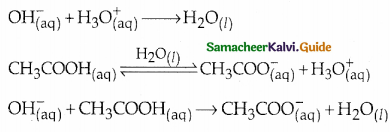
Question 4.
Derive Henderson-Hasselbalch equation.
Answer:
Consider an acidic buffer
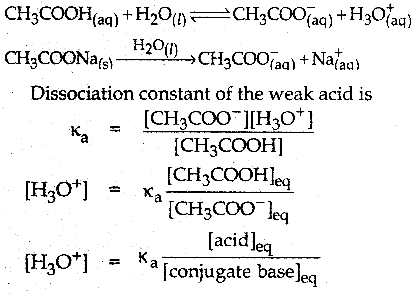
- The weak acid is dissociated only to a small extent.
- Due to the common ion effect, the dissociation is further suppressed.
- Hence the equilibrium concentration of the acid is nearly equal to the initial concentration of the unionised acid.
- Similarly, the equilibrium concentration of the conjugate base is nearly equal to the initial concentration of the added salt.
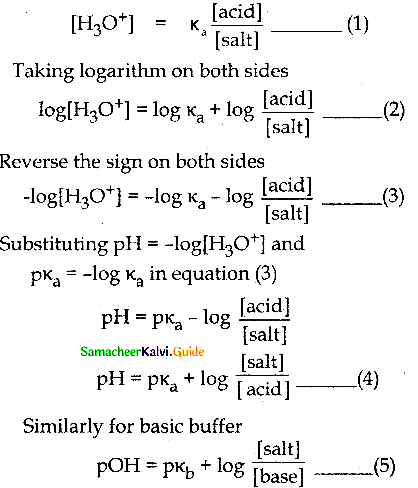
Equations (4) & (5) are known as
Henderson-Hasselbaich equations.
![]()
Question 5.
Derive an expression for the hydrolysis constant and degree of hydrolysis of salt of a weak acid and weak base.
Answer:
- Consider the reaction between the weak acid CH3COOH and the weak base NH4OH to form the salt CH3COONH4 and water.
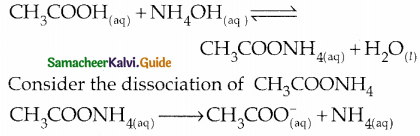
- \(\mathrm{NH}_{4}^{+}\) is the strong conjugate acid of the weak base NH4OH.
- CH3COO– is the strong conjugate base of the weak acid CH3COOH.
- Hence both \(\mathrm{NH}_{4}^{+}\) and CH3COO– have the tendency to react with water.
- The nature of the solution depends on the strength of acid or base.
- if Ka >Kb then the solution is acidic and pH > 7.
- if Ka < Kb then the solution is basic and pH > 7.1
- if Ka = Kb then the solution is neutral and pH = 7.
Consider the hydrolysis of both \(\mathrm{NH}_{4}^{+}\) and CH3COO–
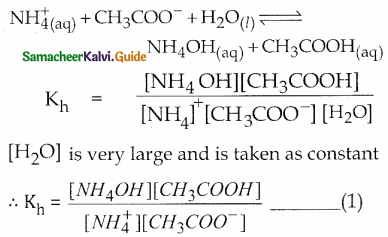
Dissociation of a weak acid
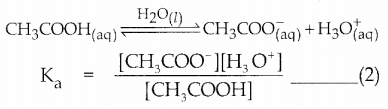
Dissociation of weak base
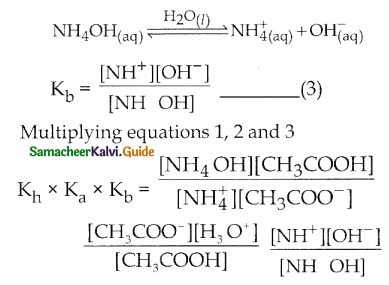
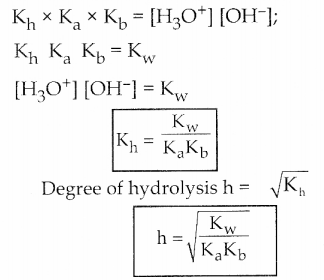
Question 6.
0.1 M Solution of HF is a weak acid. But 5M solution of HF is a stronger acid. Why?
Answer:
- Hydrofluoric acid is a much stronger acid when it is concentrated than when it is diluted. As the concentration of hydrofluoric acid approaches 100 percent.
- It’s acidity increases because of home association, where a base and conjugate acid form a bond.
- 3HF ⇌ H2F+ + HF2–
- The FHF-bifluoride anion is stabilized by a strong hydrogen bond between hydrogen and fluorine.
- The stated ionization constant of hydrofluoric acid, 10 – 3.15, does not reflect the true acidity of concentrated HF solutions.
- Hydrogen bonding also accounts for the higher boiling point of HF compared to other hydrogen halides.
IX. Additional problems:
Problems based on pH.
Question 1.
What is the pH of 0.001M NaOH?
Solution:
[OH–] = Normality = Molarity x acidity
= 0.001 x 1 = 1 x 10-3
pOH = -log 10 [OH–] = -log 1 x 10-3
= -log 1 – log 10-3 [log 1 = 0]
= 0 + 3log10 [log10 = 1]
pOH = 3
pH + pOH = 14
pH = 14 – pOH
= 14-3
pH = 11
![]()
Question 2.
What is the pH of a solution with hydronium ion concentration 6.2 x 10-9 M.
Solution:
[H3O+] = 6.2 x 10-9
pH = -log10[H3O+]
= – log 6.2 x 10-9
= – log 6.2 – log 10-9
= – log 6.2 + 9 log 10
= 9 – log 6.2
= 9 -0 .7924
pH = 8.2076
Question 3.
What is the pH of 10-2 M Ca(OH)2?
Solution:
[OH–] = Normality = Molarity x acidity
= 10-2 x 2
[OH–] = 2 x 10-2
pOH = -log10[OH– ]
= -log 2 x 10-2
= -log 2 – log 10-2
= -log 2 + 21ogl0
= 2 – log 2
= 2 – 0.3010
pOH = 1.6990
pH+pOH = 14
pH = 14 -pOH
= 14 -1.6990
pH = 12.3010
Question 4.
Calculate the hydroxyl ion concentration of a solution with pH = 5
Solution:
[H3O+] = 10pH
[H3O+] = 10-5
[H3O+][OH–] = 10-14
[OH–] = \(\frac{10^{-14}}{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]}\)
= \(\frac{10^{-14}}{10^{-5}}\)
[OH–] = 10-9
![]()
Question 5.
Calculate the pH of 10-8M HNO3
[H3O+] = 10-8 which is less than 10 7
[H3O+] from water should be added.
[H3O+] = 10 “(from water) + 10-8(from HN03)
[H3O+] = 10-7 (1+10-1)
= 10-7 (1+0.1)
[H3O+] = 1.1 x 10-7
pH = – log10 [H3O+]
= – log 1.1 x 10-7
= – log 1.1 – log 10-7
= – log 1.1 + 7 log 10
= 7 – log 1.1
= 7 – 0.0414
pH = 6.9586
Question 6.
The dissociation constant of 0.1 M weak acid is 1 x 10-5. Calculate its pH.
Answer:
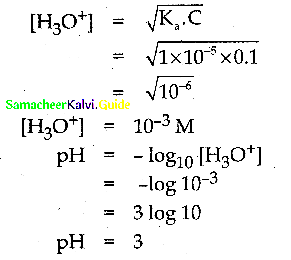
Question 7.
If a solution with pH = 5 is diluted 100 times. What will be the pH of the resulting solution?
Solution:
pH = 5; [H3O+] = 10-pH
[H3O+] = 10-5
After dilution
[H3O+] = \(\frac{10^{-5}}{100}=10^{-7} \mathrm{M}\)
As [H3O+] = 10-7 M, the [H3O+] contributed
by water should be added.
Final [H3O+] = 10-7 (from water) + 10-7 (from the solution
= 10-7(1+1)
[H3O+] = 2 x 10-7M
= – log [H3O+]
= – log 2 x 10-7
= – log 2 – log 10-7
= – log 2 + 7 log 10
= 7 – log 2
= 7 – 0.3010
= 6.6990
![]()
Question 8.
Calculate the hydrogen ion concentration in human blood. (pH of human blood is 7.4)
Solution:
pH = 7.4
pH = – logio [H3O+]
log [H3O+] = -pH
[H30+] = Antilog of (-pH)
= Antilog of (-7.4)
= Antilog of (-7-0.4)
= Antilog of (-7-0.4+1-1)
= Antilog of (-8+0.6)
= Antilog of (8.6)
[H3O+] = 3.98 x 10-8
Question 9.
Calculate the pH of a solution obtained by mixing 50ml of 0.4 N HC1 and 50ml of 0.2N NaOH.
Solution:
Milli equivalent of 50 ml, 0.4N HCl
= Vm1 x N = 50 x 0.4 = 20
Milli equivalent of 50 ml, 0.2N NaOH
= Vm1 x N = 50 x 0.2 = 10
Remaining milli equivalent of Hcl = 20-10 =10
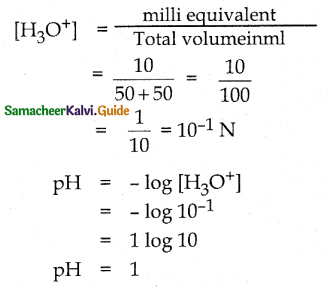
![]()
Question 10.
Calculate the pH of 10-7M HCl.
Answer:
If we do not consider [H3O+] from the ionisation of H2O, then [H3O+] = [HCl]= 10-7M)
In this case the concentration of the acid is very low (10-7M). Hence, the [H3O+] (10-7M) formed due to the auto ionisation of water cannot be neglected.
[H3O+] = 10-7 (from HCl) + 10-7 (from H2O)
= 10-7 (1+1) = 2 x 10-7
pH = -log10 [H3O+]
= log10[2 x 10-7] = – log 2 + log 10-7
= -log 2 – (-7) log1010
= 7 – log2 .
= 7 – 0.3010=6.6990
= 6.70
Problems based on Henderson – Hasselbaich equations
Question 1.
A buffer solution contains equal volumes of 0.2 M NH4OH and 0.02 M NH4Cl. Kb of NH4OH is 1 x 10-5. Calculate the pH of the buffer.
Solution:
[Base] = 0.2 M; [salt] = 0.02 M
Kb = 1 x 10-5
pKb = -log Kb = – log 1 x 10-5 = 5
For a basic buffer
\(\mathrm{pOH}=\mathrm{pK}_{\mathrm{b}}+\log \frac{[\mathrm{Salt}]}{[\text { Base }]}\)
= 5+log 0.02/0.2
= 5+1og10-1
= 5 – 1
pOH = 4
pH + pOH = 14
pH = 14 – pOH
= 14 -4
pH = 10
![]()
Question 2.
Find the pH of a buffer solution containing 0.20 mole per litre CH3COONa and 0.15 mole per litre CH3COOH, Ka for acetic acid is 1.8 x 10-5
Solution:
[acid] = 0.15 M; [salt] = 0.20 M
Ka = 1.8 x 10-5
PKa = -log Ka = – log L8 x 10-5
= – log 1.8 – log10-5
= – log 1.8 + 5 log 10
= 5 – log 1.8
= 5 – 0.2553 pKa
PKa = 4.7447
For an acidic buffer
\(\mathrm{pK}_{\mathrm{b}}=\mathrm{pOH}-\log \frac{[\text { Salt }]}{[\text { Base }]}\)
= 4.7447 + log 0.20/0.15
= 4.7447 + log 4/3
= 4.7447 + log 4 – log 3
= 4.7447+0.6021-0.4771
pH = 4.8697
![]()
Question 3.
Calculate the pKb of NH4OH, if the pH of a buffer solution containing 0.1N NH4OH and 0.1 N NH4Cl is 9.25.
Solution;)
pH of basic buffer = 9.25
∴ pOH = 14 – pH
= 14 – 9.25
pOH = 4.75
[Base] = 0.1N ; [Salt] = 0.1 N
\(\mathrm{pK}_{\mathrm{b}}=\mathrm{pOH}-\log \frac{[\text { Salt }]}{[\text { Base }]}\)
\(\mathrm{pK}_{\mathrm{b}}=\mathrm{pOH}-\log \frac{[\text { Salt }]}{[\text { Base }]}\)
PKb = 4.75 – log M = 4.75 – log 0.1/0.1 = 4.75 – 0 = 4.75
![]()
Question 4.
Calculate the pH of the solution by mixing 1.5 mole of HCN and 0.15 mole of KCN in water and making up the total volume to 0.5 litre.
Solution:
Ka = 5 x 10-10;
fAcid] = 1.5/0 5 – 3.0
[Salt] = 0.15/0.5 = 0.3
pKa = – log Ka
= – log 5 x 10-10
= – log 5 – log 10-10
= – log 5 + 10 log-10
= 10 – log 5
= 10 – 0.6990
pK-10 = 9.3010
\(\mathrm{pH}=\mathrm{pKa}+\log \frac{[\mathrm{Salt}]}{[\mathrm{Acid}]}\)
= 9.3010 + log 0.3/3.0
= 9.3010 + log 10-10
= 9.3010 -1 log 10
pH = 8.3010
Problems based on Ostwald’s dilution law
Question 1.
Calculate the degree of dissociation of 0.1 N CH3COOH if Ka for CH3COOH is 1 x 10-5.
Solution:
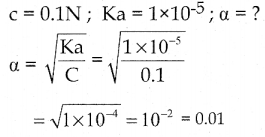
Question 2.
What is the dissociation constant of 0.1 M HCN which is 0.01% ionised?
Solution:
C = 0.1M ; α = 0.01% = \(\frac{0.01}{100}\) = 1 x 10 -4
Ka = ?
Ka = C α2 = 0.1 x (1 x 10-4)2 = 1 x 10-9
![]()
Question 3.
A weak monobasic acid is 1% ionised in 0.1M solution at 25°C. What is the percentage of ionisation in its 0.025M Solution?
Solution:
C =0.1 M; α = 1% = 1/100 = 10-2 Ka = ?
C =0.025 M % a = ?
Ka = C α2 = 0.1 x (10-2)2 = 10-5
\(\alpha=\sqrt{\frac{K a}{C}}=\sqrt{\frac{10^{-5}}{0.025}}=0.02\)
% α =100 x α = 100 x 0.02 = 2%
![]()
Question 4.
A mono basic weak acid solution has a molarity of 0.005 and pH of 5. What is its percentage ionisation in this solution?
Solution:
C = 0.005 M
pH = 5
∴ [H3O+] = 10-ph = 10-5
HA → H+ + A–
ka = \(\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}\)
[H+] = [A–] = 10-5
[HA] = 0.005M
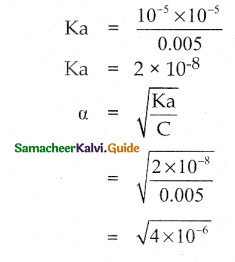
α = 2 x 10-3
% α = 100 x α
= 100 x 2 x 10-3 = 0.2%
Problems based on Solubility Product
Question 1.
Calculate the solubility product of AgCl if the solubility of AgCl is 1.3 x 10-5 moles per litre at 25°C.
Solution:
S = 1.3 x 10-5 M ; Ksp = ?
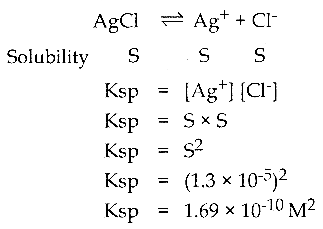
![]()
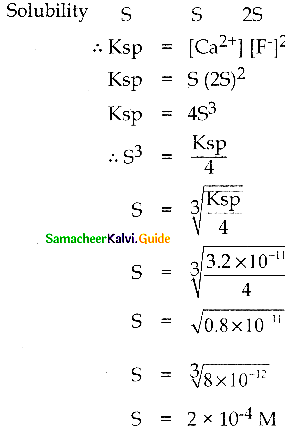
Question 2.
Calculate the solubility of Calcium fluoride in a saturated solution if its solubility product is 3.2 x 10-11 M3.
Solution:
Ksp = 3.2 x 10-11 M3; S = ?
Question 3.
The solubility of AgCl is 0.0014 g per litre at 18°C. Calculate its solubility product at 18°C. Molecular weight of AgCl is 143.5. Solubility in
Answer:
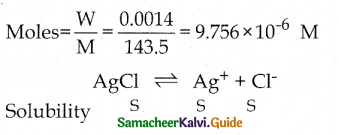
Ksp = [Ag+][Cl–]
Ksp = S x S
Ksp = S2
Ksp = (9.756 x 10-6M)2
Ksp = 95.1795 x 10-12
Ksp = 9.518 x 10-11 M2
Question 4.
The solubility product of a salt having general formula MX2 in water is 4 x 10-12. What is the concentration of M2+ ions in the aqueous solution of the salt?
Solution:
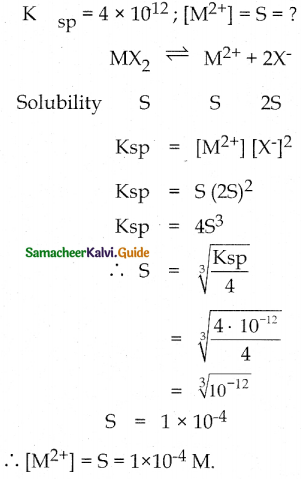
![]()
Question 5.
Solid Ba (N03)2 is gradually dissolved in 1 x 10-4 m Na2CO3 solution. At what concentration of Ba2+ will a precipitate begin to form?
(Ksp for BaCO3 = 5.1 x 10-9 )
Solution:
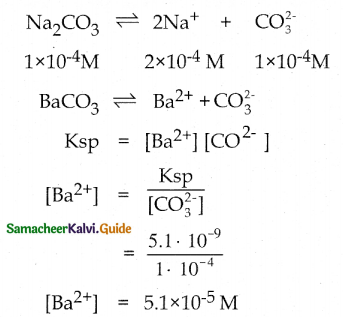
Ionic Product > Ksp, Precipitation will occur.
∴ After attaining 5.1 x 10-5 M [Ba2+] concentration, a precipitate will begin to form.
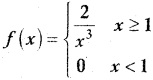
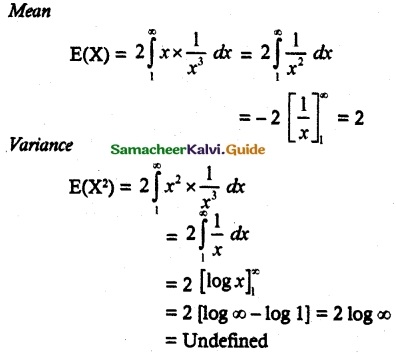
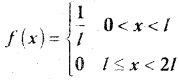
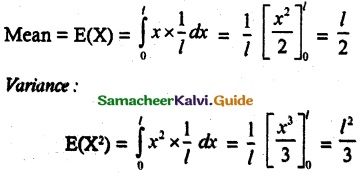
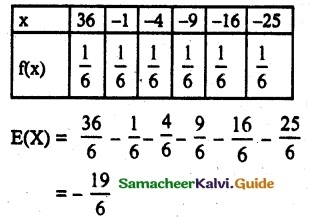
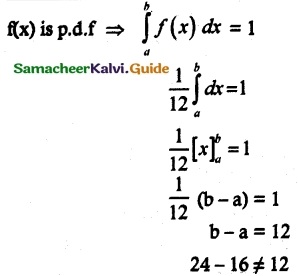

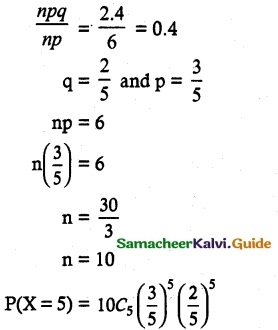
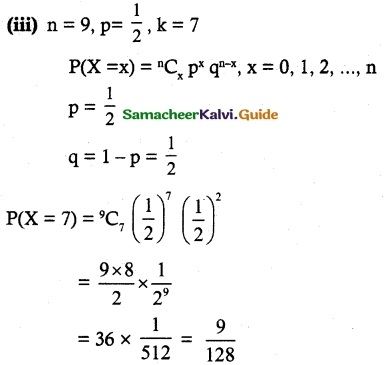
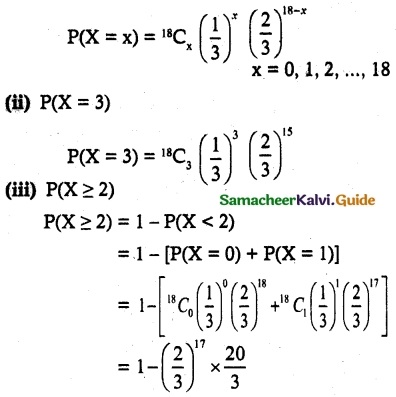
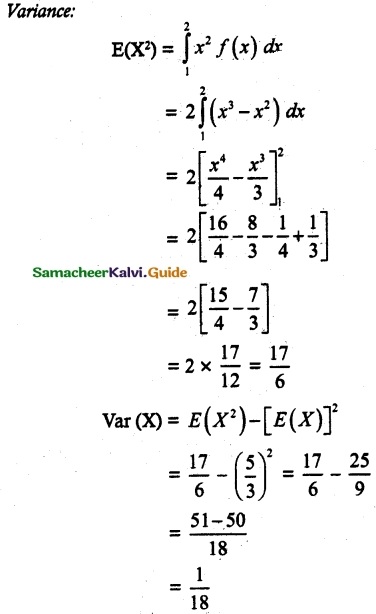
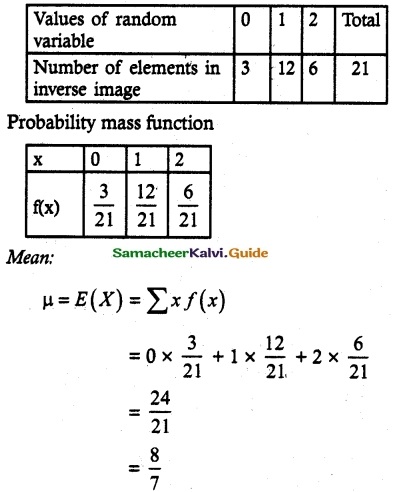
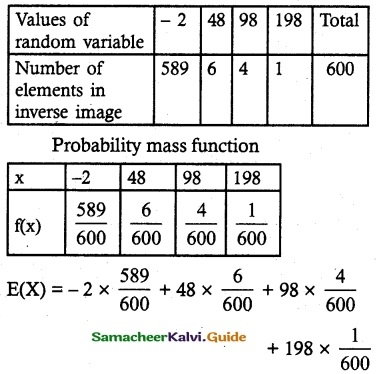
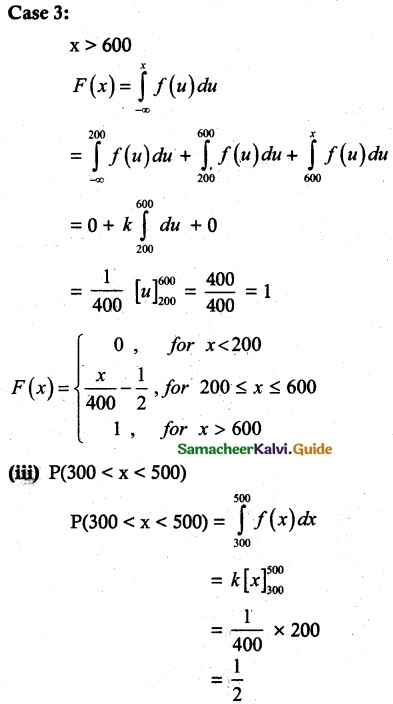
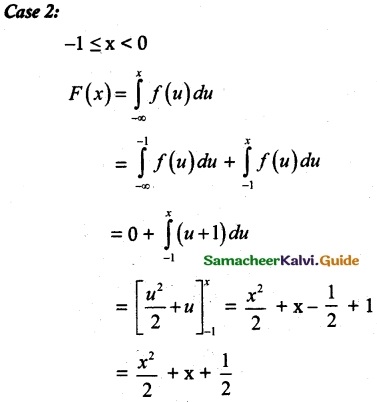
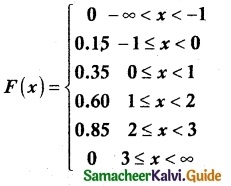
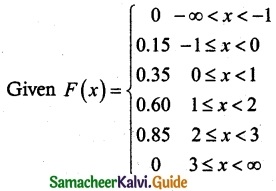
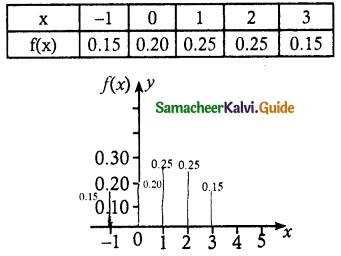
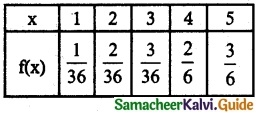
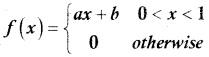 and E(X) = \(\frac { 7 }{ 12 }\) then a and b are respectively
and E(X) = \(\frac { 7 }{ 12 }\) then a and b are respectively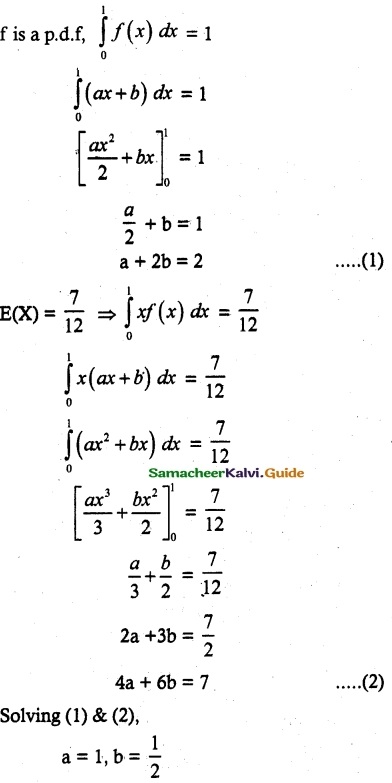
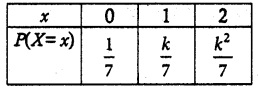
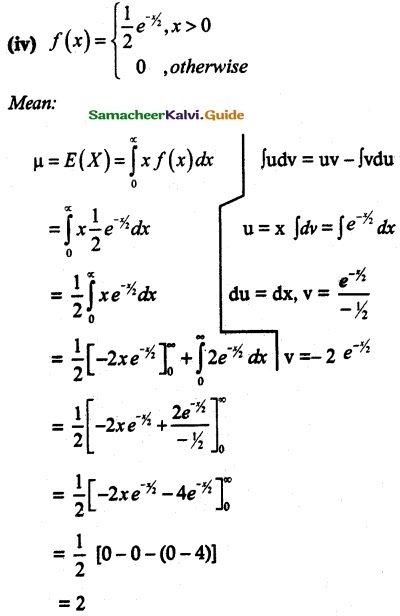
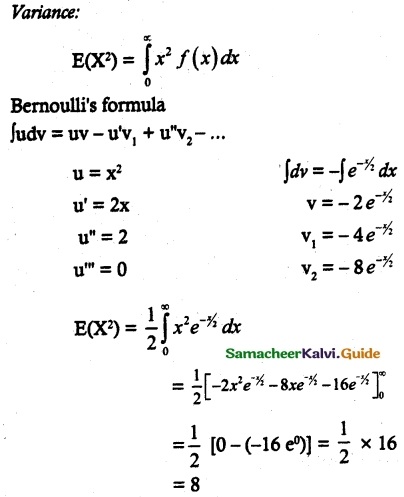
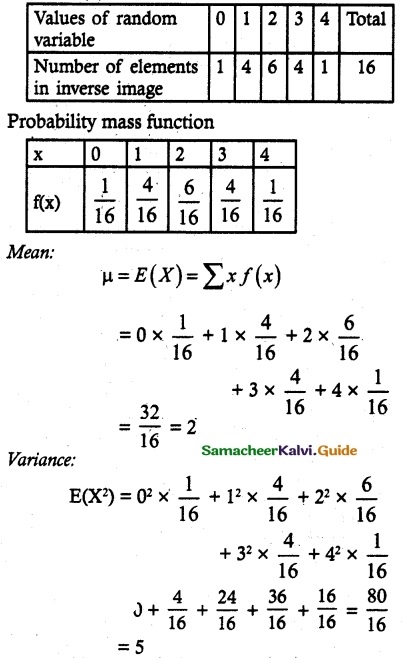
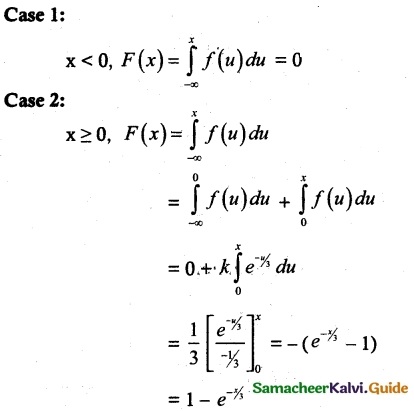
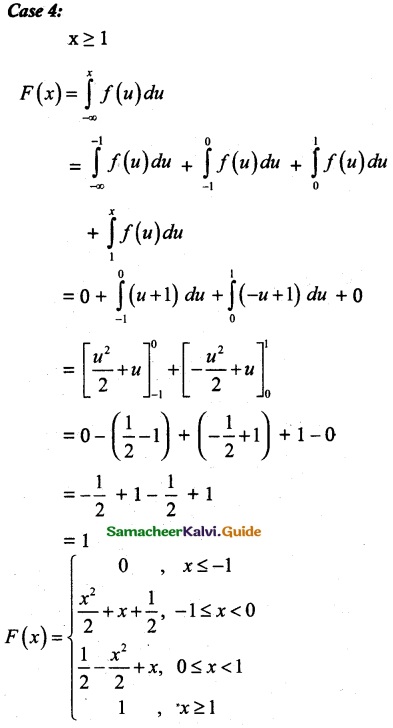
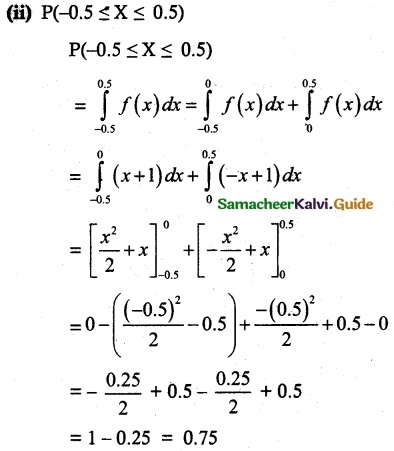
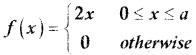 is a probability density function of a random variable, then the value of a is
is a probability density function of a random variable, then the value of a is
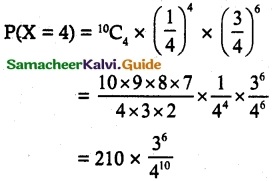
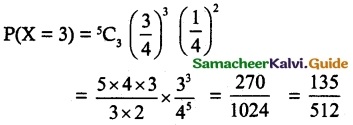
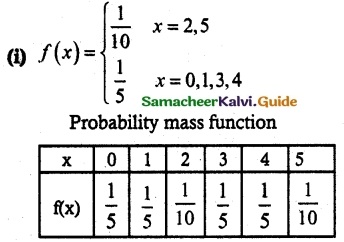
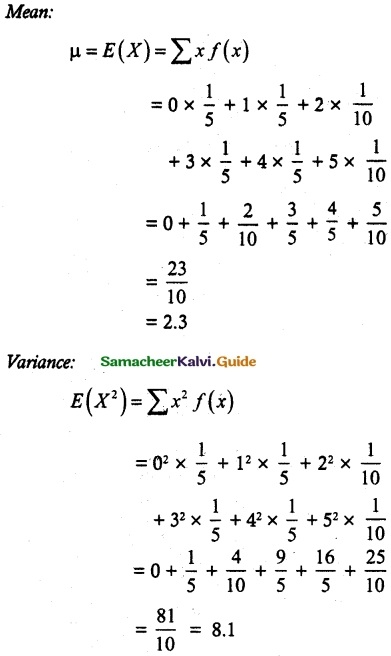
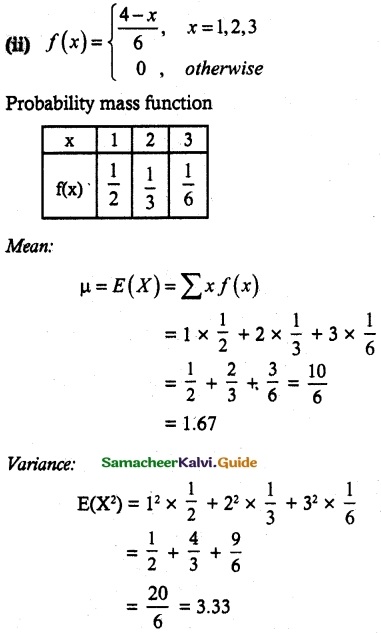
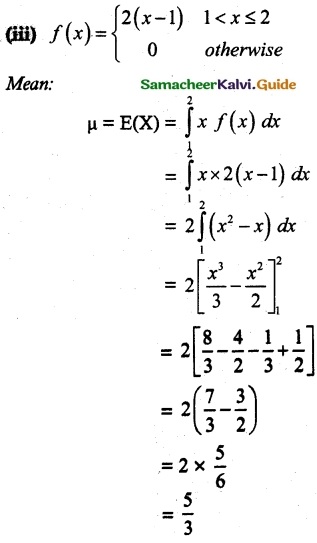
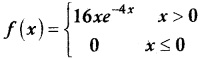
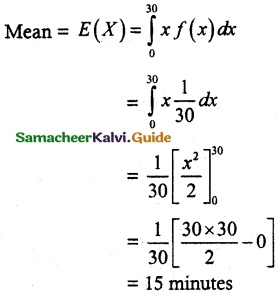
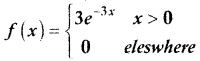
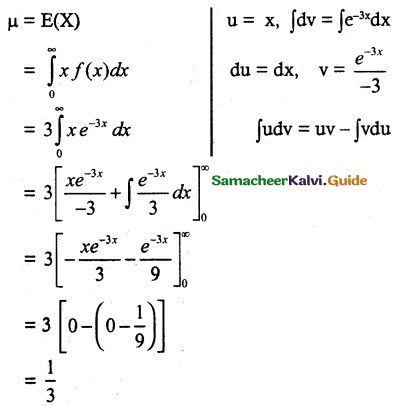
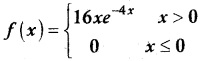
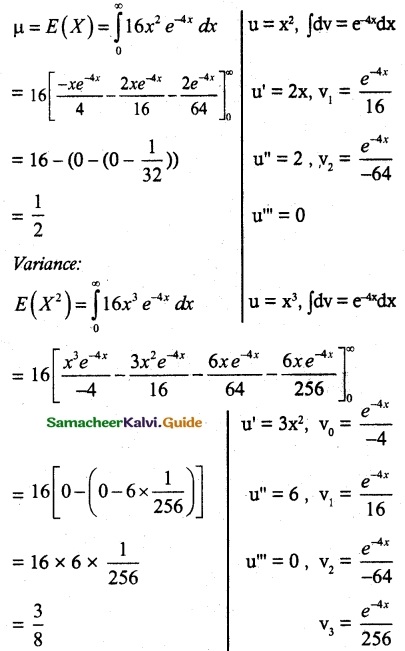

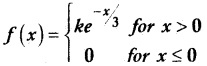
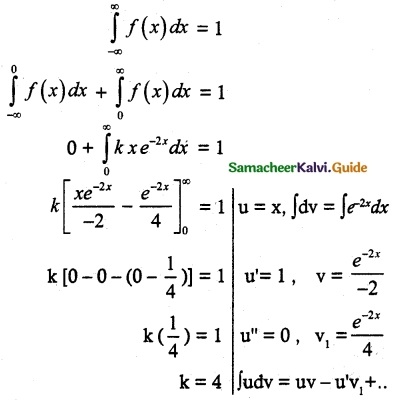
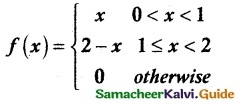
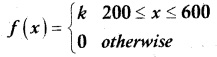
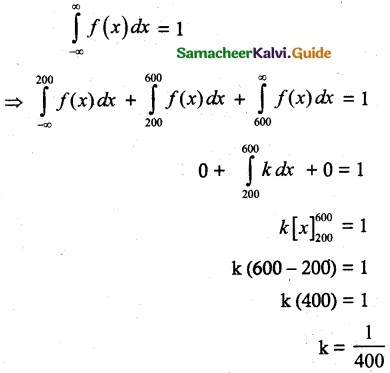
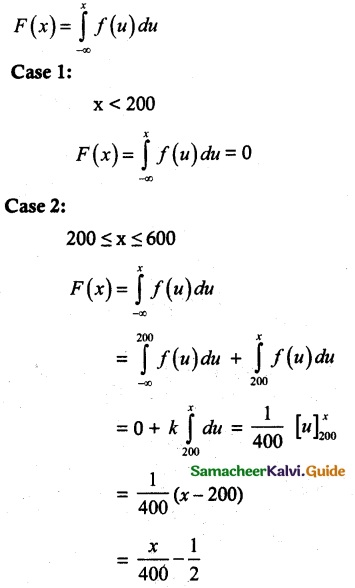
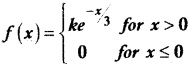
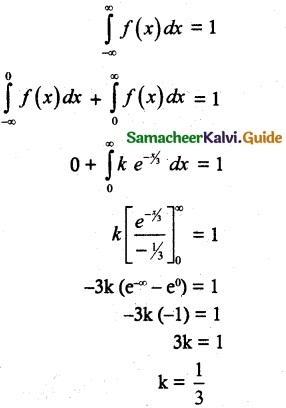
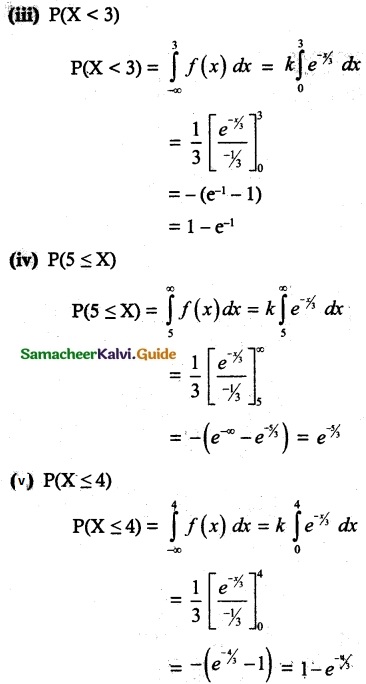
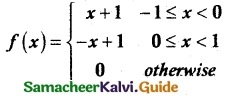
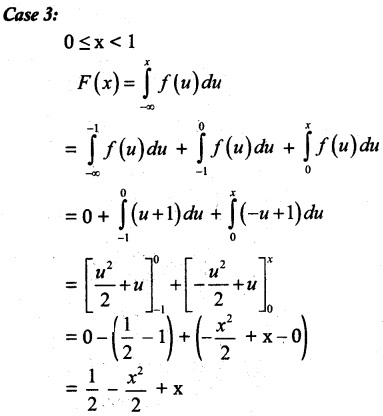

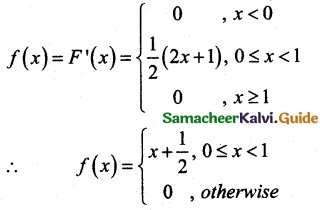
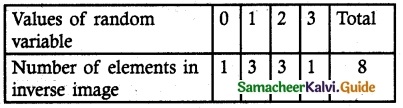
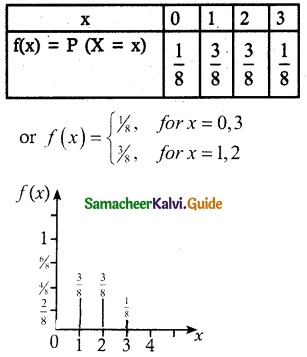
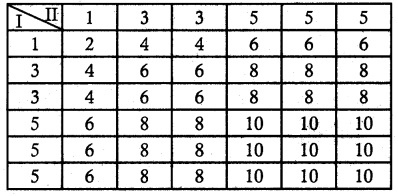
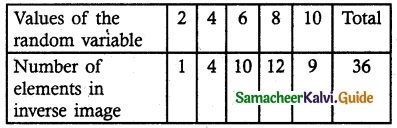
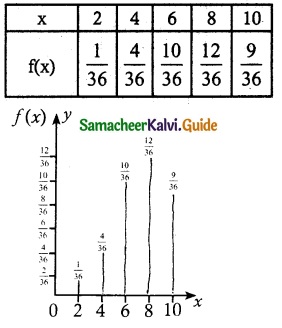
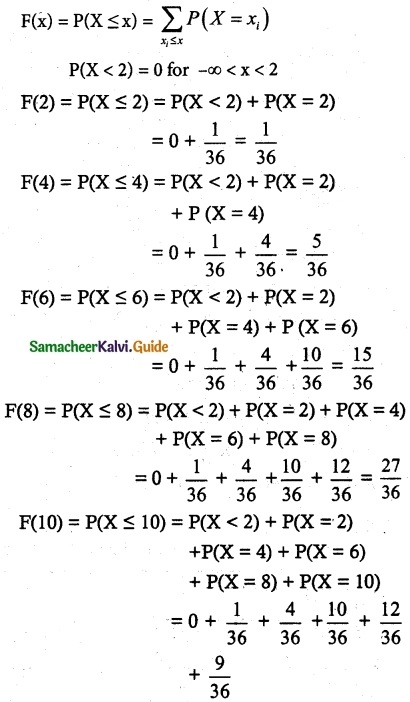
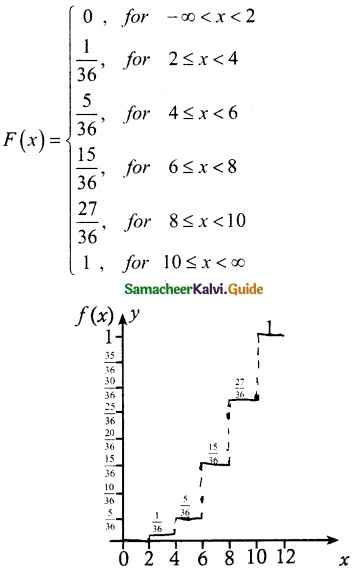
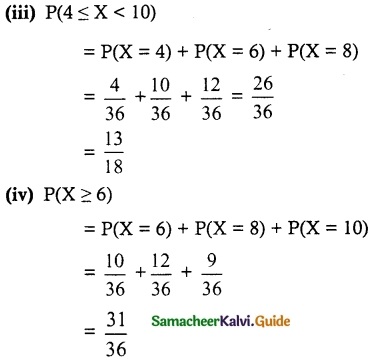
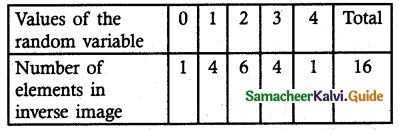
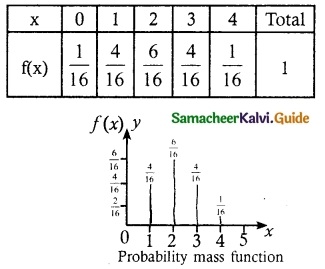
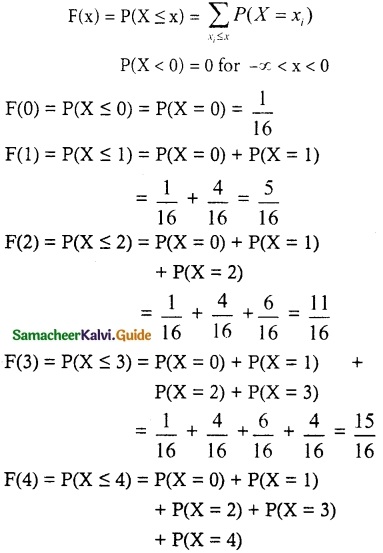
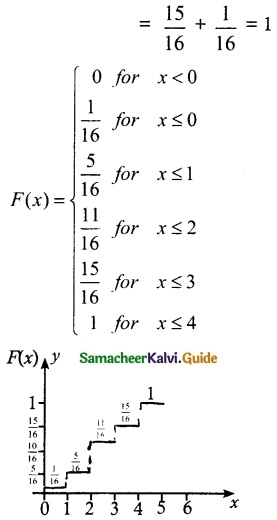

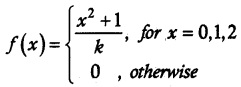
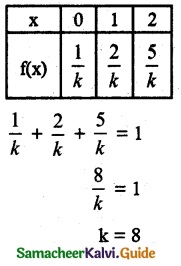
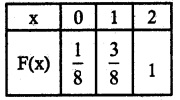

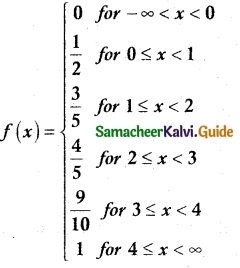
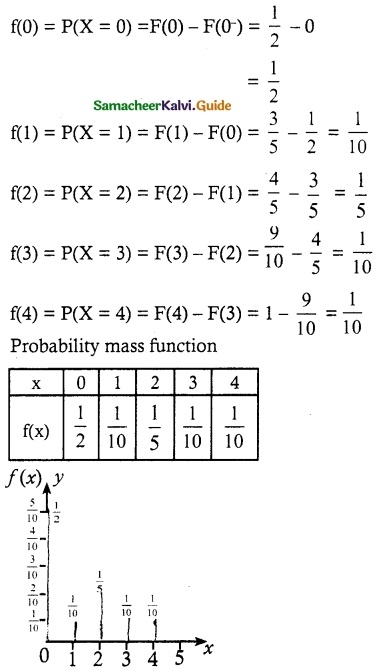




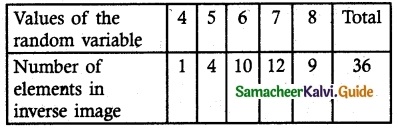
 .
.