TN State Board 12th Physics Important Questions Chapter 4 Electromagnetic Induction and Alternating Current
Question 1.
A wire which is in North-South direction is dropped freely, will any emf be induced across its ends?
Answer:
No, because both horizontal and vertical component of Earth is magnetic field are not intercepted by the falling wire.
Question 2.
Does the change in magnetic flux induce emf or current?
Answer:
emf is always induced.
Question 3.
During the change of magnetic flux when will current be induced?
Answer:
Current will be induced only when the circuit is complete.
![]()
Question 4.
A train is moving with uniform speed from north to south. Across which the ends of its axle, will the emf appear?
Answer:
Yes, emf will be appear as the train is { intercepting vertical component of Earth’s magnetic field.
Question 5.
What is meant by induced emf and induced current?
Answer:
Whenever there is a change in the magnetic flux linked with a closed circuit an emf is produced. This emf is known as the induced emf and the current that flows in the closed circuit is called induced current.
Question 6.
State Faraday’s laws of electromagnetic induction.
Answer:
Faraday’s laws of electromagnetic induction are:
First law:
Whenever the amount of magnetic flux linked with a closed circuits changes, an emf is induced in the circuit. The induced emf lasts so long as the change in magnetic flux continues.
Second law:
The magnitude of emf induced in a closed circuits is directly proportional to the rate of change of magnetic flux linked with the circuit.
![]()
Question 7.
Define self inductance. Give its unit.
Answer:
The coefficient of self induction of a coil or self-inductance of a coil is numerically equal to the opposing em/induced in the coil when the rate of change of current through the coil is unity. The unit of self inductance is henry (H).
Question 8.
What are the applications of eddy currents?
Answer:
Eddy currents are used in
- Induction stove
- Eddy current brake
- Eddy current testing
- Electromagnetic damping.
Question 9.
Define the unit of seif-inductance.
Answer:
One henry is defined as the self-inductance of a coil in which a change in current of one ampere per second produces an opposing emf of one volt.
Question 10.
Define mutual inductance in terms of magnetic flux.
Answer:
Mutual inductance is defined as the flux linkage of the second coil when a current of 1 A flows through the first coil.
![]()
Question 11.
What is the unit of mutual inductance?
Answer:
The unit of mutual inductance is henry. Mutual inductance between two coils is defined as one herny if a current of 1A in one coil produces unit flux linkage in second coil.
Question 12.
Define henry in terms of change of current.
Answer:
Mutual inductance between two coils is one henry if a current changing at the rate of 1 As-1 in the first coil induces an opposing emf of 1 V in another coil.
Question 13.
How are eddy currents used in the application of brake to electrical trains?
Answer:
A metallic drum is coupled to the wheels of a train. The drum rotates along with the wheel when the train is in motion. When the brake is applied, a strong magnetic field is developed f and hence, eddy currents are produced in the drum which oppose the motion of the drum. Hence, the train comes to rest.
![]()
Question 14.
Differentiate between self-inductance and mutual inductance.
Answer:
| Self inductance | Mutual inductance |
| Self inductance is numerically equal to the opposing emf induced in the coil when the rate of change of current through the coil is unity. | Mutual inductance is numerically equal to the emf induced in one coil, when the rate of change of current through the other coil is unity. |
| When a current of 1 ampere flows through coil in one second, an emf of e is induced. The self inductance is given by L = – e. | When a current of 1 ampere flows through a primary coil in one second and emf induced in the secondary coil is es then the mutual inductance is given bv M = – es. |
Question 15.
Explain the operation of motor used in fans.
Answer:
Eddy currents are produced in a metallic cylinder called rotor, when it is placed in a rotating magnetic field. The eddy current initially tries to decrease the relative motion between the cylinder and the rotating magnetic field. As the magnetic field continues to rotate, the metallic cylinder is set into a rotation. These motors are used in fans.
Question 16.
Mention the advantage of three phase alternation.
Answer:
- For a given dimension of the generator, three-phase machine produces higher power output than a single-phase machine.
- For the same capacity, three-phase alternator is smaller in size when compared to single phase alternator.
- Three-phase transmission system is cheaper. A relatively thinner wire is sufficient for transmission of three phase power.
![]()
Question 17.
In a step-up transformer, the primary current is more than the secondary current. Explain why?
Answer:
In any transformer the power in the primary = power in the secondary.
i.e. V1I1 = V2I2
In the step-up transformer the primary voltage is less than the secondary voltage. To maintain the power as constant the primary current in a step-up transformer is more than the secondary current.
Question 18.
Can a transformer be used for stepping up a DC? Support your answer with explanation.
Answer:
The transformer cannot be used for stepping up a DC. When the primary of the transformer is connected to DC there will be a momentary current in the secondary coil as the current in the primary builds up to its final value. Afterwards the primary current will be constant and there is no change of magnetic flux. Thus there will be no current in the secondary of the transformer.
Question 19.
What are the energy losses in a transformer?
Answer:
The energy losses in a transformer are
- Copper losses,
- Eddy current losses,
- Hysteresis losses and
- Flux leakage losses.
Question 20.
What is known as grid?
Answer:
The main transmission lines from power station to part of a common system called the grid. A large region of the country power from all the power stations in a particular region is fed into the grid. It forms a common pool from which power can be drawn where needed.
![]()
Question 21.
What are pylons?
Answer:
The cables used for transmitting power over long distances are suspended by the large porcelain insulators form large steel structures called pylons.
Question 22.
What do you mean by mutual Induction?
Answer:
The phenomenon of producing an induced emf in a coil due to the change in current in the other coil is known as mutual induction.
Question 23.
State the factors on which, the coefficient of mutual induction depends.
Answer:
The coefficient of mutual induction between a pair of coils depends on the following factors:
(i) Size and shape of the coils, number of turns and permeability of material on which the coils are wound.
(ii) Proximity of the coils.
Question 24.
What is A.C. generator?
Answer:
The ac generator is a device used for converting mechanical energy into electrical energy.
![]()
Question 25.
State and explain the principle of A.C generator.
Answer:
A.C. generator is based on the principle of electromagnetic inducion, according to which an emf is induced in a coil when it is rotated in a uniform magnetic field.
Question 26.
Mention the difference between a step up and step down transformer.
Answer:
| Step up transformer | Step down transformer |
| The current in the primary coil is more than that in the secondary coil. | The current in the primary coil is less than that in the secondary coil. |
| The number of turns in the primary coil is less than that in the secondary coil. | The number of turns in the primary coil is more than that in the secondary coil. |
| It is used to convert a low A.C. voltage into a high A.C. voltage. | It is used to convert a high A.C. voltage into a low A.C. voltage. |
Question 27.
What is a poly phase AC generator?
Answer:
If a number of armature windings are used in the alternator it is known as polyphase alternator. It produces voltage waves equal to the number of windings or phases. Thus a polyphase system consists of a numerous windings which are placed on the same axis but displaced from one another by equal angle which depends on the number of phases.
Question 28.
Why a DC ammeter cannot read AC?
Answer:
For direct current XL = 0 and XC = ∞. Alternating current varies in magnitude and direction periodically. But direct current is a unidirectional current and does not vary periodically. Hence a DC ammeter cannot read AC.
![]()
Question 29.
What is meant by RMS value of AC?
Answer:
The Root Mean Square value of an alternating current is defined as the square root of the mean squares of all currents over one cycle.
Question 30.
What is phasor diagram?
Answer:
The diagram that indicates various phasors and their phase relations is called phasor diagram.
Question 31.
Define alternating current and give its expression.
Answer:
Alternating current is defined as an electric current which is induced when a coil is rotated in a uniform magnetic field. Since the induced current varies in magnitude and direction periodically, it is called alternating current.
Alternating current is given by I = I0 sin ωt
Irms = \(\frac{\mathrm{I}_{0}}{\sqrt{2}}\)
![]()
Question 32.
What is capacitive reactance?
Answer:
Capacitive reactance is given by XC = \(\frac{1}{\mathrm{C}_{\omega}}\) = \(\frac{1}{2 \pi v C}\)
where v is the frequency of the AC supply.
For DC v = 0; XC = ∞
Thus a pure capacitor offers infinite resistance to DC. But in an AC circuit, the reactance of the coil decreases with increase in frequency.
Question 33.
What is inductive reactance?
Answer:
Inductive reactance is given by,
XL = ωL = 2πυL, where υ is the frequency of the AC supply.
For DC υ = 0;
∴ XL = 0
Thus a pure inductor offers zero resistance to DC. But in an AC circuit the reactance of the coil increases with increase in frequency.
Question 34.
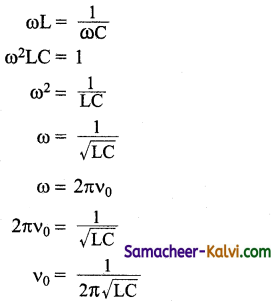
What is resonant frequency in LCR circuit?
Answer:
The particular frequency υo at which the impedance of the circuit becomes minimum and therefore the current becomes maximum is called resonant frequency of the circuit.
![]()
Question 35.
Define power factor.
Answer:
The average power dissipated in an AC circuit depends not only on the voltage and . current but also on the cosine of the phase angle Φ between them.
Pav = I2rms Z cos (Φ)
where Irms – RMS value of current
Z – impedance; cos Φ – power factor.
Hence, power factor is defined as the ratio of average power (Pav) dissipated in an AC circuit to the square of rms value of current (I2rms).
Question 36.
What happens to the value of current in RLC series circuit, if frequency of the source is increased?
Answer:
When the frequency of the source is increased, the value of current in RLC frequency, the current will be maximum since the impedance of the circuit is nearly zero.
Question 37.
Define efficiency of a transformer.
Answer:
Efficiency of a transformer is defined as the ratio of output power to the input power, output power
input power
η = \(\frac{\text { output power }}{\text { input power }}\)
= \(\frac{\mathrm{E}_{s} \mathrm{I}_{s}}{\mathrm{E}_{p} \mathrm{I}_{p}}\)
![]()
Question 38.
What is efficiency of an ideal transformer? Why?
Answer:
The efficiency of an ideal transformer is 1. i.e., 100% where there is no power loss. Because, when there is no power loss, the output power is equal to input power.
Question 39.
Why the efficiency of transformer is less than one?
Answer:
Practically there are numerous factors leading to energy loss in a transformer and hence the efficiency is always less than one.
Question 40.
What is meant by alternating emf?
Answer:
A rotating coil in a magnetic field, induces an alternating emf and hence an alternating current. Since, the emf induced in the coil varies in magnitude and direction periodically, it is called an alternating emf.
Question 41.
What is the significance of alternating emf?
Answer:
The significance of an alternating emf is that it can be changed to lower or higher voltages conveniently and efficiently using a transformer. Also the frequency of the induced emf can be altered by changing the speed of the coil. This enables us to utilise the whole range of electromagnetic spectrum for one purpose or the other.
![]()
Question 42.
How alternating current can be measured? Give reason.
Answer:
Since alternating current varies continously with time, its average value over one complete cycle is zero. Hence its effect is measured by rms value of AC.
Question 43.
What is acceptor circuit?
Answer:
The series resonant circuit is often called an ‘acceptor’ circuit. By offering minimum impedance to current at the resonant frequency it is able to select or accept most readily this particular frequency among many frequencies.
In radio receivers the resonant. frequency of the circuit is tuned on the frequency of the signal desired to be detected. This is usually done by varying the capacitance of a capacitor.
Question 44.
What is alternating current?
Answer:
When the electric current produced by a generator changes its direction of flow continuously and periodically in a circuit several times in a second, current is known as an alternating current.
Question 45.
Can a transformer be used for stepping up a DC? Support your answer with explanation.
Answer:
The transformer cannot be used for stepping up a DC. When the primary of the transformer is connected to DC there will be a momentary current in the secondary coil as the current in the primary builds up to its final value. Afterwards the primary current will be constant and there is no change of magnetic flux. Thus there will be no current in the secondary of the transformer.
![]()
Question 46.
What is inductive reactance?
Answer:
Inductive reactance plays the same role as the resistance. Hence, the inductance impedes
the flow of current in the circuit. It is given by XL = Lω = 2 π υL.
Question 47.
What is capacitive reactance?
Answer:
The capacitive reactance is the opposition offered by a capacitor to the flow of current through it. It is given by
XC = \(\frac{1}{\mathrm{C}_{\omega}}=\frac{1}{2 \pi v \mathrm{C}}\) .
Question 48.
What is resonance in LCR circuit?
Answer:
The LCR circuit is said to be in resonance when its inductance reactance is equal to its capacitive reactance i.e., XL = XC. The current in the circuit is maximum. The voltage and current are in phase when LCR circuit is in resonance.
Question 49.
What is the application of resonance in radio sets?
Answer:
In radio sets, the antenna circuit contains LCR circuit. Its capacitance is varied until the resonant frequency of the LCR circuit is the same as the carrier frequency of any particular radio station. At this state, the current in the LCR circuit is maximum and the receiver responds to the incoming signal.
![]()
Question 50.
What is meant by ‘wattful’ current?
Answer:
The component of current Irms cos (Φ) which is in phase with the voltage is called active component. The power consumed by this current = Vrms Irms cos (Φ). So that it is also known as ‘Wattfui’ current.
Question 51.
Mention various formulae for power factor.
Answer:
(i) Power factor = cos Φ = cosine of the angle of lead or lag.
(ii) Power factor = \(\frac{\mathrm{R}}{\mathrm{Z}}=\frac{\text { Resistance }}{\text { Impedance }}\)
(iii) Power factor = \(\frac{\mathrm{VI} \cos \phi}{\mathrm{VI}}=\frac{\text { True power }}{\text { Apparent power }}\)
Question 52.
What are the examples of power factors?
Answer:
Some examples for power factors:
(i) Power factor = cos 0° = 1 for a pure resistive circuit because the phase angle <|) between voltage and current is zero. (ii) Power factor = cos (± \(\frac{\pi}{2}\)) = 0 for a purely inductive or capacitive circuit because the phase angle c|> between voltage and current is ± \(\frac{\pi}{2}\)
(iii) Power factor lies between 0 and 1 for a circuit having R, L and C in varying proportions.
Question 53.
State the advantages of AC over DC.
Answer:
(i) The generation of AC is cheaper than that of DC.
(ii) When AC is supplied at higher voltages, the transmission losses are small compared to DC transmission.
(iii) AC can easily be converted into DC with the help of rectifiers.
![]()
Question 54.
What are the disadvantages of AC over DC?
Answer:
(i) Alternating voltages cannot be used for certain applications Eg: charging of batteries, electroplating, electric traction etc.
(ii) At high voltages, it is more dangerous to work with AC than DC.
Question 55.
Draw a diagram to illustrate the construction of a transformer.
Answer:
refer figure P. No: 206 – Q. No: 18
Question 56.
What are copper losses? How are they minimised?
Answer:
Transformer windings have electrical resistance. When an electric current flows through them, some amount of energy is dissipated due to Joule heating. This energy loss is called copper loss that is minimized by using wires of larger diameter.
Question 57.
What is meant by alternating voltage?
Answer:
An alternating voltage is the voltage which changes polarity at regular intervals of time and the direction of the resulting alternating current also changes accordingly.
![]()
Question 58.
Represent squared wave form of alternating current diagramatically.
Answer:
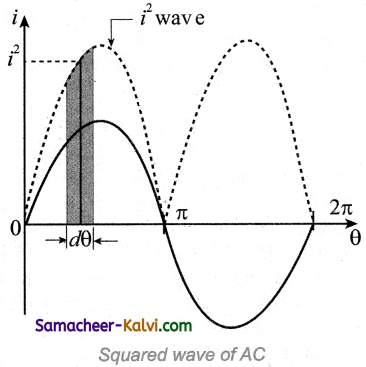
Question 59.
What is meant by sinusoidal alternating voltage?
Answer:
If the waveform of alternating voltage is a sine wave, then it is known as sinusoidal alternating voltage which is given by the relation.
υ = Vm sin ωt.
Question 60.
Draw curves explaining the variations em/in a three phase generator.
Answer:
refer figure P. No: 205 – Q. No: 17 – (ii)
Question 61.
What is a transformer?
Answer:
Transformer is a stationary device used to transform electrical power from one circuit to another without changing its frequency. The applied alternating voltage is either increased or decreased with corresponding decrease or increase of current in the circuit.
![]()
Question 62.
Draw the phasor and wave diagrams for AC circuit with R.
Answer:
refer figure P. No: 182 – (ii)
Question 63.
Draw the phasor and wave diagrams for A.C. current with L.
Answer:
refer figure P. No: 182 – (iii)
Question 64.
Draw the phasor and wave diagrams for A.C. circuit with C.
Answer:
refer figure P. No: 182 – (iv)
Question 65.
Draw a diagram to indicate the variation of (i) electrical energy and (ii) magnetic energy as a function of time.
Answer:
refer figure P. No: 188
![]()
Question 66.
As soon as current is switched on in a high voltage wire, the bird sitting on the wire flies away: Give reason.
Answer:
When current is switched on, current is induced in the body of the bird. Its wings experience mutual repulsion, due to the flow of opposite currents in them. So the bird flies away.
Question 67.
Draw the phasor diagram its represent the variation of current with voltage.
Answer:
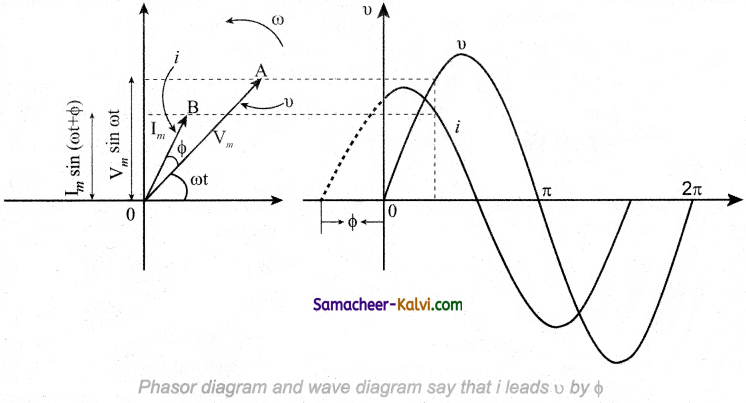
Question 68.
Prove that whenever the electric current changes in one circuit changes then the galvanometer shows a deflection using an experiment.
Answer:
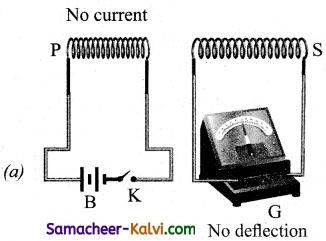
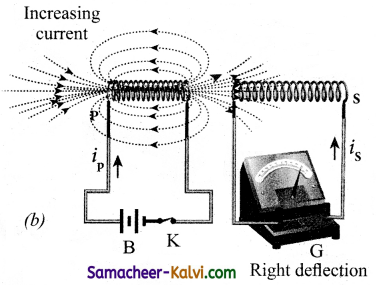
Consider two closed circuits. The circuit consisting of a coil P, a battery B and a key K is called as primary circuit while the circuit with a coil S and a galvanometer G is known as secondary circuit. The coils P and S are kept at rest in close proximity with respect to one another. If the primary circuit is closed, then electric current starts flowing in the primary circuit. Due to this, the galvanometer gives a momentary deflection.
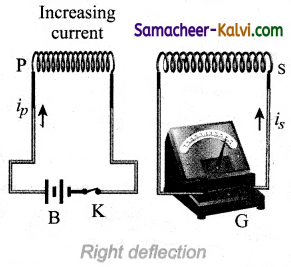
After that, when the electric current reaches a certain steady value, no deflection is observed in the galvanometer. Likewise if the primary circuit is broken, the electric current starts decreasing and there is again a sudden deflection but in the opposite direction.
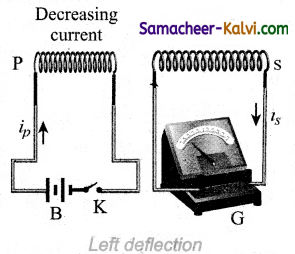
When the electric current becomes zero, the galvanometer shows no deflection. From the above observations, it is concluded that whenever the electric current in the primary circuit changes, the galvanometer shows a deflection.
![]()
Question 69.
Discuss the drawbacks of eddy currrents.
Answer:
When eddy currents flow in the conductor, a large amount of energy is dissipated in the form of heat. The energy loss due to the flow of eddy current is inevitable but it can be reduced to a greater extent with suitable measures. The design of transformer core and electric motor armature is crucial in order to minimise the eddy current loss.
To reduce these losses, the core of the transformer is made up of thin laminas insulated from one another in while for electric motor the winding is made up of a group of wires insulated from one another. The insulation used does not allow huge eddy currents to flow and hence losses are minimized.
![]()
Question 70.
Explain Faraday’s experiments that lead to Faraday’s laws of electromagnetic induction.
Answer:
In the first experiment, when a bar magnet is placed close to a coil, some of the magnetic lines of force of the bar magnet pass through the coil. It infers that the magnetic flux is linked with the coil. When the bar magnet and the coil approach each other, the magnetic flux linked with the coil increases. So this increase in magnetic flux induces an emf
Hence a transient electric current flows in the circuit in one direction.
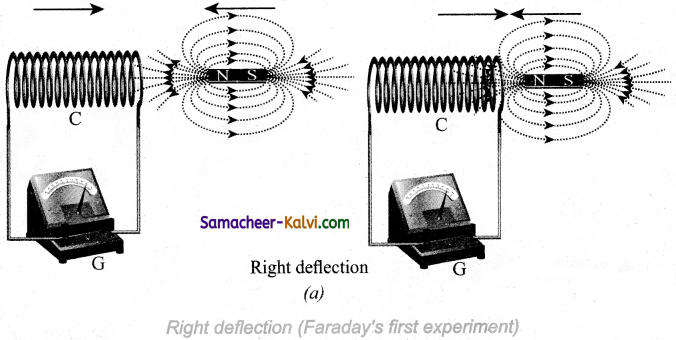
At the same time, when they recede away from one another, the magnetic flux linked with the coil decreases. The decrease in magnetic flux again induces an emf in opposite direction so an electric current flows in opposite direction.
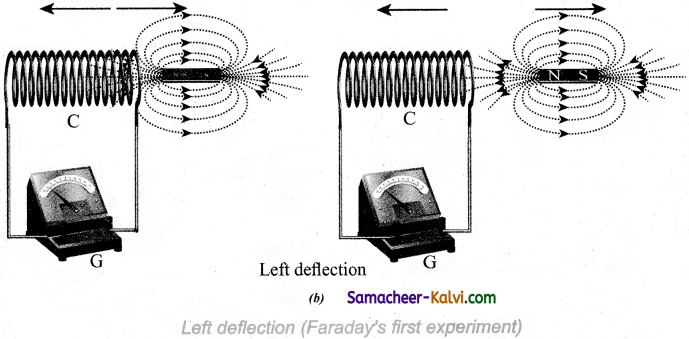
Hence there is deflection in the galvanometer when there is a relative motion between the coil and the magnet. In the second experiment, when the primary coil P carries an electric current, a magnetic field is established around it. The magnetic lines of this field pass through itself and the neighbouring secondary coil S.
When the primary circuit is open, no electric current flows in it. Hence the magnetic flux linked with the secondary coil is zero.
However, when the primary circuit is closed, the increasing current builds up a magnetic field around the primary coil. Hence, the magnetic flux linked with the secondary coil increases. This increasing flux linked induces a transient electric current in the secondary coil.
When the electric current in the primary coil reaches a steady value, the magnetic flux linked with the secondary coil does not change and the electric current in the secondary coil will disappear.
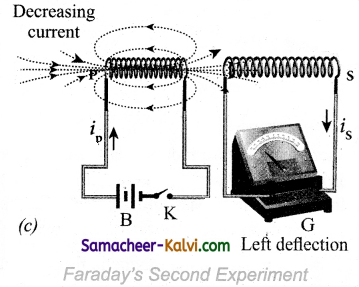
Similarly, when the primary circuit is broken, the decreasing primary current induces an electric current in the secondary coil, but in the opposite direction So there is deflection in the galvanometer whenever there is a change in the primary current.
![]()
Question 71.
Explain a simple demonstration for the production of eddy current.
Answer:
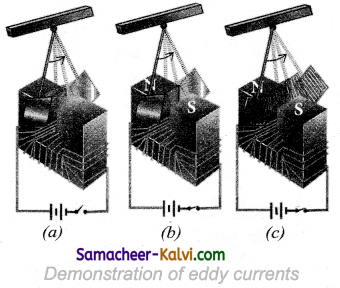
Let us consider a pendulum that can be made to oscillate between the poles of a powerful electromagnet (fig (a)).
First the electromagnet is switched off, the pendulum is slightly displaced and released. It begins to oscillate and it executes a large number of oscillations before stopping. The air friction is the only damping force. When the electromagnet is switched on and the disc of the pendulum is made to oscillate, eddy currents are produced in it which will oppose the oscillation.
A heavy damping force of eddy currents will bring the pendulum to rest within a few oscillations (figure (b)). However if some slots are cut in the disc as shown in the (figure (c) eddy currents are reduced. The pendulum now will execute several oscillations before coming to rest. This clearly demonstrates the production of eddy current in the disc of the pendulum.
Question 72.
Drive expression for mutual inductance between two long co-axial solenoids.
Answer:
Consider two long co-axial solenoids of same length l. The length of these solenoids is large when compared to their radii so that the magnetic field produced inside the solenoids is uniform and the fringing effect at the ends may be ignored. Let A1 and A2 be the area of cross section of the solenoids with A1 being greater A2.
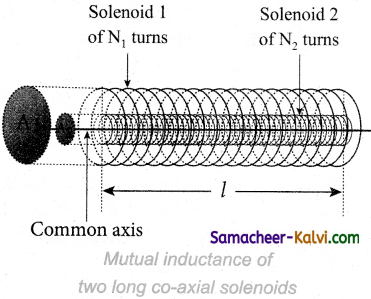
The turn density of these solenoids are n1 and n2 respectively.
Let i1 be the current flowing through solenoid 1, then the magnetic field produced inside it is B1 = µ0 n1 i1
As the field lines of \(\vec{B}\)1 are passing through the area bounded by solenoid 2, the magnetic flux is linked with each turn of solenoid 2 due to solenoid 1 and is given by
\(\Phi_{21}=\int_{A_{2}} \overrightarrow{\mathrm{B}}_{1} \cdot d \overrightarrow{\mathrm{A}}\)= B1 A2 since θ = 0° = (µ0 n1 i1)A2
The flux linkage of solenoid 2 with total turns N2 is
N2 Φ21 = (n2 l) (µ0 n1 i1) A2 since N2 = n2 l
N2 Φ21 = (µ0 n1 n2 A2 l) i1 ………..(1)
From equation.
N2 Φ21 = M21 i1 ………….(2)
Comparing the equation (1) and (2)
M21 = µ0 n1 n2 A2 l ……………..(3)
This is the expression for mutual inductance M21 of the solenoid 2 with respect to solenoid 1. In the same way, we can find mutual inductance M12 of solenoid 1 with respect to solenoid 2 as given below.
The magnetic field produced by the solenoid 2 when carrying a current i2 is
B2 = µ0n2i2
This magnetic field B2 is uniform inside the solenoid 2 but outside the solenoid 2, it is almost zero. Therefore for solenoid 1, the area A2 is the effective area over which the magnetic field B2 is present; not area A1, Then the magnetic flux Φ12 linked with each turn of solenoid 1 due to solenoid 2 is
\(\Phi_{12}=\int_{\mathrm{A}_{2}} \overrightarrow{\mathrm{B}}_{2} \cdot d \overrightarrow{\mathrm{A}}=\mathrm{B}_{2} \mathrm{~A}_{2}=\left(\mu_{0} n_{2} i_{2}\right) \mathrm{A}_{2}\)The flux linkage of solenoid 1 with total turns N1 is
N1 Φ12 = (n1 l) (µ0 n2 i2) A2 since N1 = n1 l
N1 Φ12 = (µ0 n1 n2 A2 l) i2 since N1 Φ12 = M12 i2
M12 i2 = (µ0 n1 n2 A2 l) i2
Therefore, we get
∴ M12 = µ0 n1 n2 A2 l …………(4)
From equations (3) and (4), we can write
M12 = M21 = M ………..(5)
In general, the mutual inductance between two long co-axial solenoids is given by
M = µ0 n1 n2 A2 l …….(6)
If a dielectric medium of relative permeability µr is present inside the solenoids, then
M = µ n1 n2 A2 l
(or) M = µ0 µr n1 n2 A2 l
![]()
Question 73.
Explain the applications of series RLC resonant circuit.
Answer:
RLC circuits have many applications like filter circuits, oscillators, voltage multipliers etc.. An important use of series RLC resonant circuits is in the tuning circuits of radio and TV systems. The signals from many broadcasting stations at different frequencies are available in the air.
To receive the signal of a particular station, tuning is done. The tuning is commonly achieved by varying capacitance of a parallel plate variable capacitor, thereby changing the resonant frequency of the circuit. When resonant frequency is nearly equal to the frequency of the signal of the particular station, the amplitude of the current in the circuit is maximum. Thus the signal of that station alone is received.
Question 74.
Prove that in a pure resistive circuit the current is in phase with the applied voltage.
Answer:
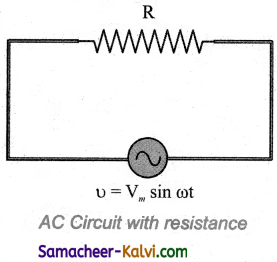
refer figure P. No: 182 – (ii)
Consider a circuit containing a pure resistor of resistance R connected across an alternating voltage source. The instantaneous value of the alternating voltage is given by
υ = Vm sin ωt ………..(1)
An alternating current i flowing in the circuit due to this voltage, develops a potential drop across R and is given by
VR = iR …………(2)
Kirchhoff ’s loop rule states that the algebraic sum of potential differences in a closed circuit is zero. For this resistive circuit,
υ – VR = 0
From equation (1) and (2),
Vm sin ωt = iR
i = \(\frac{\mathrm{V}_{m}}{\mathrm{R}}\) sin ωt
i = Im sin ωt ……………….(3)
where \(\frac{\mathrm{V}_{m}}{\mathrm{R}}\), the peak value of alternating current in the circuit. From equations (1) and (3), it is clear that the applied voltage and the current are in phase with each other in a resistive circuit. It means that they reach their maxima and minima simultaneously. This is indicated in the phasor diagram. The wave diagram also depicts that current is in phase with the applied voltage.
![]()
Question 75.
Determine the phase relationship between voltage and current in a pure capacitive circuit.
Answer:
Let us consider a circuit containing a capacitor of capacitance C connected across an alternating voltage source. The alternating voltage is given by
υ = Vm sin ωt ………….(1)
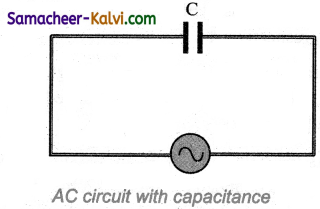
Let Question be the instantaneous charge on the capacitor. The emf across the capacitor at that instant is \(\frac{q}{\mathrm{C}}\). According to Kirchoff’s loop rule,
υ = \(\frac{q}{\mathrm{C}}\) = 0
Question = CVm sin ωt
By the definition of current,
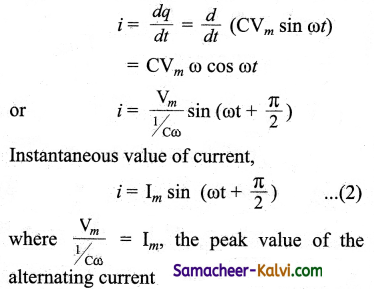
From equation (1) and (2)
It is clear that current leads the applied voltage by \(\frac{\pi}{2}\) in a capacitive circuit. This is shown pictorially in figure. The wave diagram for a capacitive circuit also shows that the current leads the applied voltage by 90°.
Capacitive reactance XC
The peak value of current Im is given by Im = \(\frac{\mathrm{V}_{m}}{1 / \mathrm{C\omega}}\).
Let us compare this equation Im = \(\frac{V_{m}}{R}\) with from resistive circuit. The quantity
\(\frac{1}{\mathrm{C} \omega}\) plays the same role as the resistance R in resistive circuit. This is the resistance offered by the capacitor, called capacitive reactance (XC). It measured in ohm.
XC = \(\frac{1}{\mathrm{C} \omega}\) …….(3)
Its unit is ohm.
The capacitive reactance (XC) varies inversely as the frequency. For a steady current, f = 0.
XC = \(\frac{1}{\omega C}=\frac{1}{2 \pi f C}=\frac{1}{0}=\infty\)
Thus a capacitive circuit offers infinite resistance to the steady current. So that steady current cannot flow through the capacitor.
![]()
Question 76.
Discuss the effects of series resonance.
Answer:
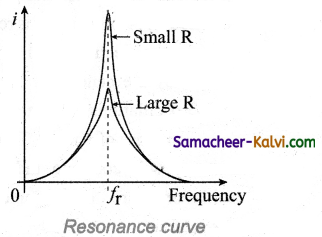
When series resonance occurs, the impedance of the circuit is minimum and is equal to the resistance of the circuit. As a result of this, the current in the circuit becomes maximum. This is shown in the -resonance curve drawn between current and frequency.
At resonance, the impedence is
Z = \(\sqrt{\mathrm{R}^{2}+\left(\mathrm{X}_{\mathrm{L}}-\mathrm{X}_{\mathrm{C}}\right)^{2}}\) = R
since XL = XC
Therefore, the current in the circuit is
Im = \(\frac{V_{m}}{\sqrt{R^{2}+\left(X_{L}-X_{C}\right)^{2}}}\)
Im = \(\frac{\mathrm{V}_{m}}{\mathrm{R}}\)
The maximum current at series resonance is limited by the resistance of the circuit. For smaller resistance, larger current with sharper curve is obtained and vice versa.
Question 77.
Derive an expression for angular frequency of LC oscillations.
Answer:
Angular frequency of LC oscillations
By differentiating equation
q(t) = Qm cos(ωt + Φ) …………(1)
twice, we get
\(\frac{d^{2} q}{d t}\) = – Qm ω2 cos(ωt + Φ) ………….(2)
Substituting equations
(1) and (2) in equation,
L\(\frac{d^{2} q}{d t^{2}}\) + \(\frac{1}{\mathrm{C}}\) Question = 0
we obtain
L[-Qm ω2 cos(ωt + Φ)] + \(\frac{1}{\mathrm{C}}\) Qm
cos (ωt + Φ) = 0
Rearranging the terms, the angular frequency of LC oscillations is given by
ω = \(\frac{1}{\sqrt{\mathrm{LC}}}\) ………..(4)
This equation is the same as that obtained from qualitative analogy.
Question 78.
Discuss the oscillations of electrical and magnetic energy.
Answer:
The electrical energy of the LC oscillator is
UE = \(\frac{q^{2}}{2 \mathrm{C}}=\frac{\mathrm{Q}_{m}^{2}}{2 \mathrm{C}} \cos ^{2}(\omega t+\phi)\)
The magnetic energy is
UB = \(\frac{1}{2} L i^{2}=\frac{Q_{m}^{2}}{2 C} \sin ^{2}(\omega t+\phi)\)
If the two energies are plotted with an assumption of Φ = 0, we obtain
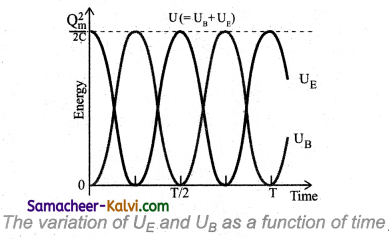
From the graph, it can be noted that
(i) At any instant UE + UB = \(\frac{\mathrm{Q}_{m}^{2}}{2 \mathrm{C}}\) = constant
(ii) The maximum values of UE and UB are both \(\frac{\mathrm{Q}_{m}^{2}}{2 \mathrm{C}}\)
(iii) When UE is Maximum, UB is zero and vice versa.
![]()
Question 79.
Explain the generation of LC oscillations.
Answer:
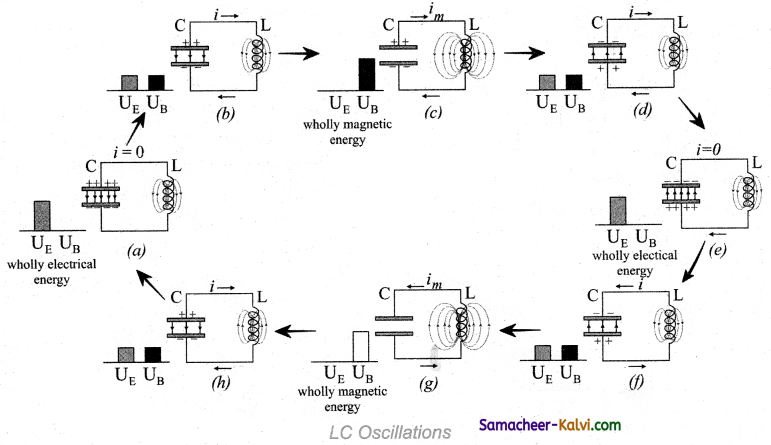
Let us assume that the capacitor is fully charged with maximum charge Qm at the initial stage. So that the energy stored in the capacitor is maximum and is given by
UE = \(\frac{\mathrm{Q}_{m}^{2}}{2 \mathrm{C}}\)
As there is no current in the inductor, the energy stored in it is zero i.e., UB = 0. Therefore, the total energy is wholly electrical (fig (a)).
The capacitor now begins to discharge through the inductor that establishes current i in clockwise direction. This current produces a magnetic field around the inductor and the energy stored in the inductor is given by
UB = \(\frac{\mathrm{L} i^{2}}{2}\). As the charge in the capacitor decreases, the energy stored in it also decreases and is given by UE = \(\frac{q^{2}}{2 C}\). Thus there is a transfer of some part of energy from the capacitor to the inductor. At that instant, the total energy is the sum of electrical and magnetic energies (figure (,b)).
When the charges in the capacitor are exhausted, its energy becomes zero i.e., UE = 0. The energy is fully transferred to the magnetic field of the inductor and its energy is maximum. This maximum energy is given
UB = \(\frac{\mathrm{LI}_{m}^{2}}{2}\)
where Im that is maximum current flowing in the circuit. The total energy is wholly magnetic (figure (c)).
Even though the charge in the capacitor is zero, the current will continue to flow in the same direction because the inductor will not allow it to stop immediately. The current is made to flow with decreasing magnitude by the collapsing magnetic field of the inductor. As a result of this, the capacitor begins to charge in the opposite direction. A part of the energy is transferred from the inductor back to the capacitor.
The total energy is the sum of the electrical and magnetic energies (figure (d)). When the current in the circuit reduces to zero, the capacitor becomes fully charged in the opposite direction. The energy stored in the capacitor becomes maximum. Since the current is zero, the energy stored in the inductor is zero. The total energy is wholly electrical (figure (e)).
The state of the circuit is similar to the initial state but the difference is that the capacitor is charged in opposite direction. The capacitor then starts to discharge through the inductor with anti-clockwise current. The total energy is the sum of the electrical and magnetic energies (figure (f)).
As already explained, the processes are repeated in opposite direction (figure (g) and (h)). Finally, the circuit returns to the initial state (figure (a)). Thus, when the circuit goes through these stages, an alternating current flows in the circuit. As this process is repeated again and again, the electrical oscillations of definite frequency are generated.
These are known as LC oscillations. In the ideal LC circuit, there is no loss of energy. Therefore, the oscillations will continue indefinitely. Such oscillations are called undamped oscillations.
Question 80.
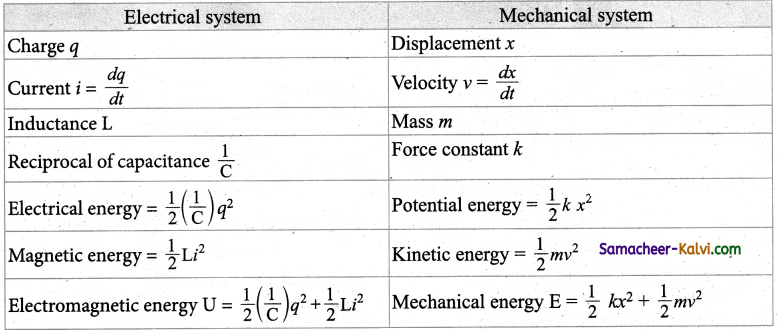
Compare the analogies between electrical and mechanical quantities.
Answer:
![]()
Question 81.
The number of turns of a long solenoid is 1000 turns. When a current of 4A flows through it the magnetic flux linked with each , turn of the solenoid ¡s 4 x iO Wb. Calculate the self inductánce of the solenoid.
Answer:
Number of turns N = 1000
Current I = 4A
Magnetic flux = 4 × 10-3
∴ self inductance of the soÌenoid L = \(\frac{\mathrm{N} \phi}{\mathrm{I}}\)
L = \(\frac{1000 \times 4 \times 10^{-3}}{4}\) = 1H
Question 82.
A coil of area of cross section 0.5 m2 with 10 turns is in a plane perpendicular to a uniform magnetic field of 0.2 Wb/m2. Calculate the flux through the coil.
Answer:
Area of a coil A = 0.5 M2
Number of turns N = 10
Magnetic field B= 0.2 Wb /m2
Magnetic flux (Φ) = ?
Φ = NBA cos θ = NBA
∵ θ = 0°
= 10 × 0.2 × 0.5 = 1 Wb
Question 83.
Air core solenoid having a diameter of 4 cm and length 60 cm is wound with 4000 turns. If a current of 5 A flows in the solenoid, calculate the energy stored in the solenoid.
Answer:
Diameter of a solenoid d = 4 × 10-2 m
Length of the solenoid / = 60 × 10-2 m
Number of turns N = 4000;
Current I = 5 A
Energy stored of a solenoid L = \(\frac{1}{2}\) LI02
Self inductance of a solenoid L = \(\frac{\mu_{0} \mathrm{~N}^{2} \mathrm{~A}}{l}\)
Self inductance of air solenoid L = \(\frac{\mu_{0} \mathrm{~N}^{2} \mathrm{~A}}{l}\)
Area of a solenoid = πr² = \(\frac{\pi d^{2}}{4}\)
Self inductance of the solenoid
L = \(\frac{4 \pi \times 10^{-7} \times(4000)^{2} \times 4 \pi \times 10^{-4}}{60 \times 10^{-2}}\)
= \(\frac{4 \times 3.14 \times 10^{-7} \times 16 \times 10^{6} \times 4 \times 3.14 \times 10^{-4}}{60 \times 10^{-2}}\)
= 42.067 × 10-7+6-4+2
= 42.067 × 10-3 henry
= 42.067 × 10-3
Energy stored E = \(\frac{1}{2}\) LI02
= \(\frac{1}{2}\) × 42.067 × 10-3 × (5)2
= 525.8375 × 10-3 J
= 0.5258375 J
Energy stored in the solenoid = 0.5258375 J
![]()
Question 84.
A magnetic field of induction 20 tesla acts at right angle to a coil of area 20 m2 with 50 turns. Find the flux linked to the coil.
Answer:
N = 50 turns;
B = 20 tesla;
A = 20 m2
Φ = NBA cos θ
= 50 × 20 × 20 × cos 0 = 20000.
Magnetic flux linked with the coil = 2 × 104 Wb.
Question 85.
When a current of 2 ampere is flowing through a coil of 1000 turns, produces a magnetic flux of 0.02 weber. Calculate the self inductance of the coil.
Answer:
I = 2 ampere N = 1000
Φ = 0.02 weber
Self inductance I = \(\frac{\mathrm{N}(\phi)}{\mathrm{I}}\)
= \(\frac{1000 \times 0.02}{2}\) = 10 henry
Self inductance = 10 henry
Question 86.
How much magnetic potential energy is stored in a 20 mH coil when it carries a current of 0.2 A?
Answer:
Magnetic potential energy stored up in the coil = \(\frac{1}{2}\) Li2
Magnetic potential energy = \(\frac{1}{2}\) × 20 × 10-3 × (0.2)2
= 4 × 10-4 joules.
![]()
Question 87.
A coil of resistance 200 Ω is placed in a magnetic field if the magnetic flux Φ linked with the coil varies with time t (sec) is Φ = 50t2 + 4. When t = 2s, calculate the current in the coil.
Answer:
Magnetic flux Φ = 50t2 + 4
Induced emf e = \(\left|-\frac{d \phi}{d t}\right|\)
= \(\frac{d \phi}{d t}\)
= \(\frac{d}{d t}\) (50t2 + 4)
e = 100t
when t = 2s, e = 100 × 2 = 200 V
Resistance of the coil = 200 Ω
∴ Current in the coil = \(\frac{e}{\mathrm{R}}=\frac{200}{200}\)
I = 1 A
Question 88.
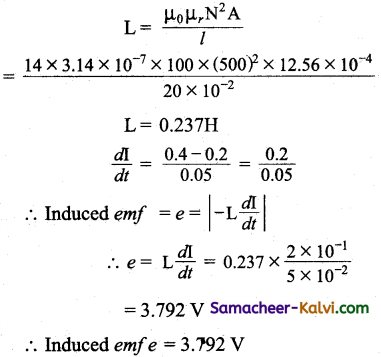
A 20 cm long solenoid having 500 turns and radius 2 cm is wound over an iron core of relative permeability 100. If the current through it changes from 0.2A to 0.4 A in 0.05 second then calculate the average emf in the solenoid.
Answer:
Length of the solenoid l = 20 cm = 20 × 10-2 m
Radius of the solenoid r = 2 cm = 2 × 10-2 m
Area of the solenoid A = πr²
A = 3.14 × (2 × 10-2)2
= 3.14 × 4 × 10-4
= 12.56 × 10-2 m2
Relative permeability µr = 100
Permeability in free space µ0 = 4π × 10-7
Number of turns of the solenoid = 500 turns
Self inductance of the solenoid
![]()
Question 89.
A rectangular coil of 2000 turns of wire has dimensions of 0.2 m × 0.1 m. It rotates at the rate of 4200 rotations per minute with its plane perpendicular to a magnetic field of strength 0.2 Wbm-2. Calculate
(i) maximum value of the emf induced in the coil,
(ii) instantaneous value of the induced emf when the coil has rotated through an angle of 60° from its initial position.
Answer:
Number of turns of the coil N = 2000
Area of the coil A = 0.2 × 0.1 m2
Speed of rotation ω = 4200 rotations per minute
= \(\frac{4200 \times 2 \pi}{60}\) rad s-1
Field strength B = 0.2 Wbm-2
Induced emf e = nAB ω . sin ωt
(a) E is maximum, when sin ωt = 1
Emax = NAB ω
= 2000 × 0.2 × 0.1 × 0.2 × \(\frac{4200 \times 2 \pi}{60}\) = 3517 V
(b) When ω t = 60°
Emax = N AB ω sin ω t
= 3517 × sin 60°
= 3517 × \(\frac{\sqrt{3}}{2}\) = 3045 V
Answer:
(a) Maximum value of em/induced in the coil = 3517 V.
(b) Instantaneous value of induced emf = 3045 V.
Question 90.
A Conducting circular loop having radius of 2 cm is placed in a magnetic field of 0.05 T with its plane perpendicular to the field. If the radius of the loop is made to shrink at a constant rate of 1 mm/s then calculate the induced emf in the circular loop.
Answer:
Magnetic field B = 0.05 T
Radius of the coil dr = 2 × 10-2 m
Change of Time dt = 1 × 10-3 s
∴ Magnetic flux (Φ) = BA = B π r2
∴ Induced emf = \(\left|-\frac{d \phi}{d t}\right|\)
= \(\frac{d \phi}{d t}=\frac{d}{d t}\left(\mathrm{~B} . \pi r^{2}\right)\)
∴ e = 2πBr\(\frac{d r}{d t}\)
= 2 × 3.14 × 0.05 × \(\frac{2 \times 10^{-2}}{1 \times 10^{-3}}\)
= 6.28 × 0.10 × 10-2+3
= 0.628 × 101 = 6.28 V
∴ Induced emf e = 6.28 V
![]()
Question 91.
Calculate the mutual inductance between two coils when a current of 4 A changing to 8 A in 0.5 s in one coil, induces an emf of 50 m V in the other coil.
Answer:
I1 = 4A; I2 = 8A; dt = 0.5s;
e = 50 mV = 50 × 10-3 V, M = ?
e = – M \(\frac{d \mathrm{I}}{d t}\)
∴ Mutual inductance = \(-\frac{e}{\frac{d I}{d t}}\)
= \(\frac{e}{\left(\frac{I_{2}-I_{1}}{d t}\right)}=-\frac{50 \times 10^{-3}}{\left(\frac{8-4}{0.5}\right)}\)
= – 6.25 × 10-3
∴ M = 6.25 mH
Mutual inductance between two coils is 6.25 mH
Question 92.
An aircraft having a wingspan of 20.48 m flies due north at a speed of 40 ms-1. If the vertical component of earth’s magnetic field at the place is 2 × 10-5 T. Calculate the emf induced between the ends of the wings.
Answer:
l = 20.48 m; v = 40 ms-1;
B = 2 × 10-5 T; e = ?
e = -B Iv
= -2 × 10-5 × 20.48 × 40
e = – 0.0164 volts
Question 93.
A helicopter rises vertically with a speed of 10 m/s. If the helicopter be a horizontal linear conductor of length 20 m and if the horizontal components of the earth’s magnetic induction be 2 × 10-3 Wb/m2, calculate the potential difference between the tips of the nose and the tail of the helicopter.
Answer:
When the helicopter cuts the magnetic lines of induction, the induced emf
e = Blv
Here B = 2 × 10-3 Wb/m2;
l = 20m; V = 10 m/s
∴ The induced emf (the potential difference between the nose and tail of the helicopter
e = 2 × 10-3 × 20 × 10 = 0.4 V
![]()
Question 94.
The co-efficient of mutual inductance is 5 H between the primary and secondary of a coil. A current of 10 A is cut off in 5 sec. Calculate the induced’emf.
Answer:
The induced emf e = – M \(\frac{d \mathrm{I}}{d t}\)
= – 5 × \(\frac{10}{0.5}\) = – 100 V.
The negative sign indicates that the induced emf opposes the cause.
Question 95.
When a current of 2 ampere is flowing through a coil of 1000 turns, produces a magnetic flux of 0.02 weber. Calculate the self inductance of the coil.
Answer:
I = 2 ampere
n = 1000 Φ = 0.02 weber
Self inductance = \(\frac{\mathrm{N}(\phi)}{\mathrm{I}}\)
= \(\frac{1000 \times 0.02}{2}\)
Self inductance =10 henry.
Question 96.
A coil of 160 turns of cross-sectional area 250 cm2 rotates at an angular velocity of 300 rad/sec about an axis parallel to the plane of the coil in a uniform magnetic field of 0.6 weber/meter2. What is the maximum emf induced in the coil? If the coil is connected to a resistance of 2 ohm what is the maximum torque that has to be delivered to maintain its motion?
Answer:
We know that emax = NAB ω.
= 160 × 0.6 × (250 × 10-4) × 300 = 720 volt.
Now imax = \(\frac{e_{\max }}{R}\)
= \(\frac{720}{2}\) = 360 Amp.
τ = NiBA sin θ
τmax = Ni BA
= 160 × 360 × 0.6 × (250 × 10-4)
Maximum torque = 864 newton metre.
This torque opposes the rotation of the coil. Hence to maintain the rotation, an equal torque must be applied in opposite direction.
![]()
Question 97.
A solenoid of self inductance 200 mH carries a current of. 1A. Calculate
(i) the energy stored in the inductance and
(ii) total magnetic flux through the solenoid.
Answer:
Self inductance L = 200 × 103
Current flowing through the solenoid I = 1 A
(i) The total energy stored E = \(\frac{1}{2}\) LI2
.-. E= \(\frac{1}{2}\) × 200 × 10-3 × (1.0)2
= 100 × 10-3 J
Energy E = 100 mJ
(ii) Total magnetic flux Φ = LI .
= 200 × 10-3 × 1
= 200 × 10-3 Wb.
Φ = 200 mW
Question 98.
A magnetic field of 2 × 10-2 T acts at right angles to a coil of area 100 cm2 with 50 turn. When the coil is removed from the field in a particular interval of time, the emf induced in the coil is 0.1 V. Then calculate that interval of Time.
Answer:
Magnetic field B = 2 × 10-2 T
Area of the coil = 100 × 10-4 m2
Number of turns of the coil N = 50
Induced emf e = 0.1 V.
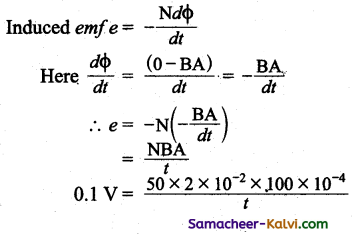
t = \(\frac{100 \times 10^{2} \times 100 \times 10^{-4}}{0.1}\)
= 1000 ×10-2 × 100 × 10-4
= 1000 × 10-4 = 0.1 s
∴ Interval of time = 0.1 s.
![]()
Question 99.
The primary of a transformer has 400 turns while the secondary has 2000 turns. If the power output from the secondary at 1100 V is 12.1 kW, calculate the primary voltage. If the resistance of primary is 0.2Ω and that of secondary is 2Ω and the efficiency of the transformer is 90% calculate
(i) heat loss in the primary coil,
(ii) heat loss in the secondary coil.
Answer:
Number of turns of primary Np = 400
Number of turns of secondary Ns = 2000
Secondary voltage Es = 1100 V
Power output Ps = 12.1 kW= 12100 W
Resistance of primary Rp = 0.2 Ω
Resistance of secondary Rs = 2 Ω
Efficiency of transformer
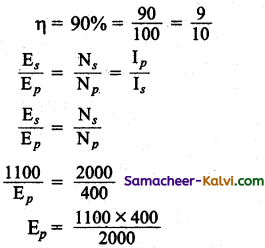
Primary Voltage Ep = 220 V
Secondary current Is = \(\frac{\mathrm{P}_{s}}{\mathrm{E}_{s}}=\frac{12100}{1100}\) = 11 A
η = \(\frac{\mathrm{E}_{s} \mathrm{I}_{s}}{\mathrm{E}_{p} \mathrm{I}_{p}}\)
Ip = \(\frac{\mathrm{E}_{s} \mathrm{I}_{s}}{\mathrm{E}_{p} \eta}\)
= \(\frac{1100 \times 11 \times 10}{220 \times 9}=\frac{121000}{1980}\) = 61.11 A
(i) Heat loss in the primary coil η = Ip2 R
= (61.11)2 × (0.2)
= 746.88 = 747 W
(ii) Heat loss in the secondary coil = Is2 R
= (11)2 × 2 = 121 × 2 = 242 W
Primary voltage = 220 V;
Heat loss in the primary coil = 747 W
Heat loss in the secondary coil = 242 W
![]()
Question 100.
A helicopter rises vertically with a speed of 10 m/s. If the helicopter be a horizontal linear conductor of length 20 m and if the horizontal components of the earth’s magnetic induction be 2 × 10-3 Wb/m2, calculate the potential difference between the tips of the nose and the tail of the helicopter.
Answer:
When the helicopter cuts the magnetic lines of induction, the induced emf e = Blv
Here B = 2 × 10-3 Wb/m2;
l = 20 m; v = 10 m/s
∴ The induced emf (the potential difference between the nose and tail of the helicopter)
e = 2 × 10-3 × 20 × 10 = 0.4 V
Potential difference e = 0.4 volts.
Question 101.
A step-up transformer operates on 220 volts and supplies a current of 2 Amp. The ratio of the primary and the secondary windings is 1 : 25. Determine the secondary voltage, primary current and the power output (Assume 100% efficiency).
Answer:
In any transformer
Here Ep = 220 volts; Is = 2 Amp.
\(\frac{\mathrm{N}_{p}}{\mathrm{~N}_{s}}=\frac{1}{25}\)∴ Ip = \(\frac{\mathrm{N}_{s}}{\mathrm{~N}_{p}}\) × I = 25 × 2 = 50
and Es = \(\frac{\mathrm{N}_{s}}{\mathrm{~N}_{p}}\) × E„ = 25 × 250 = 5500 Volts Power output
= Es × Is
= 5500 × 2 = 11000 watts.
![]()
Question 102.
A step-up transformer is used on 200 volts line to provide a potential difference of 1600 volts at 2 ampere current. Find the number of turns in the secondary when the number of turns in the primary is 100.
Answer:
In any transformer
\(\frac{\mathrm{I}_{s}}{\mathrm{I}_{p}}=\frac{\mathrm{E}_{p}}{\mathrm{E}_{s}}=\frac{\mathrm{N}_{p}}{\mathrm{~N}_{s}}\)
Here Ep = 200 V Np = 100
Es = 1600 V Ns = ?
Is = 2 Amp. Ip = ?
Again \(\frac{\mathrm{I}_{s}}{\mathrm{I}_{p}}=\frac{2}{\mathrm{I}_{p}}=\frac{1}{8}\)
Ip = 16 Amp.
\(\frac{\mathrm{N}_{p}}{\mathrm{~N}_{s}}=\frac{100}{\mathrm{~N}_{s}}=\frac{1}{8}\);
Ns = 800
Number of turns in the secondary Ns = 800.
Question 103.
The wings of an aeroplane are 10 m apart. The plane is moving horizontally towards the north at a place where the vertical component of earth’s magnetic field is 3 × 10-5 T. Calculate the induced emf set up between the tips of the wings if the velocity of the aeroplane is 720 km/hr.
Answer:
B = 3 × 10-5 T; .
v = 720 km/ hr.
= 720 × \(\frac{5}{18}\) m/s.
l = 10 m.
Induced emf e = – Blv
= – 3 × 10-5 × 10 × 720 × \(\frac{5}{18}\)
= – 600 × 10-4 V = 0.06 V.
Question 104.
A capacitor of capacitance 2 μF is in an AC circuit of frequency 1000 Hz. If the rms value of the applied emf is 10 V, find the effective current flowing in the circuit.
Answer:
C = 2 μF, v = 1000 Hz, Eeff = 10 V
Xc = \(\frac{1}{\mathrm{C} \omega}=\frac{1}{\mathrm{C} \times 2 \pi \nu}\)
= \(\frac{1}{2 \times 10^{-6} \times 2 \pi \times 10^{3}}\) = 79.6 Ω
Irms = \(\frac{\mathrm{E}_{e f f}}{\mathrm{X}_{c}}=\frac{10}{79.6}\) = 0.126 A
∴ Irms = 0.126 A
![]()
Question 105.
A bulb connected to 50 V, DC consumes 20 W power. Then the bulb is connected to a capacitor in an AC power supply of 250 V, 50 Hz. Find the value of the capacitance of the capacitor required so that the bulb draws the same amount of current.
Answer:
P = 20 Ω; V = 50 V; υ = 50Hz; C = ?
V = 250 V
P = VI
∴ I = \(\frac{P}{V}=\frac{20}{50}\) = 0.4 A
∴ Resistance,
R = \(\frac{V}{I}=\frac{50}{0.4}\) = 125 Ω
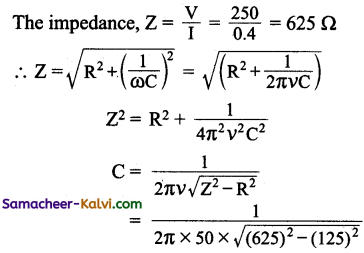
= \(\frac{1}{2 \pi \times 50 \times 612.37}\)
Capacitance of the Capacitor C = 5.198 µF
Question 106.
An AC voltage represented by e = 310 sin 314t is connected in series to a 24 W resistor, 0.1 H inductor and a 25 µF capacitor. Find the value of the peak voltage, rms voltage, frequency, reactance of the circuit, impedance of the circuit and phase angle of the current.
Answer:
R = 24 Ω, L = 0.1 H, C = 25 × 10-6 µF
e = 310 sin 314 7 ………….(1)
and e = E0 sin ωt …(2)
Comparing equations (1) and (2)
E0 = 310 V
Erms = \(\frac{\mathrm{E}_{0}}{\sqrt{2}}=\frac{310}{\sqrt{2}}\) = 219.2 V
ωt = 314 t
2πυ = 314
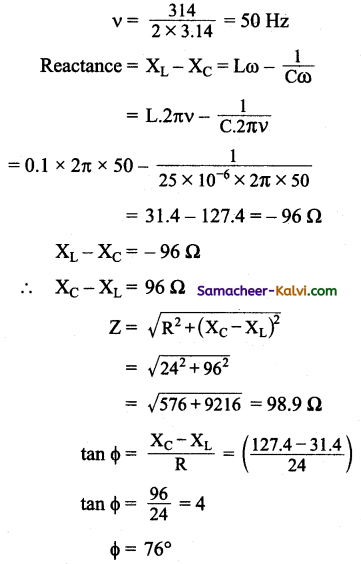
Predominance of capacitive reactance signify that current leads the emf by 76°.
![]()
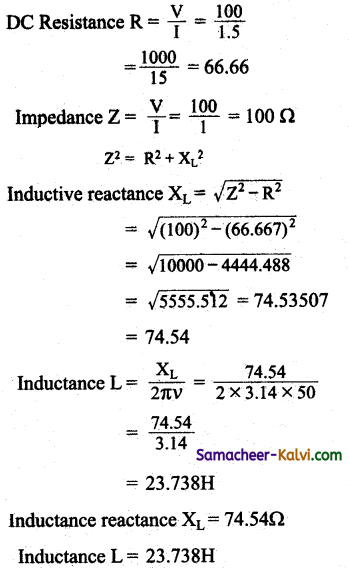
Question 107.
A student connects a long air core coil of manganin wire to a 100V DC source and records a current of 1.5A. When the same coil is connected across 100V, 50 Hz AC source, the current reduces to 1 A. Calculate the value of reactance and inductance of the coil.
Answer:
Electromotive force of a DC source V = 100 V
Current I = 1.5 A;
Electromotive force of an AC source e = 100 V;
Frequency v = 50 Hz;
Current I = 1 A V
Resistance R = \(\frac{\mathrm{V}}{\mathrm{I}}\)
Inductance L = \(\frac{X_{L}}{2 \pi V}\)
Inductance reactance XL = Lω = L × 2πυ
Inductance reactance XL = \(\frac{e}{\mathrm{I}_{0}}\)
Question 108.
An emf e = 100 sin 200 πt is connected to a circuit containing a capacitance of 0.1 μF and resistance of 500 Ω in series. Find the power factor of the circuit.
Answer:
Electromotive force is given by e = 100 sin 200 πt
Capacitance C = 0.1 μF;
Resistance R = 500 Ω
Phase angle Φ = tan-1 \(\left(\frac{\mathrm{X}_{\mathrm{L}}}{\mathrm{R}}\right)\)
XC = \(\frac{1}{\mathrm{C} \omega}\)
Power factor = cos (Φ)
Comparing the equation e = 100 sin 200 πt with general equation of emf, e = a sin ωt
we get, ω = 200 π
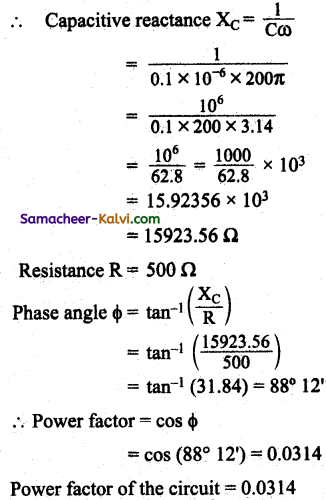
![]()
Question 109.
A radio can tune over the frequency range of a portion of broadcast band (800 kHz to 1200 kHz). If its LC circuit has an effective inductance of 200 µH, what must be the range of its variable capacitance?
Answer:
Frequency range = 800 kHz to 1200 kHz
∴ v1 = 800 × 103 Hz
∴ v2 = 1200 × 103 Hz
Effective inductance L = 200 × 10-6 H
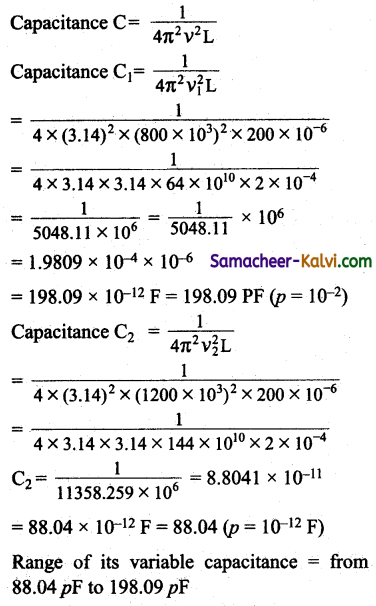
Question 110.
A transformer has an efficiency of 80 %. It is connected to a power input of at 4 KW and 100 V. If the secondary voltage is 240 V. Calculate the primary and secondary currents.
Answer:
Efficiency of a transformer η = 80% = \(\frac{80}{100}\)
Power input P = 4 kW = 4000 W
Primary voltage Ep = 100 V;
Secondary voltage Es = 240 V
Primary current Ip = \(\frac{\text { Power input }}{\text { Primary voltage }}\)
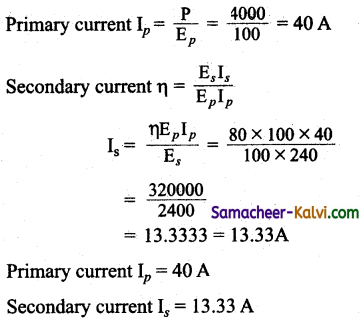
![]()
Question 111.
Why are oscillation of a copper sheet in a magnetic field highly damped?
Answer:
It is because of the development of eddy current in the copper sheet.
Question 112.
Sparking occurs in the switches when light is put off. Given reason.
Answer:
Larger amount of einf is induced at break of circuits. This causes the sparking.
Question 113.
When is the magnetic flux linked with a coil held in a magnetic field zero?
Answer:
The magnetic flux will be zero when the plane of the coil is along the magnetic field.
Question 114.
Can one coil have an inductance without a resistance?
Answer:
No. Every material of the coil has some resistance.
Question 115.
A coil of metal wire is stationary in a non-uniform magnetic field. Will any emf be induced in the coil?
Answer:
No, emf will be induced as magnetic flux linked with the stationary coil is not changing.
![]()
Multiple Choice Questions:
Question 1.
Assertion:
In a conductor, free electrons keep on moving but magnetic force does not on the conductor placed in a magnetic field.
Reason:
Force on free electrons due to magnetic field-always are perpendicular to its direction of motion.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are false and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(c) Assertion is true but reason is false.
Question 2.
Assertion:
when a magnetic dipole is placed in a non-uniform magnetic field, torque only i acts on the dipole.
Reason:
Force will also act on the dipole if magnetic field were uniform.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly
(b) Both Assertion and Reason are false and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(a) Both Assertion and Reason are true and reason explains assertion correctly.
![]()
Question 3.
Assertion:
If an electron while coming vertically from outerspace enter the earth’s magnetic field, it is deflected towards west.
Reason:
Electrons do not have angular momentum.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are false and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(d) Assertion is false but reason is true.
Question 4.
Assertion:
If a charged particle is moving in a perpendicular uniform magnetic field, its kinetic energy does not change:
Reason:
Velocity of the particle does not change in a magnetic field.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are false and reason does not explain assertion correctly. .
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(c) Assertion is true but reason is false.
Question 5.
Assertion:
A tangent galvanometer is used for measuring current.
Reason:
As it is direct reading.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are false and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(c) Assertion is true but reason is false.
![]()
Question 6.
Assertion:
current I flows along the length of an infinitely long straight and thin walled pipe. Then the magnetic field at any point inside the pipe is zero.
Reason:
\(\oint \overrightarrow{\mathrm{B}} d l=\mu_{0} I\)
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are false and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(a) Both Assertion and Reason are true and reason explains assertion correctly.
Question 7.
A wire of length 2m carries a current of 1A is bend to form a circle. The magnetic moment * of the coil is: [IIT]
(a) \(\frac{\pi}{2}\)
(b) \(\frac{2}{\pi}\)
(c) \(\frac{1}{\pi}\)
(d) π
Answer:
(c) \(\frac{1}{\pi}\)
Question 8.
A coil of area of cross section 0.5 m2 with 10 turns is in a plane which is perpendicular to an uniform magnetic field of 0.2 Wb/m2. The flux through the coil is:
(a) 100 Wb
(b) 10 Wb
(c) 1 Wb
(d) zero
Answer:
(c) 1 Wb
![]()
Question 9.
Electromagnetic induction is not used in:
(a) transformer
(b) room heater
(c) AC generator
(d) choke coil
Answer:
(b) room heater
Question 10.
In a uniform magnetic field B, if a conductor having area A is placed, then the magnetic flux linked is given by:-
(a) Φ = AB
(b) Φ = BA sin θ
(c) Φ = BA cos θ
(d) Φ =\(\frac{d \mathrm{~B}}{d t}\)
Answer:
(c) Φ = BA cos θ
Question 11.
The unit of magnetic flux is:
(a) ampere
(b) ohm
(c) weber
(d) volt
Answer:
(c) weber
![]()
Question 12.
According to Faraday’s law of electromagnetic induction:
(a) the electric field can be produced by time varying magnetic flux
(b) the magnetic field is produced by time varying electric field
(c) charge is conserved
(d) the magnetic field is associated with energy of the charged particle
Answer:
(a) the electric field can be produced by time varying magnetic flux
Question 13.
The induced emf in a conductor is:
(a) inversely proportional to the rate of change of flux
(b) directly proportional to the rate of change of flux
(c) directly proportional to the total flux associated with the conductor
(d) equal to the flux
Answer:
(b) directly proportional to the rate of change of flux
Question 14.
The magnitude of the induced emf produced in a coil when a magnet is inserted into it does not depend upon the:
(a) number of turns in the coil
(b) resistance of the coil
(c) magnetic moment of the magnet
(d) speed of approach of the magnet
Answer:
(b) resistance of the coil
![]()
Question 15.
A cylindrical bar magnet is kept along the j axis of a circular coil. If the magnet is rotated about the axis then will be induced in the coil.
(a) an emf
(b) no emf
(c) an emf whose magnitude depends upon the angular velocity
(d) a high voltage
Answer:
(c) an emf whose magnitude depends upon the angular velocity
Question 16.
Who discovered that current can be produced in a closed conductor whenever there is a relative motion between the conductor and a magnetic field?
(a) Newton
(b) Fleming
(c) Oersted
(d) Michael Faraday
Answer:
(d) Michael Faraday
![]()
Question 17.
When the bar magnet is introduced with any pole into the coil the galvanometer shows:
(a) deflection
(b) permanent deflection
(c) momentary deflection
(d) no deflection
Answer:
(c) momentary deflection
Question 18.
The emf got by varying magnetic field is known as:
(a) Thermo emf
(b) Induced emf
(c) Changing emf
(d) Stable emf
Answer:
(b) Induced emf
Question 19.
Strength of the induced current depends upon the speed with which the:
(a) emf changes
(b) magnetic field changes
(c) emf stops
(d) emf increases
Answer:
(b) magnetic field changes
![]()
Question 20.
The strength of the magnetic field determines:
(a) strength of induced current
(b) area of the coil
(c) stability of the current
(d) fall of emf
Answer:
(a) strength of induced current
Question 21.
What is the unit of magnetic induction?
(a) ampere
(b) tesla
(c) volt
(d) weber
Answer:
(b) tesla
Question 22.
The strength of the induced emf depends on of the coil.
(a) the number of turns
(b) the resistance of the wire
(c) the specific resistance of the wire
(d) the surface area
Answer:
(a) the number of turns
![]()
Question 23.
The SI unit of mutual inductance is:
(a) tesla
(b) weber
(c) henry
(d) coulomb
Answer:
(c) henry
Question 24.
The total magnetic flux linked with the solenoid area A, length l, having N turns and a current I flows is given by:
(a) Φ = \(\frac{\mathrm{N}^{2} \mathrm{IA}}{l}\)
(b) Φ = \(\frac{\mu_{0} \mathrm{~N}^{2} \mathrm{IA}}{l}\)
(c) Φ = \(\frac{\mu_{0} \mathrm{NA}}{\mathrm{Il}}\)
(d) Φ = \(\frac{\mu_{0} \mathrm{IA}}{\mathrm{N}^{2} l}\)
Answer:
(b) Φ = \(\frac{\mu_{0} \mathrm{~N}^{2} \mathrm{IA}}{l}\)
Question 25.
A solenoid has length 1, area A and number of turns N. If it is filled with material of permeability p then its self inductance is:
(a) L = \(\frac{l N^{2} A}{\mu}\)
(b) L = \(\frac{\mu \mathrm{N}^{2} \mathrm{~A}}{l}\)
(c) L = \(\frac{\mu \mathrm{NA}^{2}}{l}\)
(d) L = \(\frac{\mu l^{2} \mathrm{~A}}{\mathrm{~N}}\)
Answer:
(b) L = \(\frac{\mu \mathrm{N}^{2} \mathrm{~A}}{l}\)
![]()
Question 26.
If a current of 1 A flows through a primary coil and the flux linked with secondary coil is Φs then coefficient of mutual induction is M =
(a) \(\frac{\phi_{s}}{\mathrm{I}}\)
(b) \(\frac{\mathrm{I}}{\phi_{s}}\)
(c) Φs
(d) \(\frac{\phi_{s}}{\mathrm{~A}}\)
Answer:
(c) Φs
Question 27.
The coefficient of mutual induction between a pair of coils depends upon:
(a) size and shape of the coil
(b) number of turns of the coil
(c) proximity of the coil
(d) all the above
Answer:
(d) all the above
Question 28.
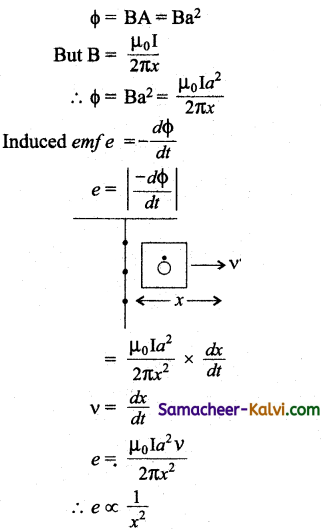
A square loop of side a is placed in such a way that its plane is the same as that of a very long straight wire carrying a current of I.
The centre O of the loop is at a distance x from the wire. A velocity is given to the loop as shown in the figure. If x > > a, the magnitude of the emf induced in the loop is proportional to:
(a) x
(b) \(\frac{1}{x^{2}}\)
(c) x2
(d) \(\frac{1}{x}\)
Answer:
(b) \(\frac{1}{x^{2}}\)
Hint:
![]()
Question 29.
A coil of metal wire is kept stationary with its plane perpendicular to a uniform magnetic field directed along the positive x-axis. The current induced in the coil.
(a) is perpendicular to the direction of the magnetic field
(b) zero
(c) circulates in anticlockwise direction when viewed from x-axis.
(d) circulates in clockwise direction when viewed from y-axis.
Answer:
(b) zero
Hint:
Since the coil is kept stationary the magnetic flux linked with the coil does not change. Hence the current induced in the coil is zero
Question 30.
Which of the following statement are not correct?
(i) A capacitor is connected in series with a bulb. When this combination is connected to an A.C. source, the bulb does not glow.
(ii) In a series A.C. circuit the applied voltage is not equal to the algebraic sum of voltages across the different elements
of the circuit.
(iii) A variable capacitor is connected in series with a bulb and this combination is connected to an A.C. source. If the
capacitance of the variable capacitor is decreased then the brightness of the bulb is reduced.
(iv) A capacitor is connected in series with a bulb and this combination is connected to a D.C. source. When the voltage of the source is increased, the brightness of the bulb wifi increase.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer:
(c) (i) and (iv)
Hint:
(i) The capacitor will offer finite resistance to A.C. Hence a current will flow in the circuit and the bulb will glow. (iv) Since the capacitor offers infinite resistance to direct current, the bulb will not glow.
![]()
Question 31.
Match the physical quantities given in Column I with their S.I. units given in Column II.
| Column – I | Column – II |
| (i) Magnetic permeability | (A) Henry |
| (ii) Magnetic field | (B) Henry / meter |
| (iii) Magnetic flux | (C) Ampere |
| (iv) Self inductance | (D) Weber |
| (E) Tesla | |
| (F) Couloumb |
(a) (i) – (F); (ii) – (E); (iii) – (C); (iv) – (B)
(b) (i) – (E); (ii) – (D); (iii) – (B); (iv) – (A)
(c) (i) – (B); (ii) – (E); (iii) – (D); (iv) – (A)
(d) (i) – (C); (ii) – (A); (iii) – (E); (iv) – (F)
Answer:
(c) (i) – (B); (ii) – (E); (iii) – (D); (iv) – (A)
Question 32.
Two ideal inductors are connected in parallel as shown in the figure. A time varying current flows as shown in the figure. The ratio of \mathrm{I}_{1} / \mathrm{I}_{2} at any time is:
(a) \(\sqrt{\frac{\mathrm{L}_{2}}{\mathrm{~L}_{1}}}\)
(b) \(\sqrt{\frac{\mathrm{L}_{1}}{\mathrm{~L}_{2}}}\)
(c) \(\frac{\mathrm{L}_{2}}{\mathrm{~L}_{1}}\)
(d) \(\frac{\mathrm{L}_{1}}{\mathrm{~L}_{2}}\)
Answer:
(c) \(\frac{\mathrm{L}_{2}}{\mathrm{~L}_{1}}\)
Hint:
Since the inductors are connected in parallel.
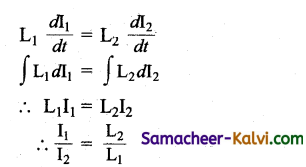
![]()
Question 33.
Which one of the following curves represents the variation of potential difference V across the inductor L with time t, the key being plugged at t = 0 in the following circuit.
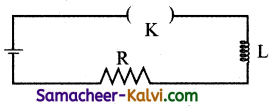
(a) 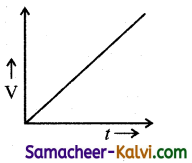
(b) 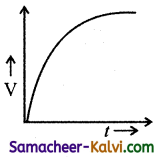
(c) 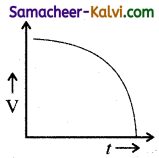
(d) 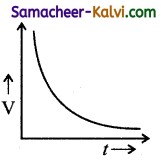
Answer:
(d)  Hint:
Hint:
Potential difference across the inductor is V = – L\(\frac{d I}{d t}\)
= – LI0 \(\frac{d}{d t}\left(1-e^{-\mathrm{R} t / \mathrm{L}}\right)\)
= – LI(\(\left.\frac{\mathrm{R}}{\mathrm{L}} e^{-\mathrm{R} t / \mathrm{L}}\right)\)
|V| = V0 e-t/L
where V0 = I0R
∴ V = V0 at t = 0 and then falls exponentially with time becoming zero at t >> e
The correct graph is d.
Question 34.
In the field of mutual induction, if the coils are wound on a soft iron core, the mutual j induction is:
(a) small
(b) large
(c) very large
(d) zero
Answer:
(c) very large
Question 35.
The generator rule is
(a) Fleming’s left hand rule
(b) Fleming’s right hand rule
(c) Maxwell’s right hand cork rule
(d) Ampere’s swimming rule
Answer:
(b) Fleming’s right hand rule
![]()
Question 36.
Which one of the following devices is based on Electromagnetic Induction?
(a) Galvanometer
(b) Ammeter
(c) Dynamo
(d) Voltameter
Answer:
(c) Dynamo
Question 37.
When a conductor of length / moving with velocity v in a uniform magnetic field B, the induced emf is given by:
(a) e = NABω
(b) e = – \(\frac{d \phi}{d t}\)
(c) e = \(\frac{d \mathrm{~B}}{d t}\)
(d) e = – Blv
Answer:
(d) e = – Blv
Question 38.
When a coil of area A is oriented through 1 an angle of orientation (0 = tot) in a uniform j magnetic field B, the induced emf is given by:
(a) e = 2 πv NAB sin ωt
(b) e = E0 cos ωt
(c) e = NAB sin θ
(d) e = NAB cos ωt
Answer:
(a) e = 2 πv NAB sin ωt
![]()
Question 39.
When the angle of orientation ωt = 3\(\frac{\pi}{2}\), the value of induced emf is given by: .
(a) E0
(b) 0
(c) ∞
(d) -E0
Answer:
(d) – E0
Question 40.
An aeroplane having a wingspan of 35m flies due north at speed 90m/s. The potential difference between the end point over a given locality if vertical components of earth’s ; magnetic field is 4 x 10-4 Wb/m2 is:
(a) 1.29 volts
(b) 1.26 volts
(c) 1.06 volts
(d) 1.66 volts
Answer:
(b) 1.26 volts
Question 41.
In a three phase A.C. generator the coils are j displaced from each other by:
(a) 90°
(b) 180°
(c) 120°
(d) 360°
Answer:
(c) 120°
![]()
Question 42.
When current flows through choke coil, the | dissipation of power is:
(a) maximum
(b) minimum
(c) zero
(d) constant
Answer:
(c) zero
Question 43.
When the current flowing through one coil changes from 11 Ato 5Aduringatime interval of 0.1 sec, an emf of 60 mv is induced in the neighbourhood coil. The mutual inductance of it will be:
(a) 0.1 H
(b) 0.01 H
(c) 0.001 H
(d) 0.002 H
Answer:
(c) 0.001 H
Question 44.
The maximum value of the induced emf is:
(a) e = E0 cos2 ωt
(b) E0 = NABω
(c) e = e sin co t
(d) e = E02 sin ωt
Answer:
(b) E0 = NABω
![]()
Question 45.
The mutual inductance between two planar concentric rings of radii r1 and r2 (r1 > r2) placed in air is given by:
(a) \(\frac{\mu_{0} \pi r_{1}^{2}}{2 r_{2}}\)
(b) \(\frac{\mu_{0} \pi r_{2}^{2}}{2 r_{1}}\)
(c) \(\frac{\mu_{0} \pi r_{2}}{r_{1}}\)
(d) \(\frac{\mu_{0} \pi}{2} \sqrt{\frac{r_{1}}{r_{2}}}\)
Answer:
(b) \(\frac{\mu_{0} \pi r_{2}^{2}}{2 r_{1}}\)
Hint:
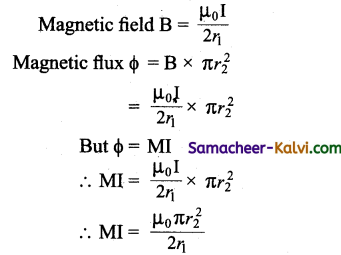
Question 46.
An A.C. source of variable frequency f is connected to an LCR series circuit. Which one of the graphs represents the variation of current I in the circuit with frequency?
(a) 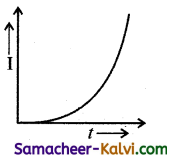
(b) 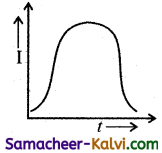
(c) 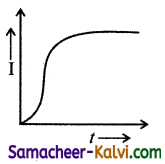
(d) 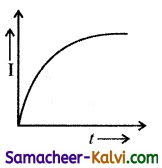
Answer:
(b) 
Hint:
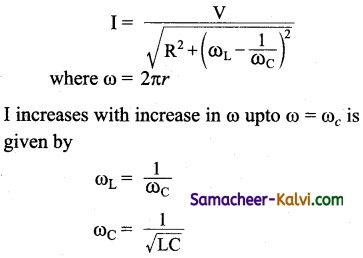
where I will be maximum.
At ω > ωc I decreases with increase in . Hence the correct graph is (b).
Question 47.
Lenz’s law is in accordance with the law of:
(a) conservation of charges
(b) conservation of flux
(c) conservation of momentum
(d) conservation of energy
Answer:
(d) conservation of energy
![]()
Question 48.
The self-inductance of a straight conductor is:
(a) zero
(b) infinity
(c) very large
(d) very small
Answer:
(a) zero
Question 49.
The unit henry can also be written as:
(a) VA s-1
(b) Wb-1 A
(c) Ω s
(d) all
Answer:
(c) Ω s
Question 50.
An emf of 12 V is induced when the current in the coil changes at the rate of 40 A s-1. The coefficient of self induction of the coil is:
(a) 0.3 H
(b) 0.003 H
(c) 30 H
(d) 4.8 H
Answer:
(a) 0.3 H
![]()
Question 51.
A DC of 5 A produces the same heating effect as an AC of
(a) 50 A rms current
(b) 5 A peak current
(c) 5 A rms current
(d) none of these
Answer:
(c) 5 A rms current
Question 52.
Transformer works on:
(a) AC only
(b) DC only
(c) Both AC and DC
(d) AC more effectively than DC
Answer:
(a) AC only
Question 53.
The part of the AC generator that passes the current from the coil to the external circuit is:
(a) fiefld magnet
(b) split rings
(c) slip rings
(d) brushes
Answer:
(d) brushes
![]()
Question 54.
The efficiency of a transformer is:
(a) η = \(\frac{\text { output power }}{\text { input power }}\)
(b) η = \(\frac{\text { input power }}{\text { output power }}\)
(c) η = input power – output power
(d) η = \(\frac{\text { input voltage }}{\text { output voltage }}\)
Answer:
(a) η = \(\frac{\text { output power }}{\text { input power }}\)
Question 55.
In an A.C circuit the applied emf e = E0 sin (ωt + π/2) leads the current I = I0 sin (ωt – π/2) by:
(a) π/2
(b) π/4
(c) π
(d) 0
Answer:
(c) π
Question 56.
Which of the following cannot be stepped up in a transformer?
(a) Input current
(b) Input voltage
(c) Input power
(d) All
Answer:
(c) Input power
![]()
Question 57.
The power loss is less in transmission lines when:
(a) voltage is less but current is nun
(b) both voltage and current are more
(c) voltage is more but current is less
(d) both voltage and current are less
Answer:
(c) voltage is more but current is less
Question 58.
Which of the following devices does not allow D.C. to pass through?
(a) Resistor
(b) Capacitor
(c) Inductor
(d) All the above
Answer:
(b) Capacitor
Question 59.
In an A C. circuit:
(a) the average value of current is zero
(b) the average value of square of current is zero
(c) the average power dissipation is zero
(d) the rms current is time of peak current
Answer:
(a) the average value of current is zero
![]()
Question 60.
Match the quantities given in Column I with the dimensions given in Column II.
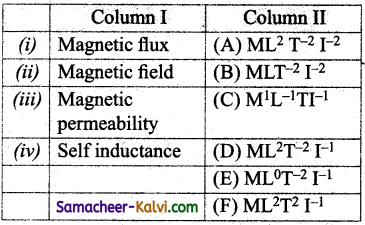
(a) (i) – (C); (ii) – (B); (iii) – (A); (iv) – (F)
(b) (i) – (E); (ii) – (D); (iii) – (F); (iv) – (C)
(c) (i) – (D); (ii) – (E); (iii) – (B); (iv) – (A)
(d) (i)- (A); (ii) – (D); (iii) – (B); (iv) – (C)
Answer:
(c) (i) – (D); (ii) – (E); (iii) – (B); (iv) – (A)
Question 61.
Select the odd man out of the following:
(a) Wb A-1
(b) Ohm – s
(c) Wb
(d) VAs-1
Answer:
(c) Wb
Hint:
(a), (b), (d) are unit of self inductance but C is not the unit of self inductance.
Question 62.
Assertion:
A spark occurs sometimes when an electric iron is switched off.
Reason:
Sparking is due to large self motived emf in the circuit during manufacture.
Which of the following is correct?
(a) Both Assertion and Reason are true and – reason explains assertion correctly.
(b) Both Assertion and Reason are true and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false reason is true.
Answer:
(c) Assertion is true but reason is false.
![]()
Question 63.
Assertion:
A step up transformer can also he used as a step down transformer.
Reason:
It is because = \(\frac{v_{s}}{v_{p}}=\frac{n_{s}}{n_{p}}\)
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are true and reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(a) Both Assertion and Reason are true and reason explains assertion correctly.
Question 64.
Assertion:
If a coil is rotated in an electric field the magnitude of induced current is zero.
Reason:
The electric flux through the loop does not change with time.
Which of the following statement is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are true but reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(b) Both Assertion and Reason are true but reason does not explain assertion correctly.
Hint:
A current is induced in a loop only if the magnetic flux linked with the coil changes.
Question 65.
Assertion:
A circular loop and a rectangular loop are moved with a constant velocity from a region of magnetic field out hits a field free region. The field is normal to the loops. A constant emf is induced in the circular loop whereas a time varying emf will be induced in the rectangular loop.
Reason:
The induced emf is constant when magnetic flux changes at a constant rate.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are true but reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(d) Assertion is false but reason is true.
Hint:
In the rectangular loop, the rate of change of area is constant. But in the circular loop rate of change of area varies.
![]()
Question 66.
Assertion:
A Magnetized iron bar is dropped vertically through a hollow region of a thick cylindrical shell made of copper. The bar will fall with an acceleration less than acceleration due to gravity.
Reason:
The emf induced in the bar causes a retarding force to act on the falling bar.
Which of the following is correct?
(a) Both Assertion and Reason are true and reason explains assertion correctly.
(b) Both Assertion and Reason are true but reason does not explain assertion correctly.
(c) Assertion is true but reason is false.
(d) Assertion is false but reason is true.
Answer:
(a) Both Assertion and Reason are true and reason explains assertion correctly.
Hint:
As per Lenz’s law and due to eddy currents the bar is falling.
Question 67.
The effective value of alternating current is:
(a) \(\frac{\mathrm{I}_{0}}{2}\)
(b) \(\frac{\mathrm{I}_{0}}{\sqrt{2}}\)
(c) √2I0
(d) 2I0
Answer:
(b) \(\frac{\mathrm{I}_{0}}{\sqrt{2}}\)
Question 68.
A fuse is having a current rating of 5 amperes. Then the peak value of the fuse wire is:
(a) 7 amperes
(b) 1 ampere
(c) 707 amperes
(d) 7.07 amperes
Answer:
(d) 7.07 amperes
![]()
Question 69.
The inductive reactance of the AC circuit is:
(a) XL = ω/L
(b) XL = Lω
(C) XL = L2ω
(d) XL = Lω2
Answer:
(b) XL = Lω
Question 70.
The capacitive reactance of the AC circuit is:
(a) Xc = Cω
(b) Xc = C/ω
(c) Xc = 1/Cω
(d) Xc > 1/Cω
Answer:
(c) Xc = 1/Cω
Question 71.
In a series resonant circuit containing an inductance ‘L’ capacitance ‘C’ and resistance ‘R’ the condition for impedance becomes minimum is:
(a) Lω > 1/Cω
(b) Lω = 1/Cω
(c) Lω < 1/Cω
(d) Lω = Cω
Answer:
(b) Lω = 1/Cω
![]()
Question 72.
The resonant frequency of an LCR circuit is:
(a) υ0 = 2π/LC
(b) υ0 = \(\frac{1}{2 \pi \sqrt{\mathrm{LC}}}\)
(c) υ0 = \(\frac{1}{2 \pi L C}\)
(d) υ0 = \(\frac{\mathrm{LC}}{2 \pi}\)
Answer:
(b) υ0 = \(\frac{1}{2 \pi \sqrt{\mathrm{LC}}}\)
Question 73.
In a LCR circuit when XL= Xc:
(a) current is minimum impedance is maximum
(b) current is maximum impedance is maximum
(c) current is maximum iiipedance is minimum
(d) current is minimum impedance is minimum
Answer:
(c) current is maximum iiipedance is minimum
![]()
Question 74.
In a LCR circuit, at the resonating frequency:
(a) XL > XC
(b) XL < XC
(c) XL XC
(d) XL = 0
Answer:
(c) XL XC
Question 75.
The current and the applied emf are in phase in an AC circuit which contains:
(a) Inductance only
(b) Resistance only
(c) Capacitance only
(d) All the three
Answer:
(b) Resistance only
Question 76.
The heat energy developed in a resistor is independent of
(a) the direction of flow of current
(b) the magnitude of flow of current
(c) the strength of emf
(d) All the three
Answer:
(a) the direction of flow of current
![]()
Question 77.
The Q-factor of an AC circuit consisting of resistance R, an inductor L and a capacitor C is given by:
(a) Question = \(\frac{1}{\sqrt{\mathrm{LC}}}\)
(b) Question = \(\frac{1}{\mathrm{R}} \sqrt{\frac{\mathrm{C}}{\mathrm{L}}}\)
(c) Question = \(\frac{1}{\mathrm{R}} \sqrt{\frac{\mathrm{L}}{\mathrm{C}}}\)
(d) Question = \(\frac{1}{\sqrt{\mathrm{LR}}}\)
Answer:
(c) Question = \(\frac{1}{\mathrm{R}} \sqrt{\frac{\mathrm{L}}{\mathrm{C}}}\)
Question 78.
The apparent power in an AC circuit is given by:
(a) E0 I0
(b) Erms Irms
(c) \(\frac{E_{0} I_{0}}{2}\)
(d) Erms + Irms
Answer:
(b) Erms Irms
Question 79.
The power factor of a choke coil having inductance L and resistance r is given by :
(a) \(\sqrt{r^{2}+\omega^{2} L^{2}}\)
(b) \(\sqrt{\frac{r^{2}+\omega^{2}+L^{2}}{r}}\)
(c) \(\frac{r}{\sqrt{r^{2}+\omega^{2} L^{2}}}\)
(d) r2 + ω 2 L2
Answer:
(c) \(\frac{r}{\sqrt{r^{2}+\omega^{2} L^{2}}}\)
![]()
Question 80.
The power generated by a choke coil having resistance r, inductance L is given by:
(a) Erms Irms
(b) \(\frac{E_{0} I_{0}}{2}\)
(c) Erms Irms \(\frac{r}{\sqrt{r^{2}+\omega^{2} L^{2}}}\)
(d) E0 I0 \(\frac{r}{\sqrt{r^{2}+\omega^{2} L^{2}}}\)
Answer:
(c) Erms Irms \(\frac{r}{\sqrt{r^{2}+\omega^{2} L^{2}}}\)
Question 81.
Choke coils are commonly seen in:
(a) incandescent bulbs
(b) fluorescent tubes
(c) stabilizer circuits
(d) radio
Answer:
(b) fluorescent tubes
Question 82.
A current of 1 A flowing through ;i oil of inductance 1 henry is switched off in one milli second. Then the induced emf is:
(a) – 100 V
(b) + 100 V
(b) -1000 V
(d) – 10000 V
Answer:
(c) – 1000 V
![]()
Question 83.
The coefficient of self induction of a coil by 1000 turns which produces a magnetic flux of 0.5 Wb for a constant current of 2.5 A is:
(a) 0.2 H
(b) 0.4 H
(c) 0.04 H
(d) 0.02 H
Answer:
(d) 0.02 H
Question 84.
The mutual inductance is 4 mH between the primary and secondary of an induction coil. A current of 4 A cuts off in 10-3 s. The magnitude of the induced emf is:
(a) 1.6 V
(b) 16 V
(c) – 1.6 × 10-3 V
(d) – 1.6 V
Answer:
(b) 16 V
Question 85.
An aeroplane having a wingspan of 35 m flies at a speed of 100 m/s. If the vertical component of earth’s magnetic field is 4 × 10-4 T, then the induced emf across the wingspan is:
(a) 28 V
(b) 2.8 V
(c) 14 V
(d) 1.4 V
Answer:
(d) 1.4 V
![]()
Question 86.
The emf in an AC containing only resistance will:
(a lag behind the current by \(\frac{\pi}{2}\)
(b) have current in phase with the applied voltage
(c) be ahead of current by \(\frac{\pi}{2}\)
(d) always be out of phase
Answer:
(b) have current in phase with the applied voltage
Question 87.
In an AC circuit with an inductor:
(a) voltage lags current by \(\frac{\pi}{2}\)
(b) voltage and current are in phase
(c) voltage leads current by TC
(d) current lags voltage by \(\frac{\pi}{2}\)
Answer:
(d) current lags voltage by \(\frac{\pi}{2}\)
Question 88.
The unit of capacitive reactance is:
(a) weber
(b) ohm
(c) volt
(d) tesla
Answer:
(b) ohm
![]()
Question 89.
The frequency at which a 1 henry inductor has a reactance of 314 Ω:
(a) 500 Hz
(b) 50 Hz
(c) 5 Hz
(d) 0.05 Hz
Answer:
(b) 50 Hz
Question 90.
If the frequency of AC is such that XL = XC then the current in the circuit is:
(a) zero
(b) minimum
(c) maximum
(d) negative
Answer:
(c) maximum
Question 91.
The phenomenon of resonance is used in :
(a) Radio
(b) Capacitor
(b) Transformer
(d) Amplifier
Answer:
(a) Radio
![]()
Question 92.
Inductive reactance to a direct current is:
(a) finite
(b) infinity
(c) zero
(d) – 1
Answer:
(c) zero
Question 93.
Inductive capacitance to a direct current is
(a) finite
(b) infinity
(c) zero
(d) unity
Answer:
(b) infinity
Question 94.
The frequency of unidirectional current is:
(a) infinity
(b) zero
(c) unity
(d) finite
Answer:
(b) zero
![]()
Question 95.
An emf of 12 V is induced when the current in the coil changes from 2 A to 6 A in 0.5 s. The coefficient of self inductance of the coil is;
(a) 1.5 H
(b) 6 H
(c) 0.3 H
(d) 3 H
Answer:
(a) 1.5 H
Question 96.
In LCR series AC circuit,the phase difference between current and voltage is 30°. The reactance of the circuit is 17.32 Ω. The value of resistance is:
(a) 30 Ω
(b) 10 Ω
(c) 17.32 Ω
(d) 1.132 Ω
Answer:
(a) 30 Ω
Question 97.
A generator produces an emf given by e = 141 sin 88 t. Then the frequency and the rms value of voltage are:
(a) 50 Hz and 100 V
(b) 1 Hz and 50 V
(c) 14 Hz and 100 V
(d) 50 Hz and 50 V
Answer:
(c) 14 Hz and 100 V
![]()
Question 98.
The output voltage of a transformer connected to 240 V mains supply is 24 V. When this transformer is used to operate a bulb with rating 24 V, 24 W, then the current flowing in the primary of the transformer is:
(a) 0.01 A
(b) 1A
(c) 10 A
(d) 0.1 A
Answer:
(d) 0.1 A
Question 99.
The peak values of voltage and current in a circuit contains resistor alone are 240 V and 0.5 amp. Then the power is:
(a) 0.002 watts
(b) 240 watts
(c) 60 watts
(d) 120 watts
Answer:
(d) 120 watts
Question 100.
The number of lines of force crossing unit area normally is:
(a) magnetic flux
(b) magnetic induction
(c) induced emf
(d) total flux
Answer:
(b) magnetic induction
![]()
Question 101.
Lenz’s law is in accordance with:
(a) conservation of momentum
(b) conservation of energy
(c) conservation of fuel
(d) conservation of angular momentum
Answer:
(b) conservation of energy
Question 102.
A signal voltage V(t) = V0 sin ωt is applied across an ideal capacitor ‘C’
(a) current I (t) is in phase with voltage V (t)
(b) current I (t) is leads with voltage V (t) by 180°
(c) current I (t) is lags with voltage V (t) by 180°
(d) over a fully cycle, the capacitor does not consume any energy from the voltage source.
Answer:
(d) over a fully cycle, the capacitor does not consume any energy from the voltage source.
Question 103.
A coil has resistance of 30 ω and inductive reactance of 20 ω at 50 Hz frequency. If an AC source of 200V, 100 Hz is connected across the coil, the current to the coil will be:
(a) \(\frac{20}{\sqrt{13}}\) A
(b) 2.0 A
(c) 8.0 A
(d) 4.0 A
Answer:
(d) 4.0 A
Hint:
when f’ = 100 Hz
f’ = 2f i.e., XL‘ = 2 XL
Z’ = \(\sqrt{900+1600}\)
= \(\sqrt{2500}\) = 50 Ω
∴ I = \(\frac{\mathrm{V}_{0}}{\mathrm{Z}^{\prime}}\)
= \(\frac{200}{50}\) = 4 A
![]()
Question 104.
An ideal transformer has a power input of 10 kW. The secondary current is 25 A. If the ratio of the number of turns in the primary and the secondary coils is 5 : 1 then the potential difference applied to the primary is:
(a) 100 V
(b) 200 V
(c) 2000 V
(d) 1500 V
Answer:
(c) 2000 V
Question 105.
The frequency at which a 2 henry inductor | has a reactance of 20 nQuestion in an A.C. circuit j with the inductor only is:
(a) 50 Hz
(b) 5 Hz
(c) 15 Hz
(d) 100 Hz
Answer:
(b) 5 Hz
Question 106.
420 kW of electric power is supplied to a small town at a distance of 8 km away from the power plant. The transmission wire has a total resistance of 0.33 ohm. If the power is transmitted at 21,000 V then the power loss is:
(a) 0.825 kW
(b) 0.132 kW
(c) 1.32 kW
(d) 132 kW
Answer:
(b) 0.132 kW
![]()
Question 107.
In an LCR circuit in series with a 220 V, 50 Hz, AC. it is observed that. L = 0.2 henry, C = \(\frac{1}{2 \times 10^{3} \pi^{2}}\) F and R = 35 ohm. Then in the circuit:
(a) current leads voltage by 60°
(b) voltage leads current by 60°
(c) current leads voltage by 30°
(d) current and voltage are in phase
Answer:
(d) current and voltage are in phase
Question 108.
The average power consumed over one cycle j in an AC. circuit is: j
(a) Erms Irms
(b) Erms Irms cos Φ
(c) Erms Irms sin Φ
(d) E0 I0 cos Φ
Answer:
(b) Erms Irms cos Φ
Question 109.
In LCR circuit when XL = XC the current:
(a) is zero
(b) is in phase with the voltage
(c) leads the voltage
(d) lags behind the voltage
Answer:
(b) is in phase with the voltage
![]()
Question 110.
In an AC circuit with capacitor only, if the j frequency of the signal is zero, then the j capacitive reactance is : .
(a) infinity
(b) zero
(c) finite maximum
(d) finite minimum
Answer:
(a) infinity
Question 111.
In step-up transformer the output voltage is 11 kV and the input voltage is 220 V. The ratio of number of turns of secondary to primary is:
(a) 20 : 1
(b) 22 : 1
(c) 50 : 1
(d) 1 : 50
Answer:
(c) 50 : 1
Question 112.
The reactance offered by 300 mH inductor to an AC supply of frequency 50 Hz is:
(a) 1046 Ω
(b) 94.2 Ω
(c) 9420 Ω
(d) 104.6 Ω
Answer:
(b) 94.2 Ω
![]()
Question 113.
The rms value of an AC. voltage with a peak value of 311 V is:
(a) 110 V
(b) 220 V
(c) 50 V
(d) 70.7 V
Answer:
(b) 220 V
Question 114.
In a transformer, eddy current loss is minimised by using:
(a) laminated core made of Mumetal
(b) laminated core made of stelloy
(c) shell type core
(d) thick copper wires
Answer:
(b) laminated core made of stelloy
Question 115.
A power of 11,000 W is transmitted at 220 V. The current through line wires is :
(a) 50 A
(b) 5 A
(c) 500 A
(d) 0.5 A
Answer:
(a) 50 A
![]()
Question 116.
The dimensional formula of impedance is:
(a) ML2T-3I-2
(b) M-1L2T3I2
(c) M-1 L-2T-3I2
(d) MLT-2I-1
Answer:
(a) ML2T-3I-2
Hint:
|Z| = \(\frac{V}{I}=\frac{M L^{2} T^{-3} I^{-1}}{I}\)
= [ML2T-3I-2] .
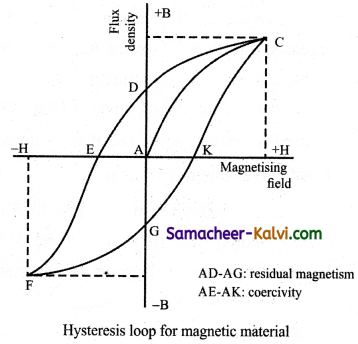
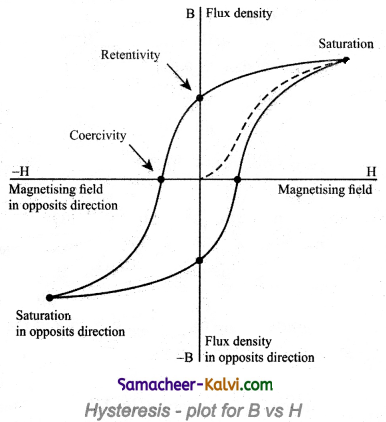
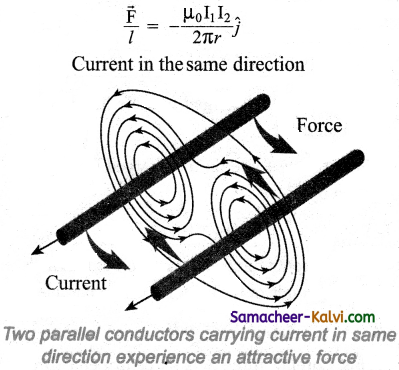
 Rectangular coil placed in a magnetic field
Rectangular coil placed in a magnetic field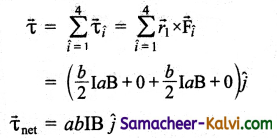
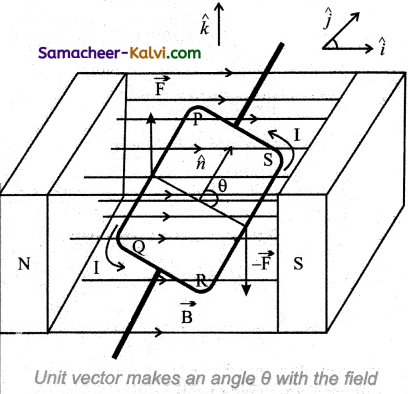
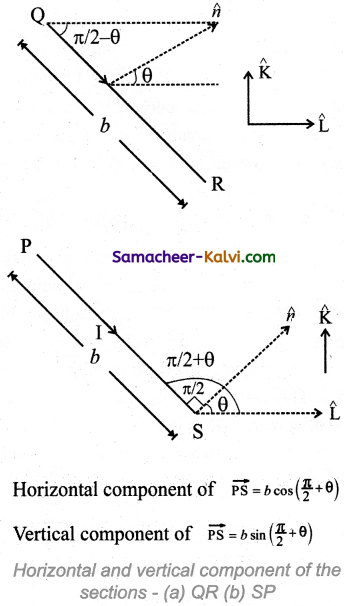
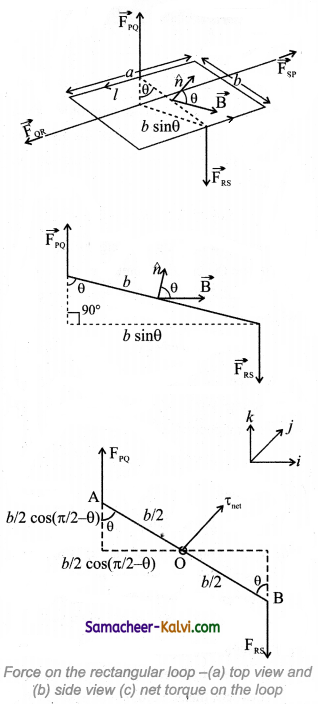
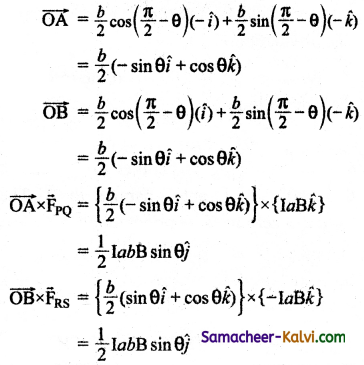
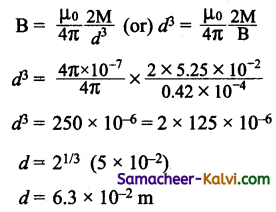
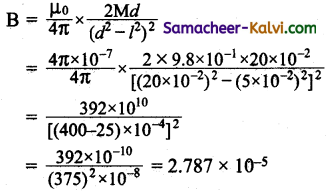
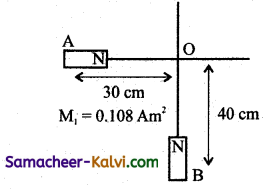
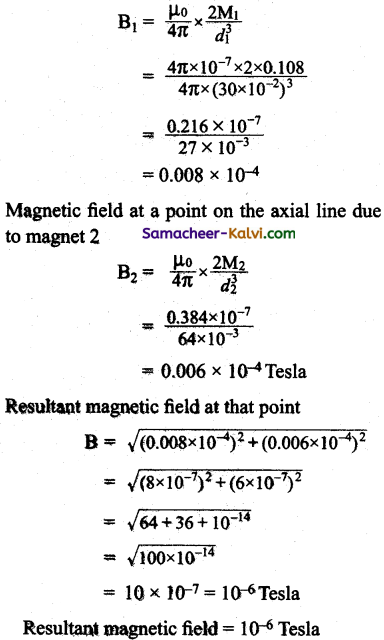
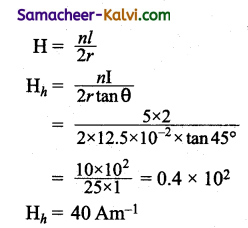
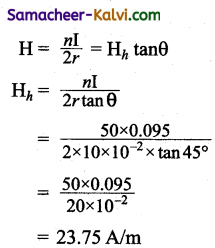
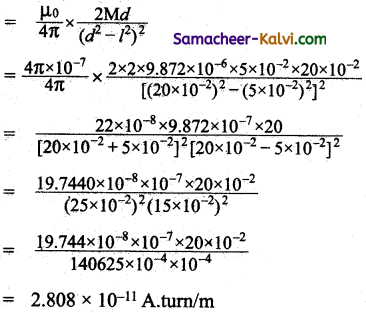
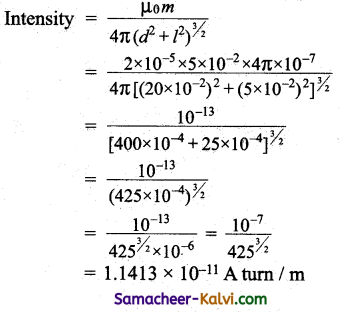
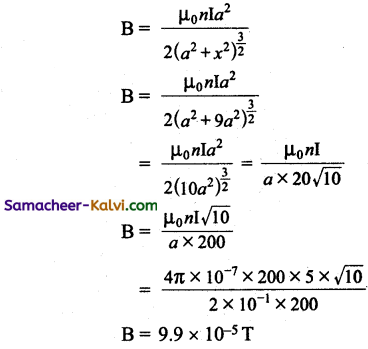
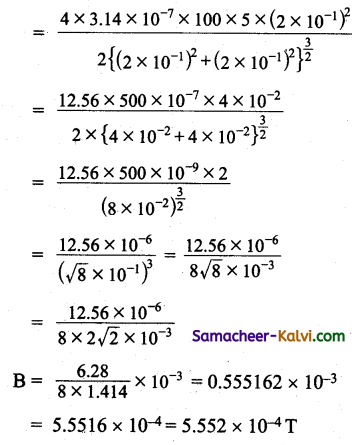
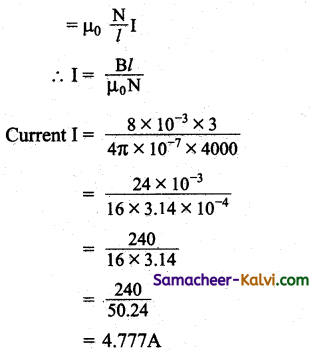
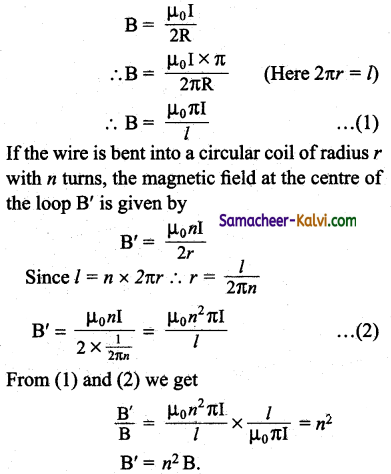
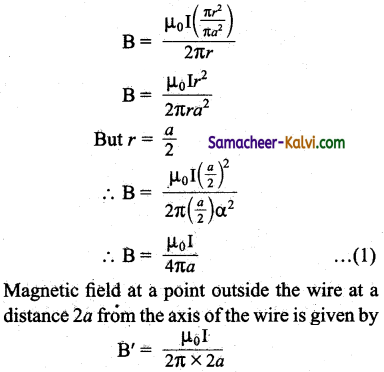
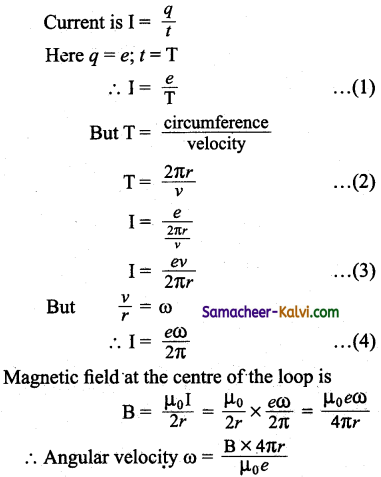
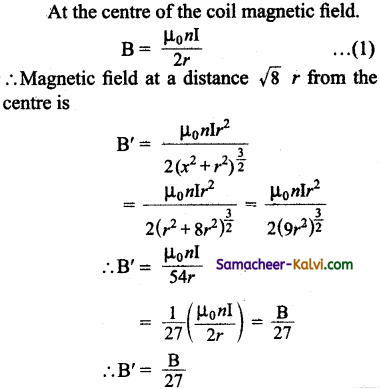
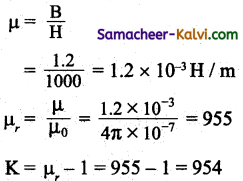
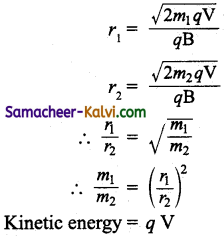
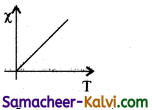
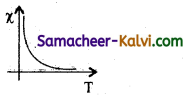
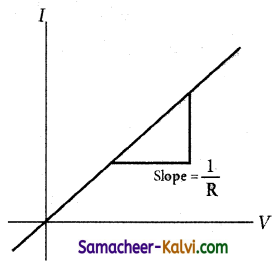
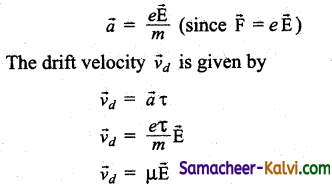
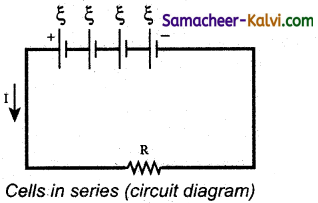
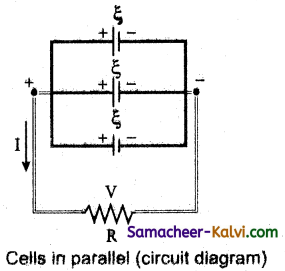
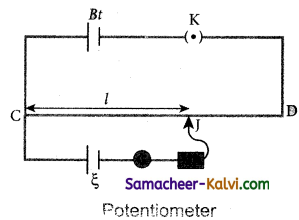
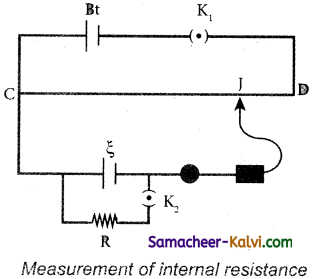
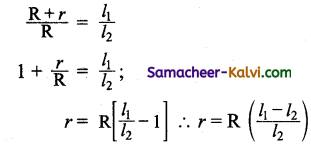
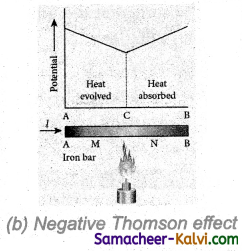
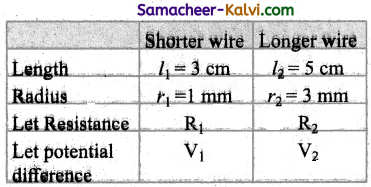
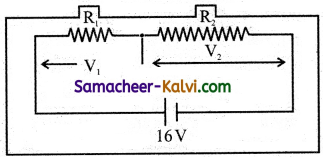
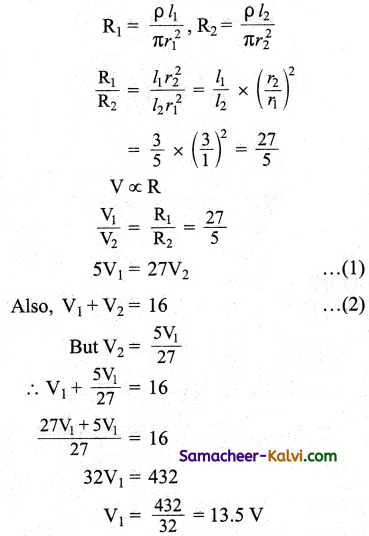
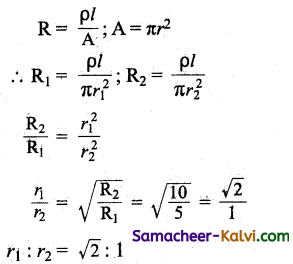
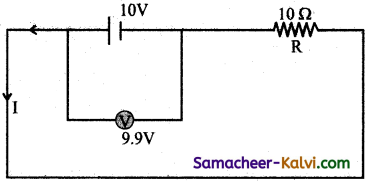
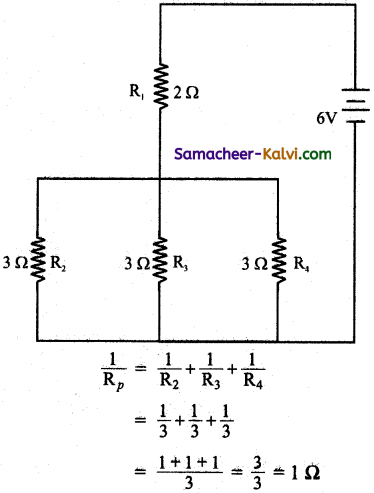
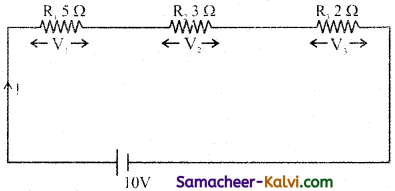
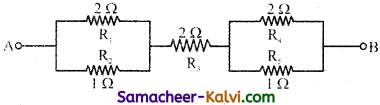
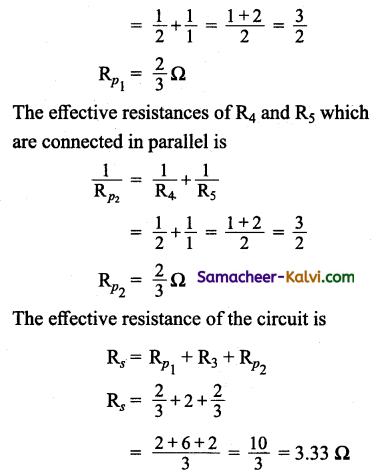
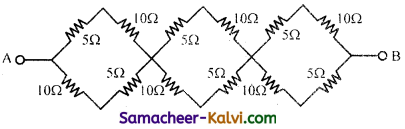
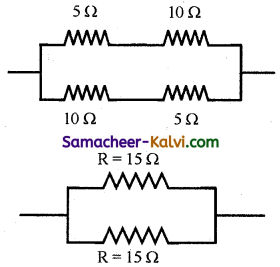

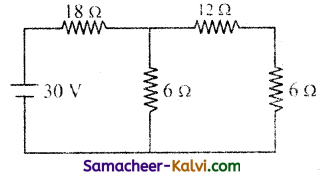
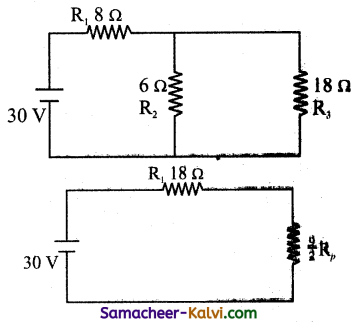
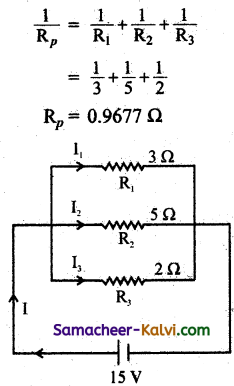
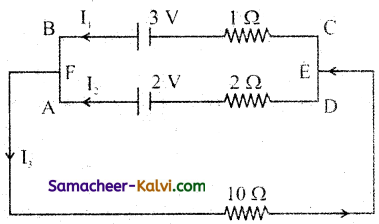
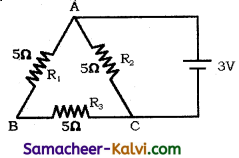
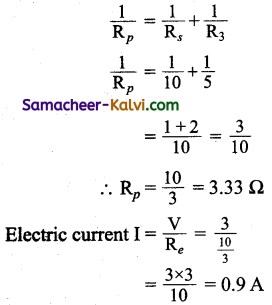
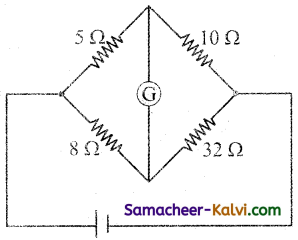
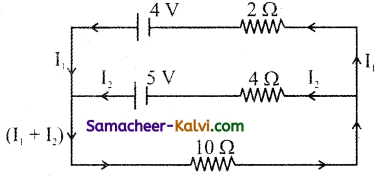
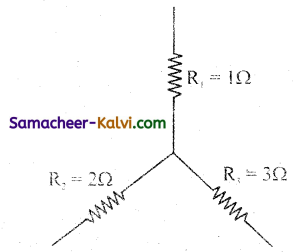
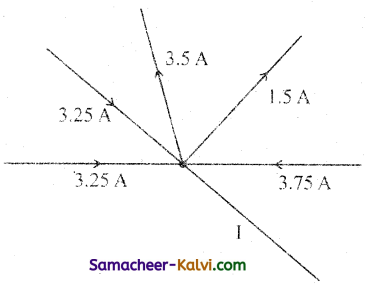
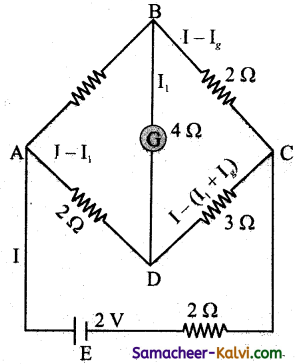
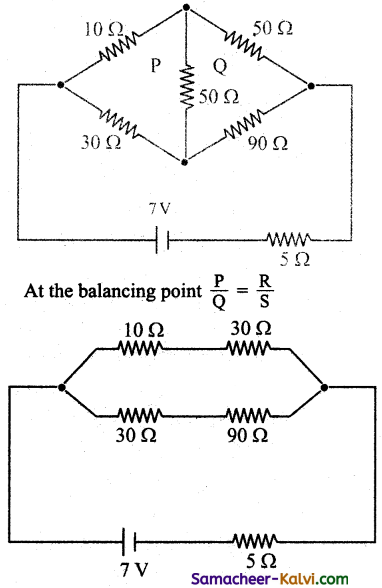
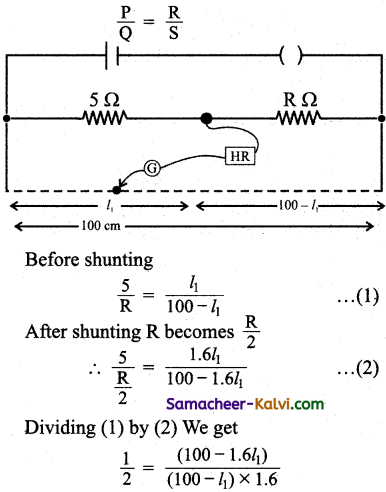

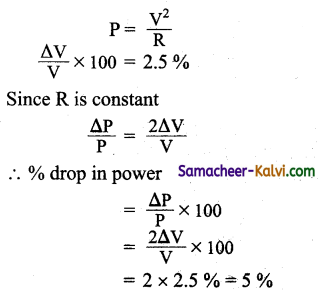
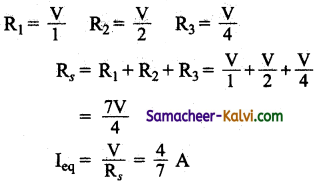
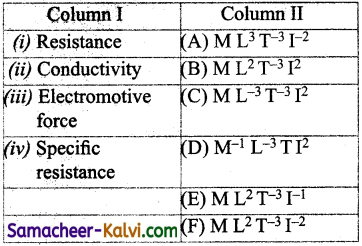
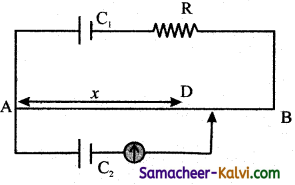
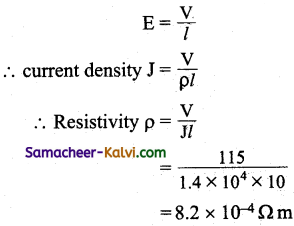
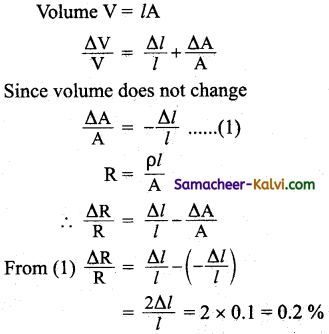
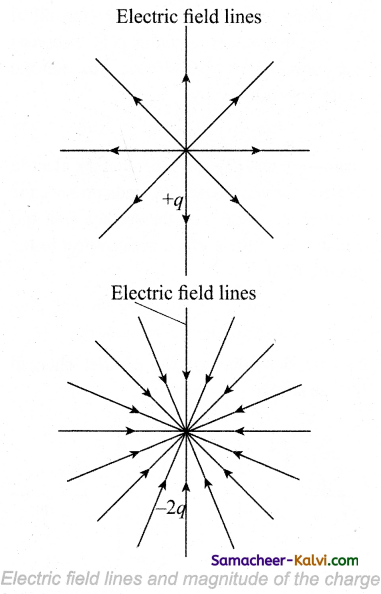
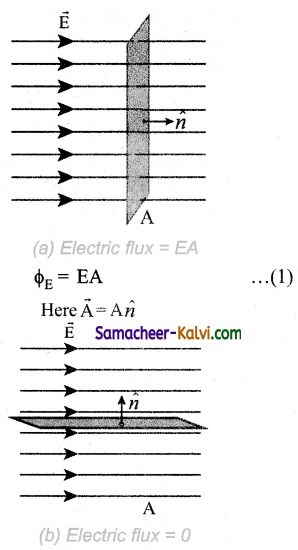
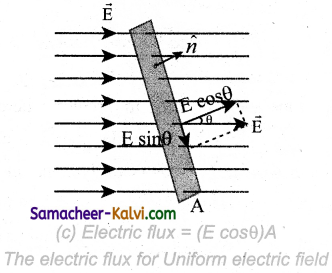
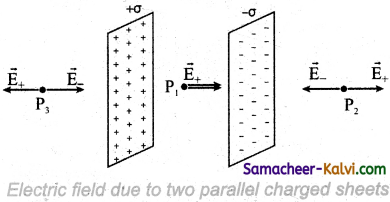
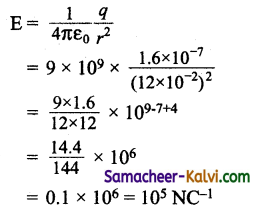
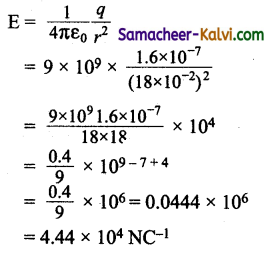
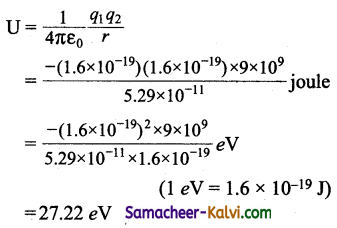
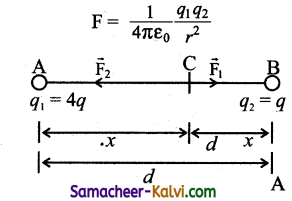
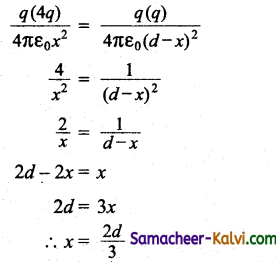
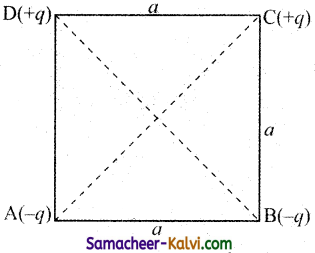
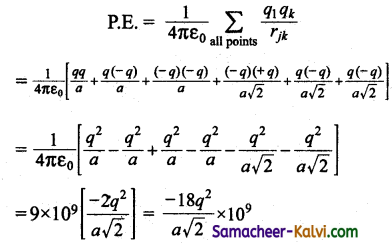
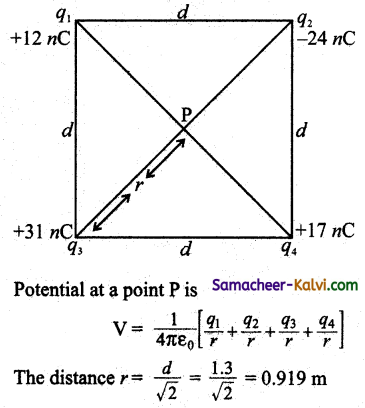
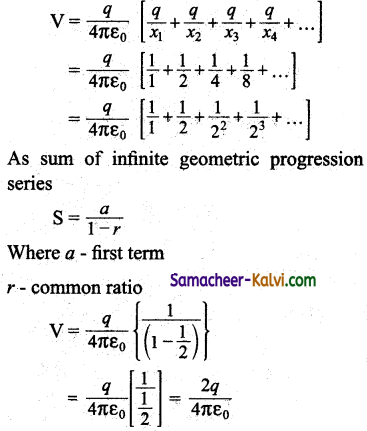
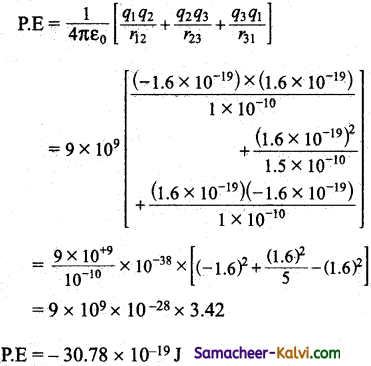
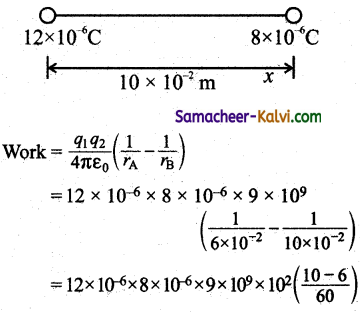
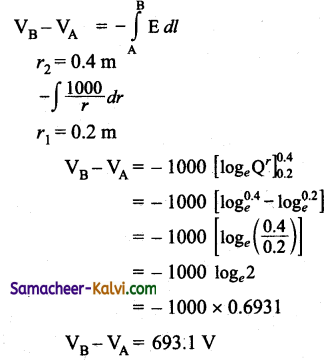
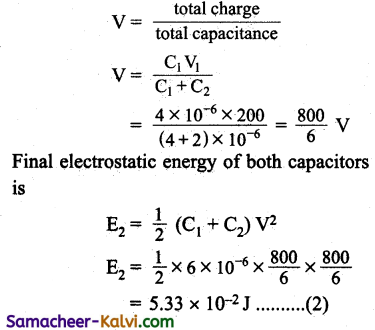
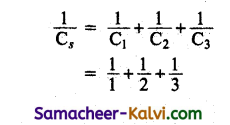
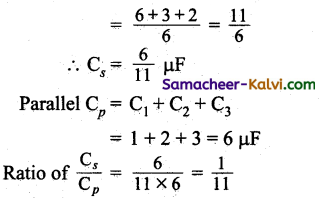
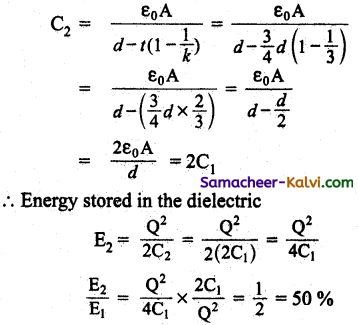
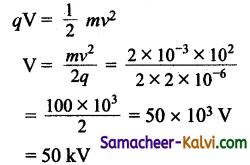
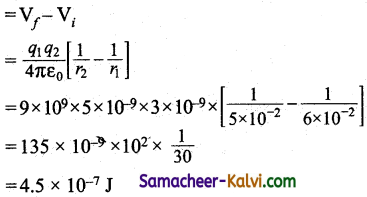
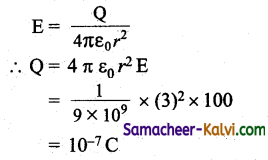
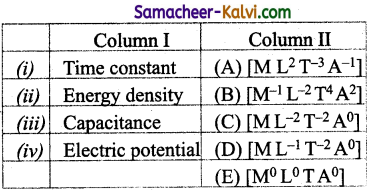







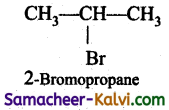
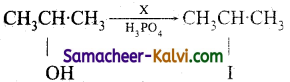
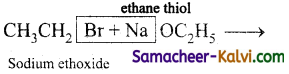 →
→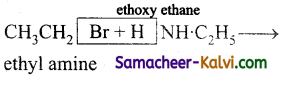 →
→
 →
→
 →
→
 →
→ →
→
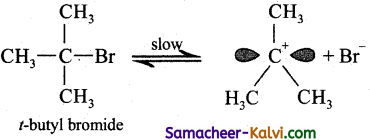
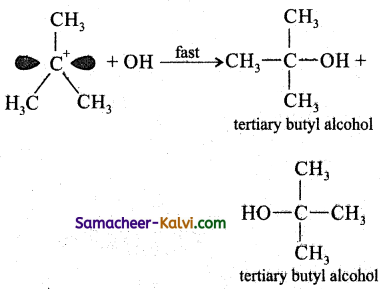
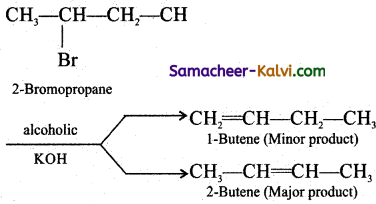
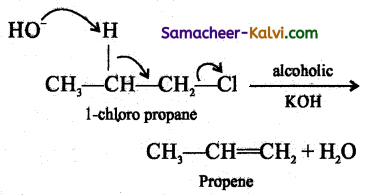

 is:
is:

 CH3Cl (methyl chloride) + HCl
CH3Cl (methyl chloride) + HCl


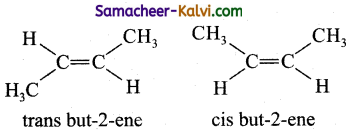
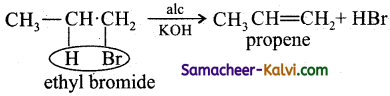
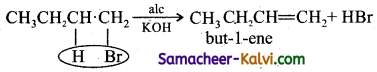
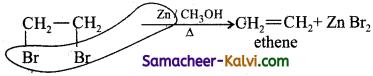

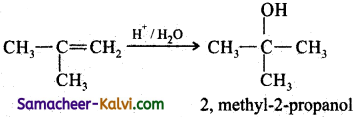

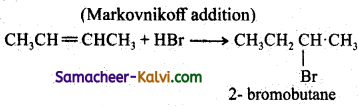
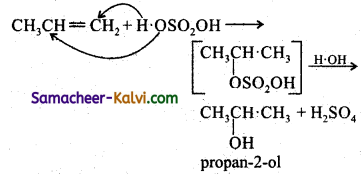
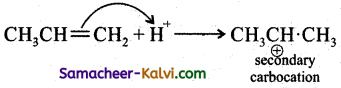



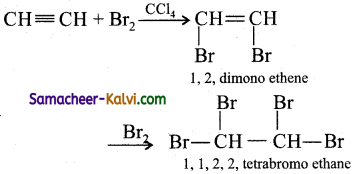
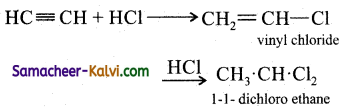
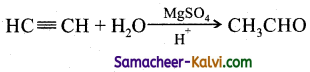
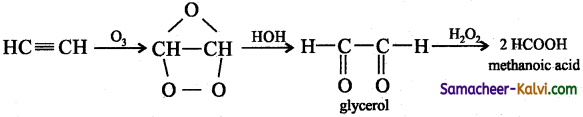




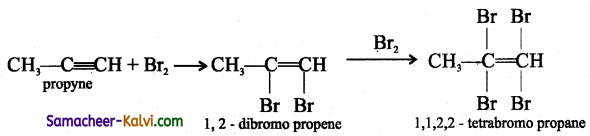
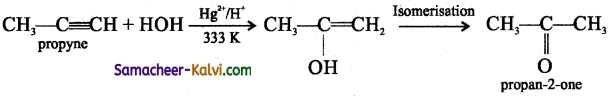
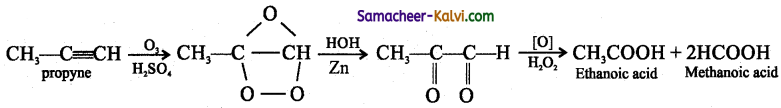
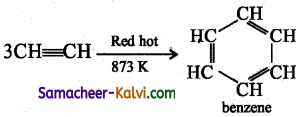





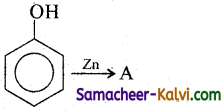
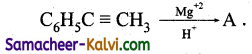
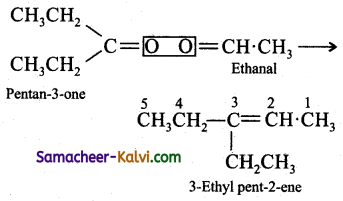
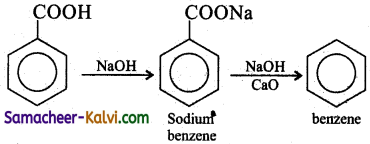
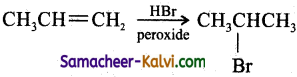
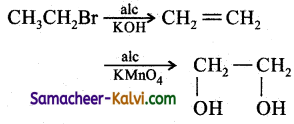
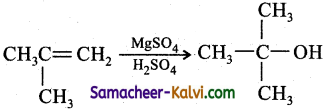
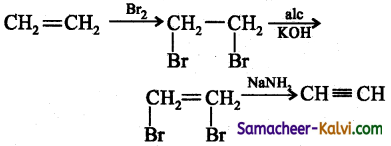
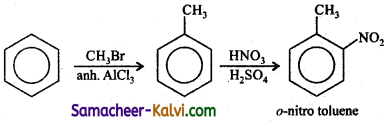
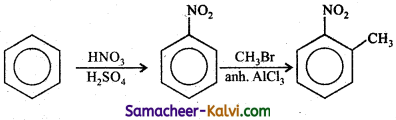
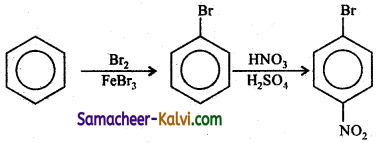
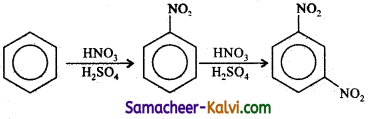
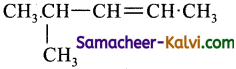
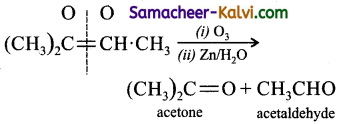
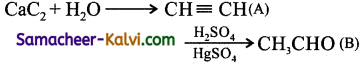
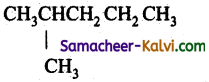
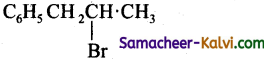

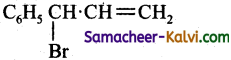
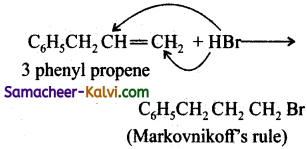
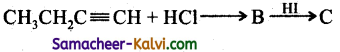




 is aromatic because it has:
is aromatic because it has:
 are chain isomers.
are chain isomers.



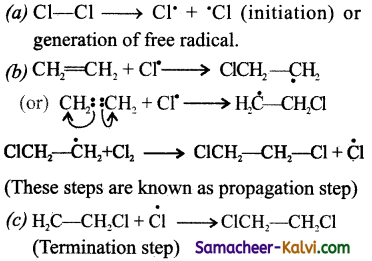
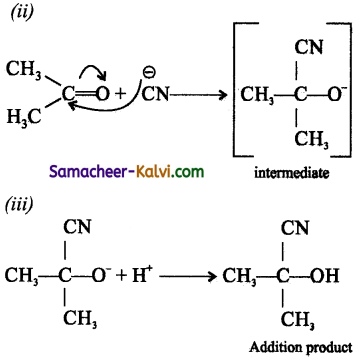
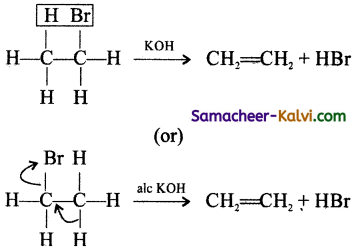
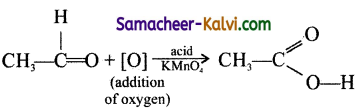
 bond undergoes heterolytic fission. The species formed are:
bond undergoes heterolytic fission. The species formed are:


 are the canonical structure of the compound.
are the canonical structure of the compound.
 group, adjacent to C = C, is involved hyper conjugation (σ electrons).
group, adjacent to C = C, is involved hyper conjugation (σ electrons).
 is an example of substitution reaction.
is an example of substitution reaction.



 group at the terminal carbon atom
group at the terminal carbon atom