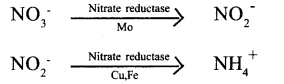
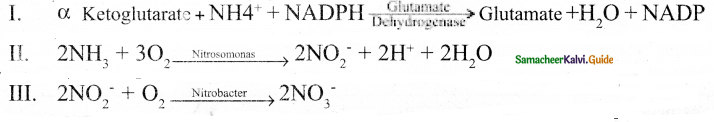Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Bio Botany Guide Pdf Chapter 14 Respiration Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 14 Respiration
11th Bio Botany Guide Respiration Text Book Back Questions and Answers
Part-I.
Question 1.
The number of ATP molecules formed by complete oxidation of one molecule of pyruvic acid is:
(a) 12
(b) 13
(c) 14
(d) 15
Answer:
(a) 12
![]()
Question 2.
During oxidation of two molecules of cytosolic NADH + H+, number of ATP molecules produced in plants are
a) 3
b) 4
c) 6
d) 8
Answer:
b) 4
Question 3.
The compound which links glycolysis and Krebs cycle is:
(a) succinic acid
(b) pyruvic acid
(c) acetyl CoA
(d) citric acid
Answer:
(c) acetyl CoA
Question 4.
Assertion (A): Oxidative phosphorylation takes place during the electron transport chain in mitochondria.
Reason (R): Succinyl Co A is phosphorylated into succinic acid by substrate phosphorylation.
a) A and R is correct. R is correct explanation of A
b) A and R is correct but R is not the correct explanation of A,
c) A is correct but R is wrong
d) A and R is wrong
Answer:
c) A is correct but R is wrong
![]()
Question 5.
Which of the following reaction is not involved in Krebs cycle.
(a) Shifting of phosphate from 3C to 2C
(b) Splitting of Fructose 1,6 bisphosphate of into two molecules 3C compounds.
(c) Dephosphorylation from the substrates
(d) All of these
Answer:
(d) All of these
Question 6.
What are enzymes involved in phosphorylation and dephosphorylation reactions in EMP pathway?
Answer:
(i) Enzymes involved in phosphorylation are
a) Hexokinase and phospnofructio kinase.
(ii) Enzymes involved in dephosphorylation are
a) Phosphoglycerate Kinase
b) Pyruvate Kinase
Question 7.
The respiratory quotient is zero in succulent plants. Why?
Answer:
In some succulent plants like Opuntia, Bryophyllum carbohydrates are partially oxidised to organic acid, particularly malic acid without the corresponding release of CO2 but O2 is consumed hence the RQ value will be zero.
Question 8.
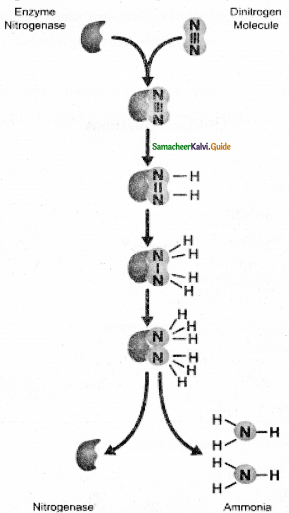
Explain the reactions taking place in mitochondrial inner membrane.
Answer:
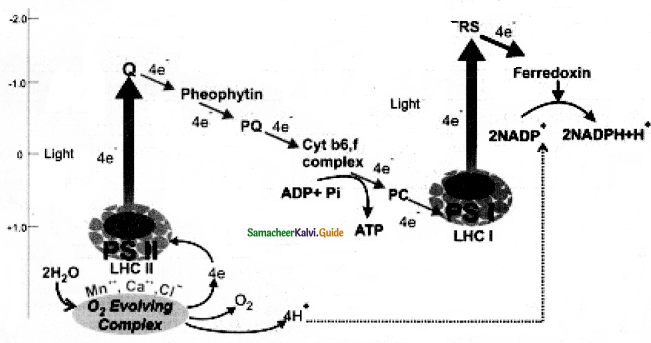
Electron and hydrogen (proton) transport takes place across four multiprotein complexes (I-IV). They are.
1. Complex-I (NADH dehydrogenase).
It contains a flavoprotein (FMN) and associated with non-heme iron Sulphur protein (Fe-S). This complex is responsible for passing electrons and protons from mitochondrial NADFI (Internal) to Ubiquinone (UQ)
NADH+H+UQ ⇌ NAD–+UQH2
2. In plants, an additional NADH dehydrogenase (External) complex is present on the outer surface of inner membrane of mitochondria which can oxidise cytosolic NADH + H+.
Ubiquinone (UQ) or Coenzyme Quinone (CoQ) is a small, lipid-soluble electron, proton carrier located within the inner membrane of mitochondria).
3. Complex-II (succinic dehydrogenase) It contains FAD flavoprotein is associated with non-heme iron Sulphur (Fe-S) protein. This complex receives electrons and protons from succinate in Kerbs cycle and is converted into fumarate and passes to ubiquinone.
Succinate + UQ Fumaraic LQH2
4. Complex-III (Cytochrome bcj complex) This complex oxidises reduced ubiquinone (ubiquinol) and transfers the electrons through Cytochrome bc1 Complex (Iron Sulphur centci bcl complex) to cytochrome c.
5. Complex IV (Cytochrome c oxidase) Complex IV is the terminal oxidase and brings about the reduction of 1/2 O2 to H2O. TWO protons are needed to form a molecule of H2O (terminal oxidation).
![]()
Question 9.
What is the name of alternate way to glucose breakdown? Explain the process in involved in it?
Answer:
- Pentose phosphate pathway is the alternate pathway for breakdown of glucose.
- Pentose phosphate pathway was described by Warburg, Dickens and Lipmami (1938).
- It is also known as Hexose monophosphate shunt (HMP shunt) or Direct oxidative phase and non – oxidative phase.
- The oxidative phase convert six molecules of six carbon Glucose 6 phosphate to 6 molecules of five-carbon sugar Ribulose – 5 Phosphate with loss of 6CO2 and generation of 12 NADPH + H+
Non oxidative pathway convert Ribulose – 5 – phosphate molecules to various intermediates such as
Ribose – 5 – phosphate (5C)
Xylulose – 5 – phosphate (5C)
Glyceraldehyde – 3 – phosphate (3C)
Sedoheptulose – 7 – phosphate (7C) and
Erythrose – 4 – phosphate (4C)
Finally five molecules of glucose 6 – phosphate is regenerated
6 x Glucose – 6 – phosphate + 12NADP+ + 6H2O
↓
5 x glucose – 6 – phosphate + 6CO2 + Pi + 12 NADPH + 2H+
The net result of complete oxidation of one glucose – 6 – phosphate yield 6CO2 and 12 NADPH + H+. The oxidative pentose phosphate pathway is controlled by glucose – 6 – phosphate dehydrogenase enzyme which is inhibited by high ratio of NADPH to NADP+.
Question 10.
How will you calculate net products of one sucrose molecule upon complete oxidation during aerobic respiration as per recent view?
Answer:
When the cost of transport of ATPs from the matrix into the cytosol is considered, the number will be 2.5 ATPs for each NADH + H+ and 1.5 ATPs for each FADH2 oxidized during the electron transport system. Therefore, in plant cells net yield of 30 ATP molecules for complete aerobic oxidation of one molecule of glucose. But in those animal cells (showing malate shuttle mechanism) net yield will be 32 ATP molecules. Since the sucrose molecule gives, two molecules of glucose and net ATP in plant cell will be 30 × 2 = 60.
In an animal cell, it will be 32 × 2 = 64.
![]()
Part-II.
11th Bio Botany Guide Respiration Additional Important Questions and Answers
I. Choose The Correct Answer
Question 1.
The term respiration was coined by:
(a) Lamark
(b) Kerb
(c) Pepys
(d) Blackman
Answer:
(c) Pepys
Question 2.
Black man divided respiration into floating respiration and protoplasmic respiration based on respiratory ………..
a) Quotient
b) respiratory reaction
c) respiratory pathway
d) substrate
Answer:
d) substrate
Question 3.
The discovery of ATP was made by:
(a) Lipman
(b) Hans Adolt
(c) Warburg
(d) Karl Lohman
Answer:
(d) Karl Lohman
Question 4.
The type of respiration which is rare and liberates toxic ammonia
a) Protoplasmic respiration
b) floating respiration
c) Aerobic respiration
d) Anaerobic respiration
Answer:
a) Protoplasmic respiration
![]()
Question 5.
On hydrolysis, one molecule of ATP releases energy of:
(a) 8.2 Kcal
(b) 32.3 kJ
(c) 7.3 Kcal
(d) 7.8 Kcal
Answer:
(c) 7.3 Kcal
Question 6.
To convert Kcal to KJ multiply by 4.18(100 Kcal=418 KJ) calculate the amount KJ energy for 7.3 Kcal
a) 30.6 KJ
b) 32.06 KJ
c) 29.03 KJ
d) 5.01 KJ
Answer:
a) 30.6 KJ
Question 7.
Identify the link reaction:
(a) conversion of glucose into pyruvic acid
(b) conversion of glucose into ethanol
(c) conversion of acetyl CoA into CO2 and water
(d) conversion of pyruvic acid into acetyl coenzyme – A
Answer:
(d) conversion of pyruvic acid into acetyl coenzyme – A
Question 8.
Food materials like carbohydrate, fat and proteins are completely oxidised into CO2, H2O and energy in ………………. respiration
a) anaerobic
b) aerobic
c) Bacterial respiration
d) Facultative
Answer:
b) aerobic
Question 9.
Kreb’s cycle is a:
(a) catabolic pathway
(b) anabolic pathway
(c) amphibolic pathway
(d) hydrolytic pathway
Answer:
(c) amphibolic pathway
![]()
Question 10.
Which process is occur in yeast and some bacteria and 2 ATP molecules are produced during this process.
a) Anaerobic respiration
b) aerobic respiration
c) mixed fermentation
d) CAC cycle
Answer:
a) Anaerobic respiration
Question 11.
The oxidation of one molecule of NADH + H+ gives rise to:
(a) 2 ATP
(b) 3 ATP
(c) 4 ATP
(d) 2.5 ATP
Answer:
(b) 3 ATP
Question 12.
Net product in Glycolysis are
a) 4ATP and 2NADH + H+
b) 2 ATP and 2NADH + H+
c) 6ATP
d) 24 ATP
Answer:
b) 2ATP and 2NADH + H+
Question 13.
Cyanide acts as electron transport chain inhibitor by preventing:
(a) synthesis of ATP from ADP
(b) flow of electrons from NADH + H+
(c) flow of electrons from cytochrome a3 to O2
(d) oxidative phosphorylation
Answer:
(c) flow of electrons from cytochrome a3 to O2
Question 14.
Which is the raw material for the formation of chlorophylls, cytochrome, phytochrome and pyrrole substance
a) acetyl COA
b) Pyruvic acid
c) Malic acid
d) Succinyl COA
Answer:
d) Succinyl COA
Question 15.
End products of fermentation in yeast is:
(a) pyruvic acid and CO2
(b) lactic acid and CO2
(c) ethyl alcohol and CO2
(d) mixed acid and CO2
Answer:
(c) ethyl alcohol and CO2
![]()
Question 16.
Which cycle is considered as amphibolic pathway.
a) Calvin cycle
b) Glycolysic
c) ETS chain
d) Kreb cycle
Answer:
d) Kreb cycle
Question 17.
The external factors that affect respiration are:
(a) temperature, insufficient O2 and amount of protoplasm
(b) temperature, insufficient O2 and high concentration of CO2
(c) temperature, high concentration of CO2 and respiratory substrate
(d) temperature, high concentration of CO2 and amount of protoplasm
Answer:
(b) temperature, insufficient O2, and high concentration of CO2
Question 18.
The complex system responsible for passing electrons and protons from mitochondria to ubiquinone is ………………..
a) Complex I
b) Complex II
c) Complex III
d) Complex IV
Answer:
a) Complex I
Question 19.
The oxidative pentose phosphate pathway is controlled by the enzyme:
(a) glucose, 1, 6 diphosphate dehydrogenase
(b) glucose 6 phosphate dehydrogenase
(c) fructose – 6 – phosphate dehydrogenase
(d) none of the above
Answer:
(b) glucose 6 phosphate dehydrogenase
Question 20.
Which are the high energy phosphate groups in ATP
a) adenine
b) Pentose sugar
c) Last two phosphate group
d) First two phosphate group
Answer:
c) Last two phosphate group
![]()
Question 21.
In-plant tissue erythrose is used for the synthesis of:
(a) Erythromycin
(b) Xanthophyll
(c) Erythrocin
(d) Anthocyanin
Answer:
(d) Anthocyanin
Question 22.
How many ATP molecules are produced when a molecule of glucose undergo fermentation?
a) TwoATPs
b) Six ATPs
c) Eight ATPs
d) one ATP
Answer:
a) Two ATPs
Question 23.
Identify the electron transport inhibitor:
(a) phosphoenol
(b) dinitrophenol
(c) xylene
(d) indol acetic acid
Answer:
(b) dinitrophenol
Question 24.
Enzymatic reaction for partial oxidation of glucose in the absence of oxygen is present in
a) Some Bacteria
b) Yeast fungus
c) A and B
d) Bryophytes
Answer:
c) A and B
![]()
Question 25.
Cyanide resistant respiration is known to generate heat in thermogenic tissues as high as:
(a) 35° C
(b) 38° C
(c) 40° C
(d) 51° C
Answer:
(d) 51° C
Question 26.
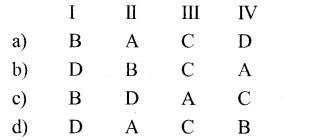
Match the Column I with the enzyme responsible for its production in column II
Answer:
| Column I | Column II |
| A. Citric acid | 1. Hexose Kinase |
| B. Glucose 6-Phosphate | 2. Lactate dehydrogenase |
| C. Lactic acid | 3. Pyruvate dehydrogenase |
| D. Acetvl CO.A | 4. Citric acid Synthetase |

Answer:
a) A-4,B -1,C-2,D-3.
![]()
Question 27.
Which one is wrongly matched
| Column I | Column II |
| A. NADH +H+ | Three ATP |
| B. Glycolysis | Twenty four ATP |
| C. FAD | Two ATP |
| D. Cytoplasmic NADH+H+ | Two ATP |
Answer:
B. Glycolysis – Twenty four ATP
II. 2 Marks Questions
Question 1.
Define respiration?
Answer:
Respiration is a biological process in which oxidation of various food substances like carbohydrates, proteins, and fats take place and as a result of this, energy is produced where O2 is taken in and CO2 is liberated.
Question 2.
Name some High energy compounds present in a cell
Answer:
- ATP → Adenosine Tri Phosphate
- GTP → Guanosine Tri Phosphate
- UTP → Uridine Tri Phosphate
![]()
Question 3.
What do you understand by compensation of point?
Answer:
The point at which CO2 released in respiration is exactly compensated by CO2 fixed in photosynthesis that means no net gaseous exchange takes place, it is called the compensation point.
Question 4.
What is Anaerobic respiration? What are its steps?
Answer:
- In the absence of molecular oxygen-glucose is incompletely degraded into either ethyl alcohol (or) Lactic acid.
- It includes two steps (i) Glycolysis (ii) Fermentation
Question 5.
What is anaerobic respiration?
Answer:
In the absence of molecular oxygen-glucose is incompletely degraded into either ethyl alcohol or lactic acid. It includes two steps:
- Glycolysis
- Fermentation
![]()
Question 6.
What is Link reaction?
Answer:
In aerobic respiration, Conversion of Pyruvic acid into acetyl coenzyme – A in the mitochondrial matrix with two molecules of NADH + H+ and 2 CO2. This is called Link reaction (or) transition reaction.
Question 7.
Who is Sir Hans Adolf Krebs?
Answer:
Sir Hans Adolf Krebs was born in Germany on 25th August 1900. He was awarded Nobel Prize for his discovery of Citric acid cycle in Physiology in 1953.
Question 8.
Why Kreb cycle is called as citric acid cycle (or) Tri Carboxylic acid Cycle?
Answer:
- TCA cycle starts with condensation of acetyl COA with oxaloacetate in the presence of water to yield Citri acid (or) Citrate.
- So it is also known as citric acid cycle (or) Tricarboxylic acid cycle.
![]()
Question 9.
Mention the role of NADH dehydrogenase enzyme in the electron transport system.
Answer:
NADH dehydrogenase contains a flavoprotein (FMN) and associated with non – heme iron Sulphur protein (Fe – S). This complex is responsible for passing electrons and protons from mitochondrial NADH (Internal) to Ubiquinone (UQ).
Question 10.
Which cycle is amphibolic pathway? Why? The Krebs cycle is called an amphibolic pathway.
Answer:
- Kreb cycle is primarily a catabolic pathway Later it is an anabolic pathway too.
- Hence it is called amphibolic pathway.
Question 11.
Mention any two electron transport chain inhibitors.
Answer:
Two electron transport chain inhibitors:
- 2, 4 DNP (Dinitrophenol) – It prevents synthesis of ATP from ADP, as it directs electrons from Co Q to O2.
- Cyanide – It prevents flow of electrons from Cytochrome a3 to O2.
Question 12.
How many ATP molecules are produced in aerobic respiration present in plants?
Answer:
In aerobic respiration net gain of 36 ATP molecules produced in complete oxidation of glucose.
Question 13.
What are the significances of Respiratory Quotient?
Answer:
The significances of Respiratory Quotient:
- RQ value indicates which type of respiration occurs in living cells, either aerobic or anaerobic.
- It also helps to know which type of respiratory substrate is involved.
Question 14.
Who was awarded the Nobel Prize for coupling of oxidation and phosphorylation in mitochondria?
Answer:
Peter Mitchell, a British Biochemist received Nobel Prize for chemistry in 1978.
Question 15.
Mention any two industrial uses of alcoholic fermentation.
Answer:
Two industrial uses of alcoholic fermentation:
- In bakeries, it is used for preparing bread, cakes, biscuits.
- In beverage industries for preparing wine and alcoholic drinks.
![]()
Question 16.
Define mixed acid fermentation.
Answer:
- Formation of Lactic acid, ethanol, formic acid and gases like CO2 and H2 from pyruvic acid.
- eg. Enterobacteriaceae.
Question 17.
Mention any two internal factors, that affect the rate of respiration in plants.
Answer:
Two internal factors, that affect the rate of respiration in plants:
- The amount of protoplasm and its state of activity influence the rate of respiration.
- The concentration of respiratory substrate is proportional to the rate of respiration.
Question 18.
Why microorganisms respire an anaerobically?
Answer:
- Bacteria are prokaryotes and they are devoid of membrane-bound organelle mitochondria.
- So they are respire anaerobically.
Question 19.
Write down any two significance of the pentose phosphate pathway.
Answer:
Two significance of pentose phosphate pathway:
- HMP shunt is associated with the generation of two important products.
- Coenzyme NADPH generated is used for reductive biosynthesis and counter damaging the effects of oxygen-free radicals.
Question 20.
Complete the following Picture.
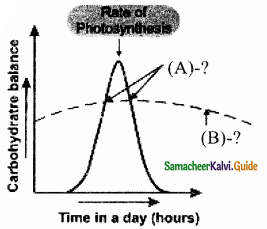
Answer:
A. Compensation point
B. Rate of Respiration
![]()
Question 21.
Write the missing A and B.
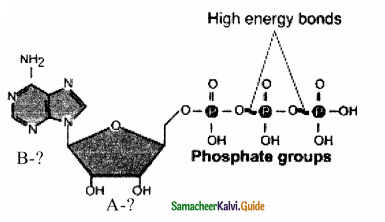
Answer:
A. Ribose
B. Adenine
III. 3 Mark Questions
Question 1.
In the biosphere how do plants and animals are complementary systems, which are integrated to sustain life?
Answer:
In plants, oxygen enters through the stomata and it is transported to cells, where oxygen is utilized for energy production. Plants require carbon dioxide to survive, to produce carbohydrates, and to release oxygen through photosynthesis, these oxygen molecules are inhaled by humans through the nose, which reaches the lungs where oxygen is transported through the blood and it reaches cells. Cellular respiration takes place inside or the cell for obtaining energy.
Question 2.
What is Respiration?
Answer:
- Breaking of C-C bonds of complex organic compounds through oxidation within the cells.
- The energy released during respiration is stored in the form of ATP and heat is liberated.
- It occurs in all the living cells of organisms.
![]()
Question 3.
What are the factors associated with the compensation point in respiration?
Answer:
The two common factors associated with compensation points are CO2 and light. Based on this there are two types of compensation points. They are the CO2 compensation point and light compensation point. C3 plants have compensation points ranging from 40 – 60 ppm (parts per million) CO2 while those of C4 plants range from 1 – 5 ppm CO2.
Question 4.
Differentiate floating respiration and protoplasmic respiration.
Answer:
| Floating respiration | Protoplasmic respiration |
| Carbohydrate (or) fat (or) organic acid serves as a respiratory substrate | Whereas protein is a respiratory substrate. |
| It is a common mode of respiration and does not produce any toxic product. | It is rare and liberates toxic ammonia. |
Question 5.
What is a redox reaction?
Answer:
NAD+ + 2e– + 2H+ → NADH + H+
FAD + 2e– + 2H+ → FADH2
When NAD+ (Nicotinamide Adenine Dinucleotide – oxidized form) and FAD (Flavin Adenine Dinucleotide) pick up electrons and one or two hydrogen ions (protons), they get reduced to NADH + H+ and FADH2 respectively. When they drop electrons and hydrogen off they go back to their original form. The reaction in which NAD+ and FAD gain (reduction) or lose (oxidation) electrons are called redox reactions (Oxidation-reduction reactions). These reactions are important in cellular respiration.
Question 6.
Define ETS (or) Electron transport chain (or) What is the importance of ETS and oxidative Phosphorylation in respiration.
Answer:
- Electron transport chain and oxidative phosphorylation remove hydrogen atoms from the products of glycolysis, link reaction, and Kreb cycle.
- It releases water molecule with energy in the form of ATP molecules in the mitochondrial inner membrane.
Question 7.
Mention the significance of Kreb’s cycle.
Answer:
The significance of Kreb’s cycle:
- TCA cycle is to provide energy in the form of ATP for metabolism in plants.
- It provides carbon skeleton or raw material for various anabolic processes.
- Many intermediates of the TCA cycle are further metabolised to produce amino acids, proteins, and nucleic acids.
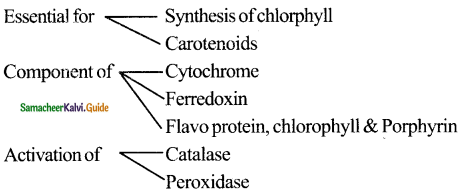
- Succinyl CoA is the raw material for the formation of chlorophylls, cytochrome, phytochrome, and other pyrrole substances.
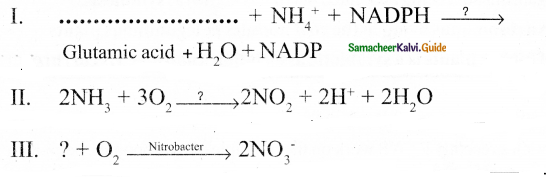
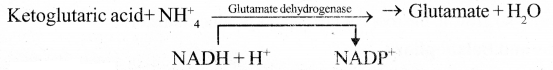
- α – ketoglutarate and oxaloacetate undergo reductive amination and produce amino acids.
- It acts as a metabolic sink which plays a central role in intermediary metabolism.
![]()
Question 8.
Write the differences between ubiquinone and Cytochrome C.
Answer:
| Ubiquinone | Cytochrome C |
| It is a small, lipid-soluble electron, proton carrier located within the inner membrane of mitochondria. | It is a small protein attached to the outer surface of inner membrane of mitochondria |
| It is associated with ETS – complex I | It is associated with ETS – complex IIII |
Question 9.
Write down the characteristic of Anaerobic respiration.
Answer:
The characteristic of Anaerobic respiration:
- Anaerobic respiration is less efficient than aerobic respiration.
- A limited number of ATP molecules is generated per glucose molecule.
- It is characterized by the production of CO2 and is used for Carbon fixation in photosynthesis.
Question 10.
RQ will be less than one in Red colour Parts Present in Plants? Why?
Answer:
- Red colour parts present in plants is due to the presence of anthocyanin
- Synthesis of anthocyanin require more O2 than CO2 evolved.
- So RQ will be less than one.
Question 11.
Write down any three external factors, that affect respiration in plants.
Answer:
Three external factors, that affect respiration in plants:
- The optimum temperature for respiration is 30°C. At low temperatures and very high temperatures rate of respiration decreases.
- When a sufficient amount of O2 is available the rate of aerobic respiration will be optimum and anaerobic respiration is completely stopped. This is called the Extinction point.
- The high concentration of CO2 reduces the rate of respiration.
Question 12.
Define Lactic acid fermentation.
Answer:
Formation of Lactic acid from pyruvic acid is Lactic acid fermentation.
Eg. Bacillus bacteria, fungi, muscles of vertebrates.

![]()
Question 13.
What is the Pentose phosphate pathway?
Answer:
- It is an alternate pathway for break down of glucose.
- It takes place in the cytoplasm of mature plant cells.
- In this pathway glucose 6 phosphate molecule is converted to Ribulose 5 phosphate with CO2 and NADPH– + H+.
Question 14.
How alcoholic beverages like beer and wine is made?
Answer:
- The conversion of pyruvate to ethanol takes place in malted barley and grapes through fermentation.
- Yeast Carryout this process under anaerobic conditions and this conversion increases ethanol concentration.
- If the concentration increases It’s toxic effect kills yeast cells and the left out is called beer and wine respectively.
IV. 5 Mark Questions
Question 1.
Ambulate the differences between aerobic and anaerobic respiration.
Answer:
| Aerobic respiration | Anaerobic respiration |
| 1. It occurs in all living cells of higher organisms. | It occurs yeast and some bacteria. |
| 2. It requires oxygen for breaking the respiratory substrate | Oxygen is not required for breaking the respiratory substrate. |
| 3. The end products are CO2 and H2O | The end products are alcohol and CO2 (or) lactic acid |
| 4. Oxidation of one molecule of glucose produces 36 ATP molecules | Only 2 ATP molecules are produced. |
| 5. It consists of four stages – glycolysis, link reaction, TCA cycle and electron transport chain. | It consists of two stages – glycolysis and fermentation. |
| 6. It occurs in cytoplasm and mitochondria | It occurs only in cytoplasm |
Question 2.
Draw the flow chart diagram For Glycolysis (or) EMP pathway.
Answer:
![]()
Question 3.
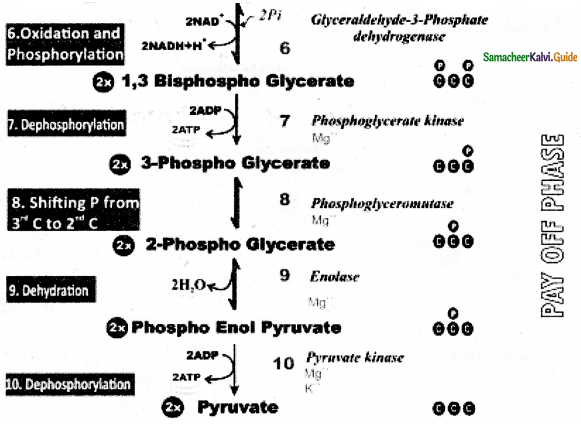
Explain the Pay – off phase of EMP Pathway of Glycolysis (or) Explain the oxidative phase of Glycolysis (or) Triose phase of Glycolysis.
Answer:
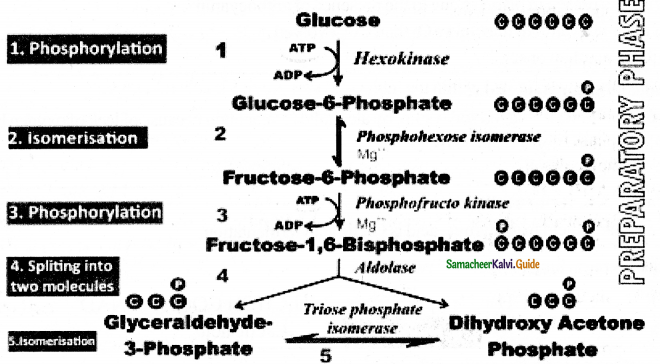
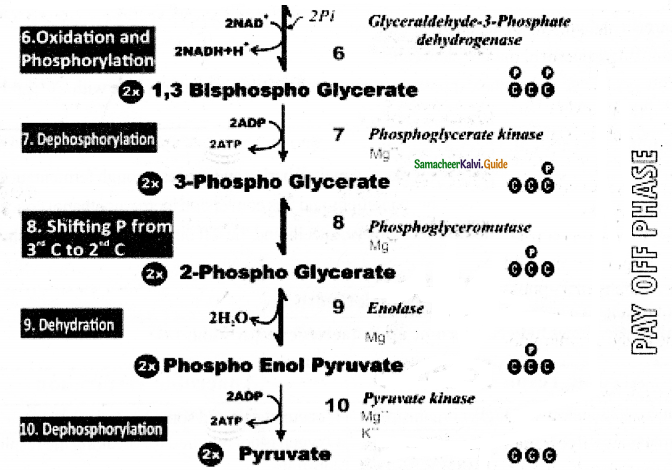
- Two molecules of glyceraldehyde 3 – phosphate oxidatively phosphorylated into two molecules of 1-3
bisphospho glycerate. - During this reaction 2 NAD+ is reduced to 2NADH+ H+ by glyceraldehyde 3-phosphate dehydrogenase.
- Further reactions are carried out by different enzymes at the end two molecules of pyruvate are produced.
- In this phase 4 ATPS are produced (at step 7 and step 10)
- Through Direct transfer of phosphate from substrate molecule to ADP and is converted into ATP is called substrate Phosphotylation. (or) Direct Phosphorylation (or) transphosphorylation.
- During the reaction (at step 9)2 phospo glycerate dehydrated into phosphoenol pyurvate, a water molecule is removed by the enzyme enolase.
- As a result enol group is formed within the molecule. This process is called Enolation.
Energy Budge of pay off phase:
- In the payoff phase totally 4 ATP and 2NADH + H+ molecules are produced.
- Since 2 ATP molecules are already consumed in the preparatory phase the net products in glycolysis are 2ATP and 2NADH + H+
Question 4.
Explain the preparatory phase of Glycolysis (or) EMP pathway (or) Describe the energonic phase phase of Glycolysis (or) EMP pathway. Describe the hexose phase of Glycolysis (or) EMP pathway.
Answer:
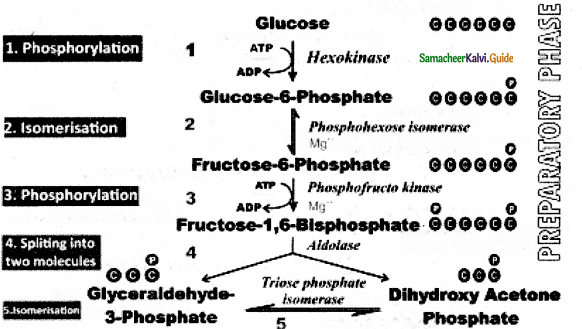
Glycolysis is a linear series of reactions in which 6- carbon glucose split into two molecules of 3 carbon pyruvic acid.
Preparatory phase:
- Glucose enters glycolysis which is the end product of photosynthesis.
- Glucose is phosphorylated into glucose 6 phosphate by the enzyme hexokinase and subsequent reactions are carried out by different enzymes.
- At the end of this phase fructose 1,6 – bisphote is cleaved into glyceraldehyde 3- phosphate and dihydroxyacetone phosphate by the enzyme aldolase.
- These two are Isomers.
- Dihydroxyacetone phosphate is isomerised into glyceraldehyde 3- phosphate by the enzyme triose phosphate isomerase.
- Now two molecules of glyceraldehyde 3 phosphate enter into pay off phase.
During the preparatory phase, two ATP molecules are àonsumed.
![]()
Question 5.
Explain pyruvate oxidation (or) Link reaction of Glycolysis.
Answer:
- Two molecules of pyruvate formed by glycolysis in the cytosol enter into mitochondnalrnatrxi.
- In aerobic respiration this pyruvate with coenzyme A is oxidatively decarboxylated into acetyl CoA by pyruvate dehydrogenase complex. .
- It produces two molecules of NADH + H+ and 2CO2
It is also called transition reaction (or) Link reaction.

The pyruvate dehydrogenase complex consists of three distinct enzymes.
- Pyruvate dehydrogenase
- Dihydroiipoyil transacetylase
- Dihydrolipoyil dehydrogenase and 5 coenzymes TPP (thymine pyrophosphate)
- NAD+
- FAD
- COA and lipoate.
Question 6.
Draw the flow chart diagram for the Kreb cycle (or) Citric acid cycle.
Answer:
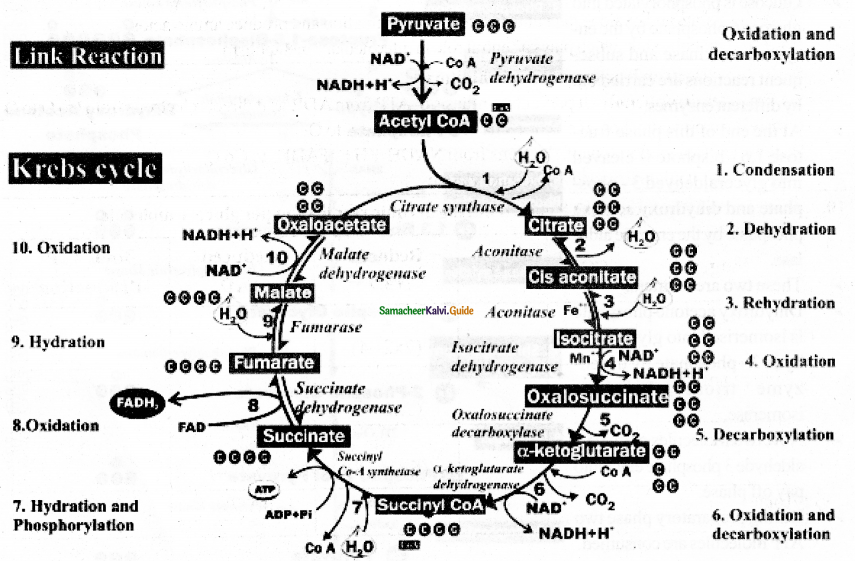
Question 7.
Explain Kreb cycle (or) Citric acid cycle (or) TCA cycle.
Answer:
- Two molecules of acetyl CoA formed from link reaction now enter into Kreb Cycle.
- It is named after its discoverer German Biochemist Sir Hans Adolf Kreb (1937).
- It is takes place in the mitochondrial matrix and inner membrane of mitochondria.
- The enzymes needed for TCA cycle are found in the mitochondrial matrix except for succinate dehydrogenase which is found in the mitochondrial inner membrane.
- First step starts with condensation of acetyl CoA with oxaloacetate in the presence of water to yield citric acid (or) citrate.
- It is followed by the action of different enzymes in cyclic manner.
- During the conversion of succinyl CoA to succinate by the enzyme succinyl CoA synthetase a molecule of ATP Synthesis from Substrate without entering the electron transport chain is called substrate-level phosphorylation.
- Kreb Cycle is repeated twice for every glucose molecule.
- Where two molecules of pyruvic acid produces six molecules of CO2, eight molecules of NADH+H+ two molecules of FADH2 and two molecules of ATP.
![]()
Question 8.
Significance of Kreb Cycle.
Answer:
- TCA cycle is to provide energy in the form of ATP for metabolism in plants.
- It provides carbon skeleton or raw material for various anabolic process.
- many intermediates of TCA cycle are further metabolised to produce amino acids, proteins and nucleic acids.
- Succinyl CoA is raw material for formation of chlorophyll, cytochrome, phytochrome and other pyrroles
substances. - α – ketoglutarate and oxaloacetate undergo reductive amination and produce amino acids.
- it acts as metabolic sink which plays a central role in intermediary metabolism.
Question 9.
Write four Electron transport chain in hibitors.
Answer:
- 2,4 DNP (Dinitrophenol) – It prevents the synthesis of ATP from ADP, as it directs electrons from CoQ to O2
- Cyanide – It prevents the flow of electrons from Cytochrome a3 to O2
- Rotenone – It prevents flow of electrons from NADH + H+ / FADH2 to Co Q
- Oligomycin – It inhibits oxidative phosphorylation
Question 10.
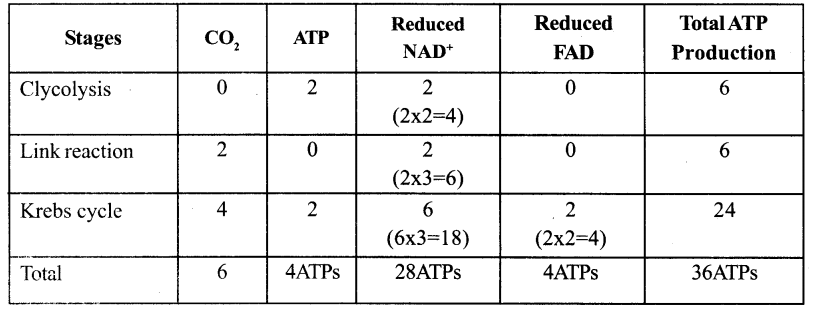
Tabulate Net Products of ATP gained during aerobic respiration per glucose molecule.
Answer:
Question 11.
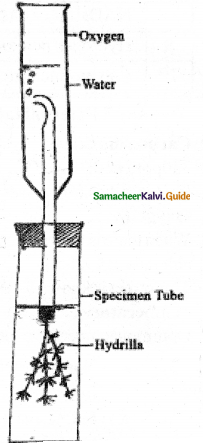
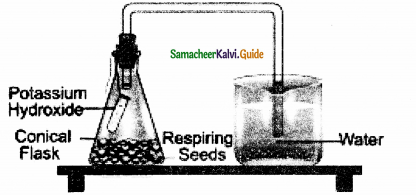
Experiment to demonstrate the production of CO2 in aerobic respiration.
Answer:
- Take small quantity of any seed (groundnut or bean seeds) and allow them to germinate by imbibing them.
- While they are germinating place them in a conical flask.
- A small glass tube containing 4 ml of freshly prepared Potassium hydroxide (KOH) solution is hing into the conical flask with the help of a thread and tightly close the one holed cork.
- Take a bent glass tube, the shorted end of which is inserted into the conical flask through the hole in the cork.
- The longer end is dipped in a beaker containing water.
- Observe the position of initial water level in bent glass tube.
- This experimental setup is kept for two hours.
- After two hours, the level of water rises in the glass tube. It is because the CO2 evolved during aerobic
respiration by germinating seeds will be absorbed by KOH solution and the level of water will rise in the glass tube. - CO2 + 2KOH → K2CO3 + H2O
![]()
Question 12.
Compare Alcoholic fermentation.
Answer:
| Alcoholic fermentation | Lactic acid fermentation |
| 1. it produces alcohol and releases CO2 from pyruvic acid | It produces lactic acid and does not release CO2 from pyruvic acid |
| 2. It takes place in two steps. | It takes place in single steps. |
| 3. It involves two enzymes, pyruvate decarboxylase with Mg++ and alcohol dehydrogenase | It uses one enzyme, lactate dehydrogenase with Zn++ |
| 4. It forms acetaldehyde as an intermediate compound | Does not form an intermediate compound. |
| 5. It commonly occurs in yeast. | Occurs in bacteria, some fungi, and vertebrate muscles. |
Question 13.
Write the Industrial uses of alcoholic fermentation.
Answer:
- In bakeries, it is used for preparing bread, cakes, biscuits.
- In beverage industries for preparing wine and alcoholic drinks.
- In producing vinegar and in tanning, curing of leather.
- Ethanol is used to make gasohol (a fuel that is used for cars in Brazil).
Question 14.
Tabulate the comparison between glycolysis and fermentation.
Answer:
| Glycolysis | Fermentation |
| 1. Glucose is converted into pyruvic acid | Stars from pyruvic acid and is converted into alcohol or lactic acid. |
| 2. It takes place in the presence or absence of oxygen. | it takes place in the absence of oxygen. |
| 3. Net gain is 2ATP. | No net gain of ATP molecules. |
| 4. 2NADH + H+ molecules are produced. | 2NADH+ H+molecules are utilised |
| 5. It commonly occurs in yeast. | Occurs in bacteria, some fungi and vertebrate muscles. |
Question 15.
Explain the demonstration of alcoholic fermentation.
Answer:
- Take a Kuhne’s fermentation tube which consists of an upright glass tube with a side bulb
- Pour 10% sugar solution mixed with baker’s yeast into the fermentation tube the side tube is filled plug the mouth with lid.
After some time, the glucose solution will be fermented. The solution will give out an alcoholic smell. - The level of the solution in the glass column will fall due to the accumulation of CO2 gas.
- It is due to the presence of zymase enzyme yeast which converts the glucose solution into alcohol and CO2
- Now introduce a pellet of KOH into the tube, the KOH will absorb CO2 and the level of solution will rise in the upright tube.
- This experiment proves during fermentation CO2 gas is evolved.
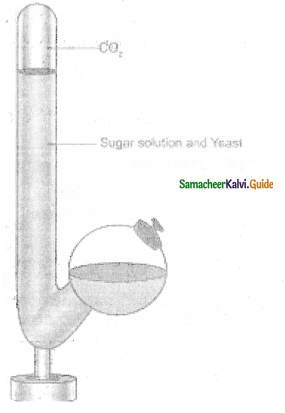
![]()
Question 16.
Write the Factors (Internal and External) which affect the process of respiration.
Answer:
External Factors:
- The optimum temperature for respiration is 30°C. At low temperatures and very high temperatures rate, respiration decreases.
- When sufficient amount of O2 is available the rate of aerobic respiration will be optimum and anaerobic respiration is completely stopped. This is called Extinction point.
- The high concentration of CO2 reduces the rate of respiration.
- A plant or tissue transferred from water to salt solution wi li increase the rate of respiration. It is called silt respiration.
- Light is an indirect factor affecting the rate of respiration.
- Wounding of plant organs stimulates the rate of respiration in that region.
Internal Factors:
- The concentration of respiratory substrate is proportional to the rate of respiration
- The amount of protoplasm and its state of activity influence the rate of respiration.
Question 17.
Write about the alternate pathway for glucose break down (or) Write about pentose phosphate pathway. (or) Phosphogluconate pathway (or) War burg – Dickens Lipmann pathway (or)Hexose Monophosphate pathway (or) HMP Shunt pathway.
Answer:
- The pentose phosphate pathway was described by Warburg, Dickens, and Lipmann (1938). Hence, it is also called Warburg – Dickens Lipmann pathway.
- It takes place in the cytoplasm of mature plant cells. It is an alternate way for break4own of glucose.
- It consists of two phases, oxidative phase, and non-oxidative phase.
- The oxidative events concert six molecules of six carbon Glucose 6 phosphate to 6 molecules of five-carbon sugar Ribulose -5 phosphate with loss of 12 NADPH + H+ (not NADH).
- The remaining reactions known as non oxidative pathway, covert Rihulose 5phosphate molecules to various intermediates such as Ribose – 5 – phosphate (5C), Xylulose – 5 – phosphate (5C), Glyceraldehyde – 7 – Phosphate (7C), and Eiythrose -4- phosphate (4C).
- Finally, five molecules of glucose -6- phosphate is regenerated. The overall reaction is:
6 x Glucose – 6 – Phosphate + 12NADP+ + 6H2O
↓
5 x Glucose-6- Phosphate + 6CO2 + Pi + 12NADPH + 12H+
- The net result of complete oxidation of one glucose-6-phosphate yield 6CO2 and12NADPH+H+
![]()
Question 18.
Draw the cycle for pentsoe phosphate pathway (or) Draw the flow chart for HMP Shunt.
Answer:
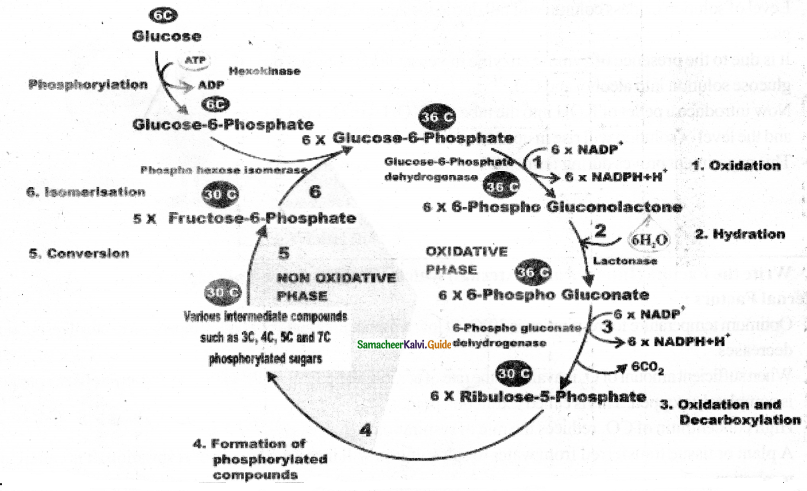
Question 19.
Write about the Significance of Pentose Phosphate pathway.
Answer:
- HMP shunt is associated with the generation of two important products. NADPH and pentsoe sugars, which play a vital role in anaholic reactions.
- Coenzyme NADPH generated is used by reductive bisynthesìs and counter damaging the effects of oxygen-free radicals.
- Ribose – 5 – phosphate and its derivatives are used in the synthesis of DNA, RNA, ATP, NAD, FAD and
Coenzynie A. . - Erythrose is used for the synthesis of anthocyanin Jignin and other aromatic compounds.