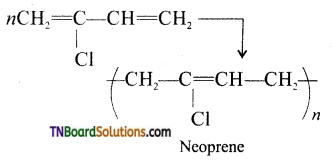
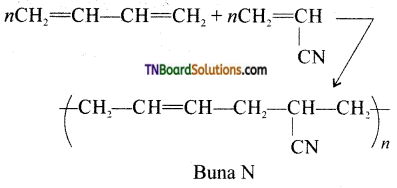
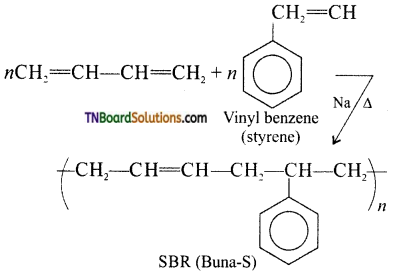
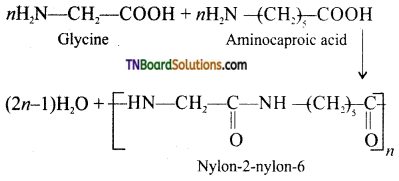
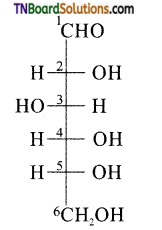
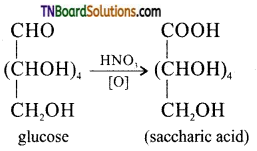
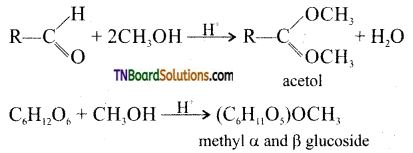
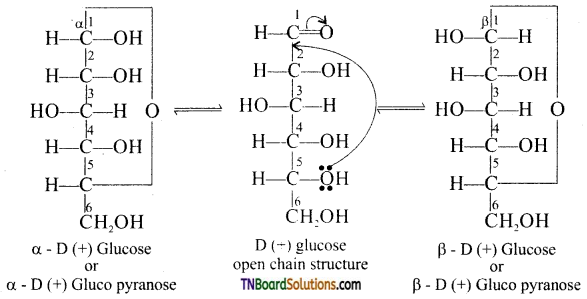
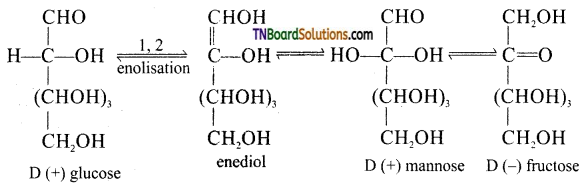
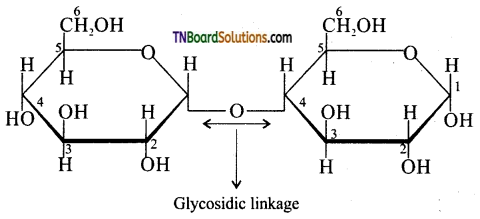
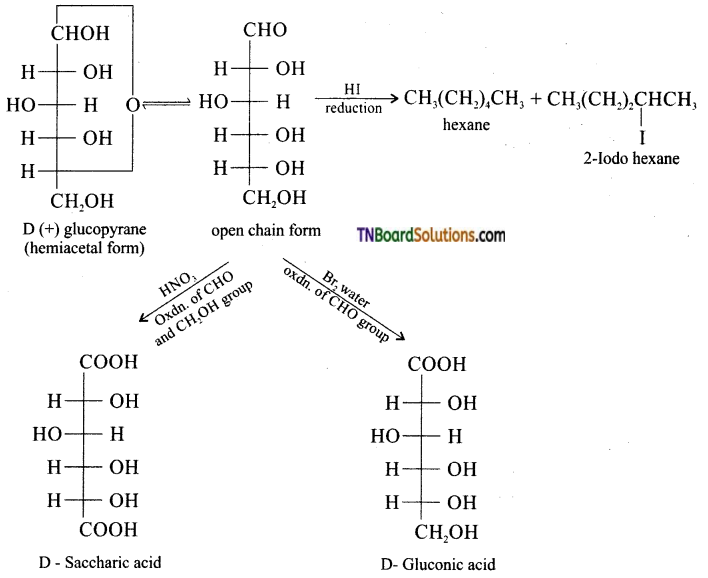
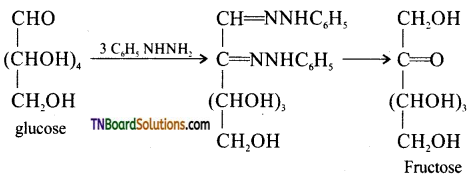
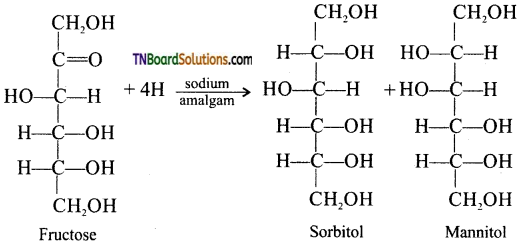
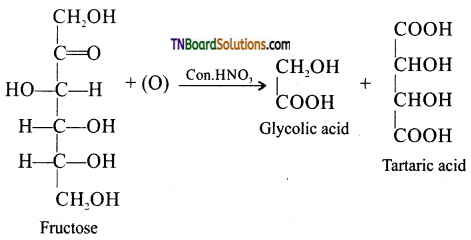
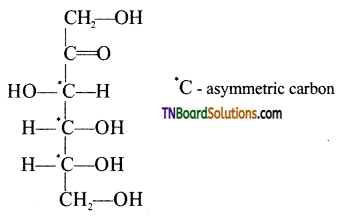
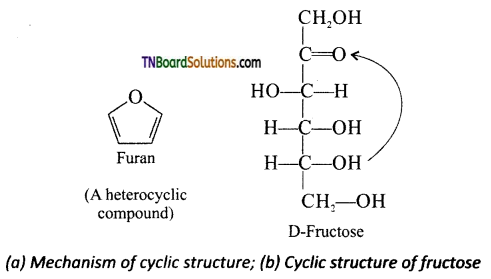
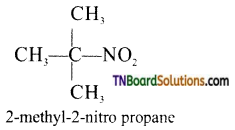
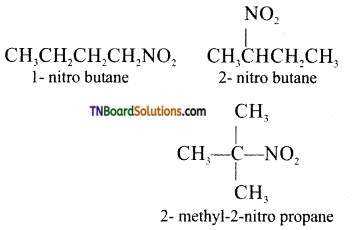
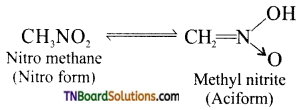
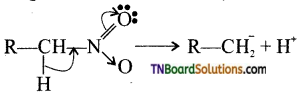
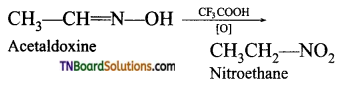
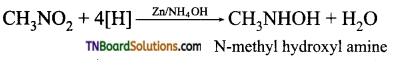
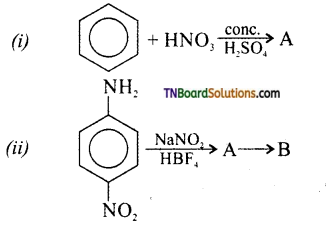
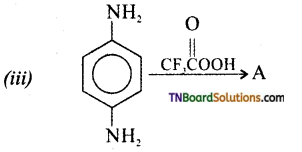
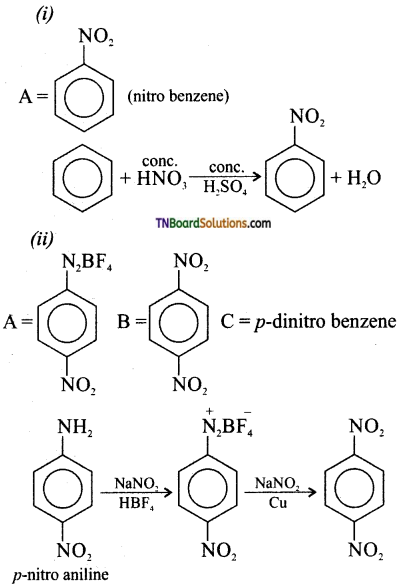
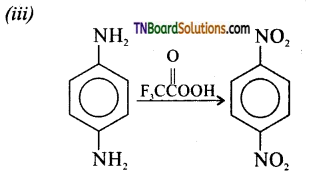
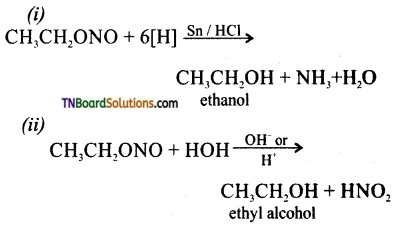
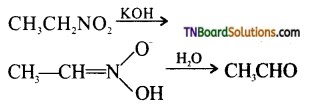
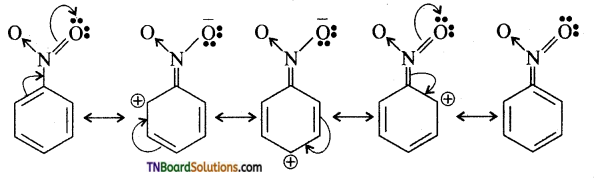
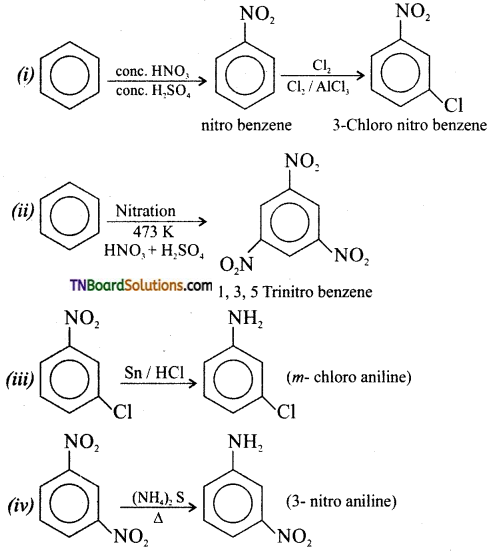
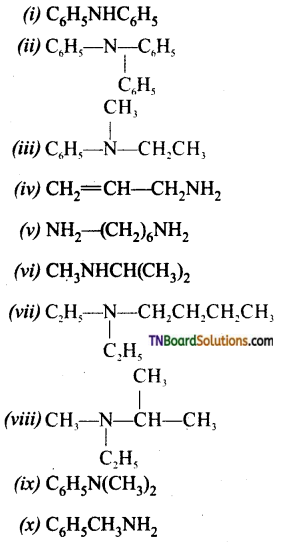
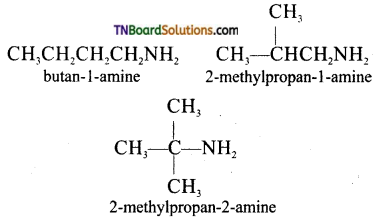
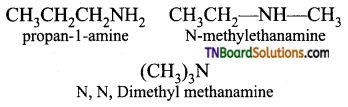
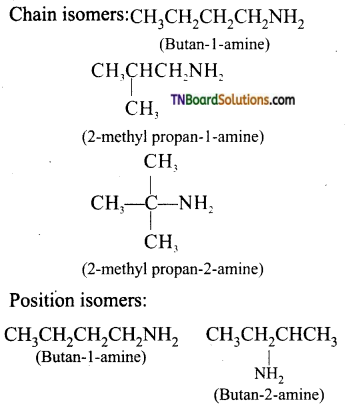
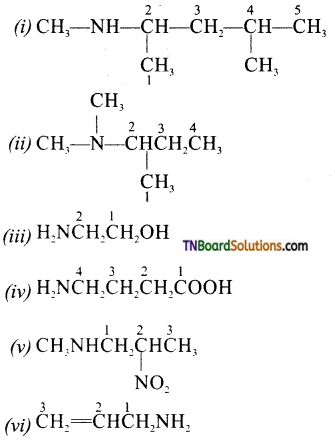
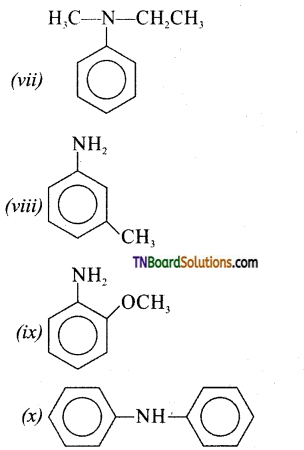
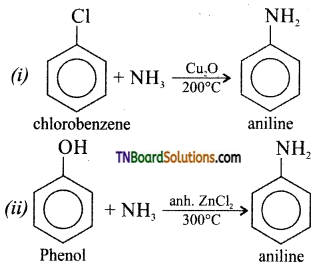
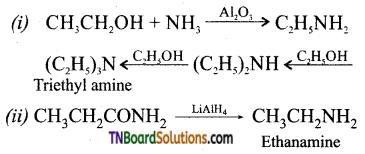
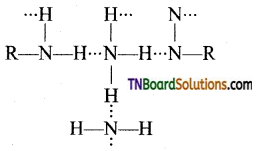
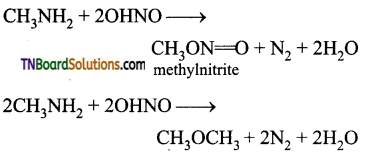
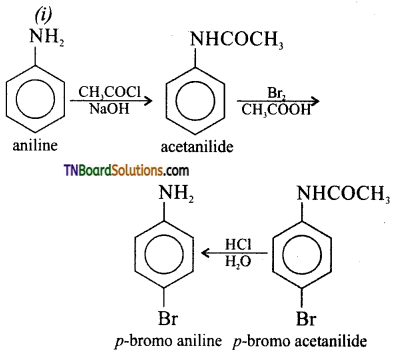
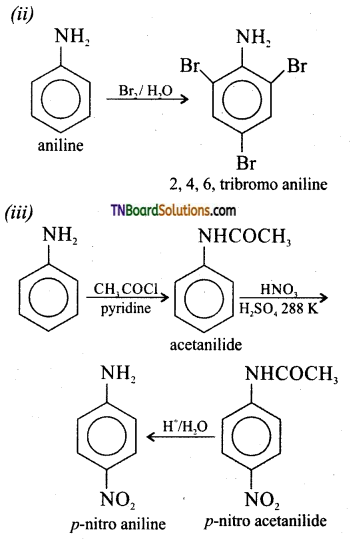
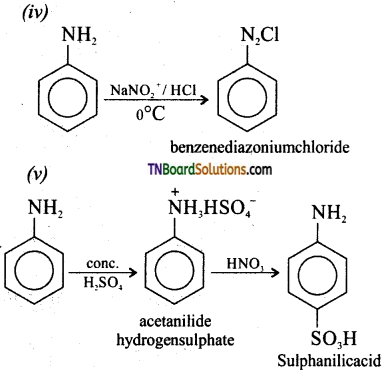
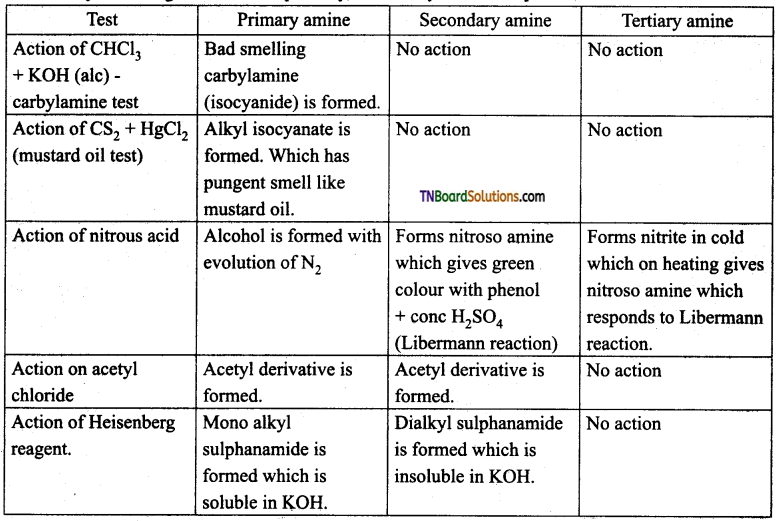
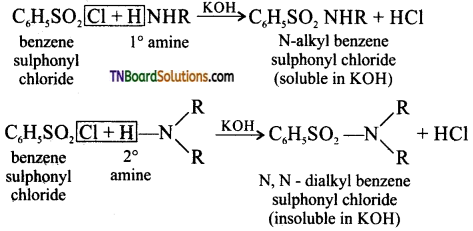
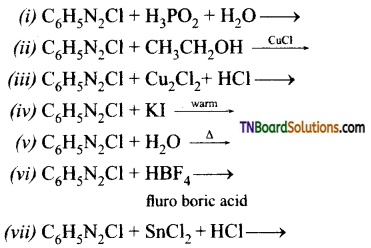
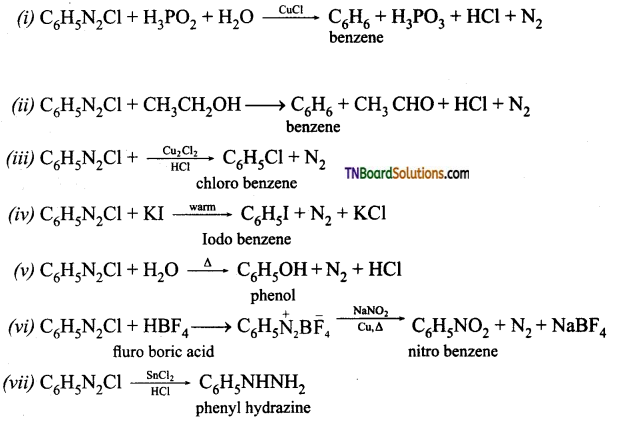
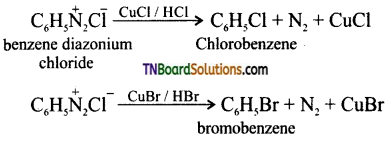
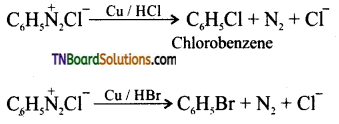
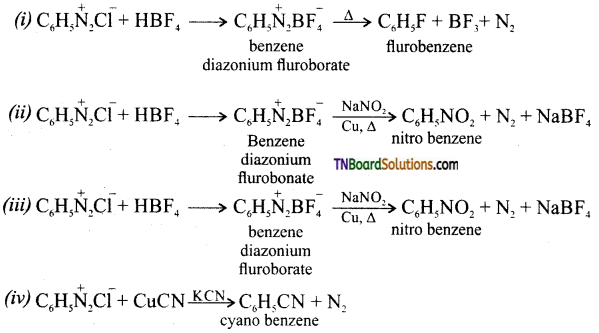
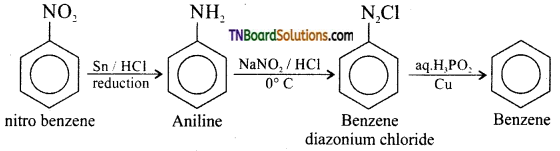
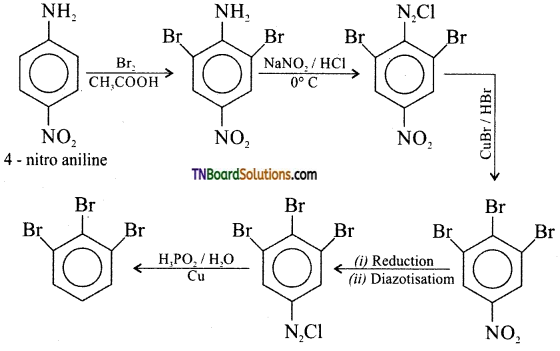
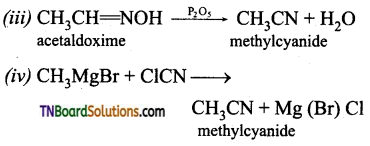
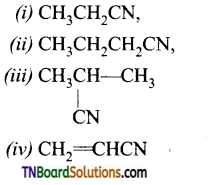
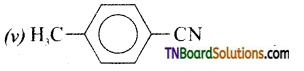
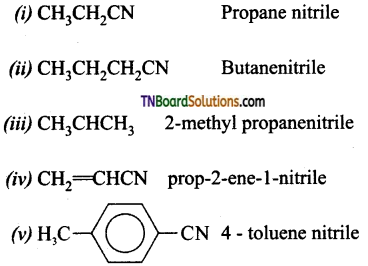
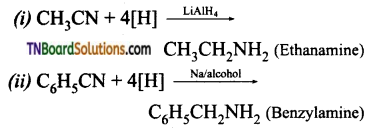
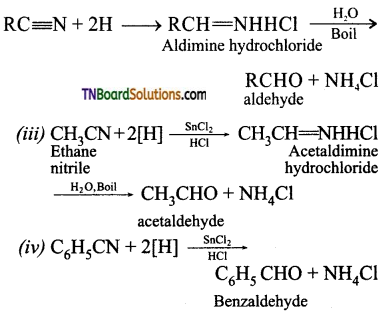
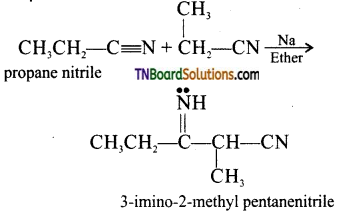
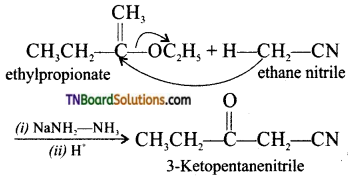
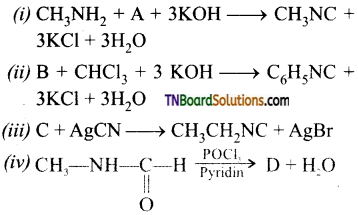
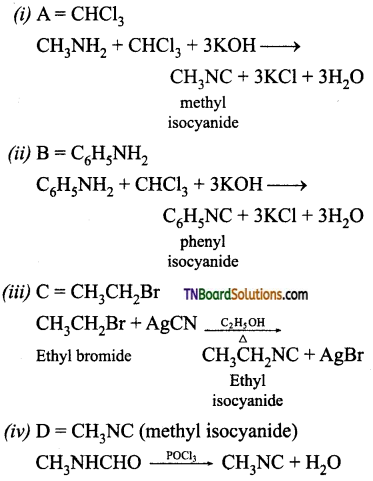
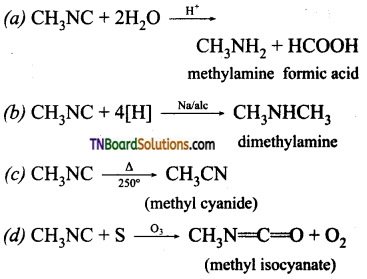
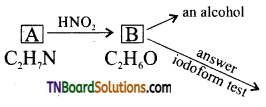
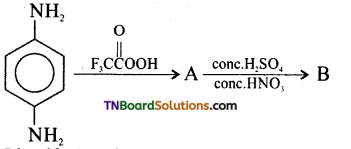
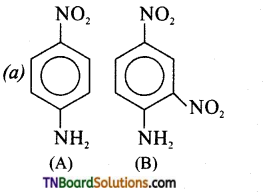
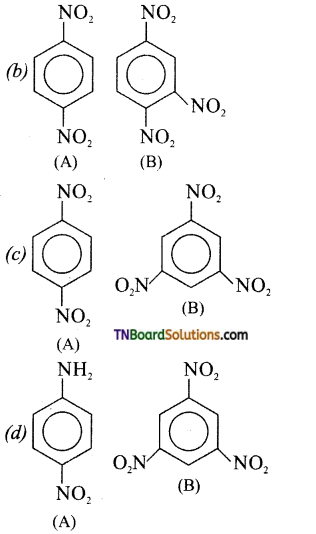
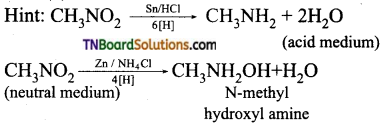
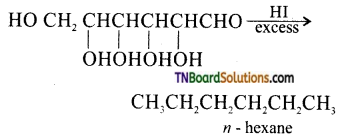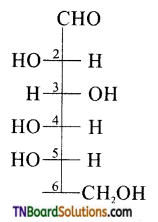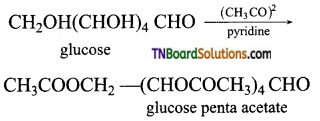Samacheer Kalvi 3rd Standard Science Book Solutions Term 2 Chapter 3 Plants
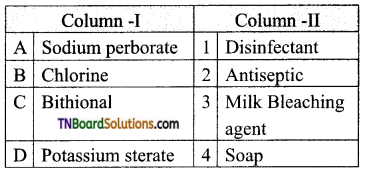
I. Choose the correct answer.
Question 1.
The function of leaf is to ________.
(a) Give Support
(b) Fix the plant firmly
(c) Produce food
(d) None of the above
Answer:
(c) Produce food
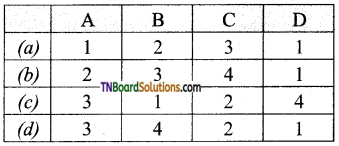
![]()
Question 2.
An example for taproot is _______.
(a) Paddy
(b) Grass
(c) Mango
(d) Ragi
Answer:
(c) Mango
Question 3.
________ supports the plant.
(a) Root
(b) Leaf
(c) Flower
(d) Stem
Answer:
(d) Stem
Question 4.
Most plants grow from the ________.
(a) root
(b) leaf
(c) flower
(d) seed
Answer:
(d) seed
Question 5.
Roots are poorly developed in _________.
(a) agaya thamarai
(b) neem
(c) teak
(d) date palm
Answer:
(a) agaya thamarai
![]()
Question 6.
If part X will be absent in a plant, new plants will not be produced. X is _________.
(a) stem
(b) root
(c) flower
(d) leaves
Answer:
(c) flower
Question 7.
The plant is adapted to grow in deserts as it has ________.
(a) fleshy stem
(b) needle like roots
(c) leaves changed to spine
(d) both a and c
Answer:
(d) both a and c
Question 8.
An example of many seeded fruit is ________.
(a) pomegranate
(b) mango
(c) apricot
(d) peach
Answer:
(a) pomegranate
Question 9.
Which of the following plant parts take part in absorption of water and exchange of gases respectively?
(a) P and R
(b) R and S
(c) S and Q
(d) T and P
Answer:
(d) T and P
![]()
II. Find the odd one.
Question 1.
(a) Carrot
(b) Radish
(c) Tomato
(d) Beetroot
Answer:
(c) Tomato
Question 2.
(a) Cabbage
(b) Greens
(c) Turmeric
(d) Spinach
Answer:
(c) Turmeric
Question 3.
(a) Neem
(b) Aloe vera
(c) Datepalm
(d) Opuntia
Answer:
(a) Neem
Question 4.
(a) Coconut
(b) Mango
(c) Apricot
(d) Orange
Answer:
(d) Orange
Question 5.
(a) Hydrilla
(b) Opuntia
(c) Water hyacinth
(d) Vallisneria
Answer:
(b) Opuntia
![]()
III. Short answers.
Question 1.
Name the parts of a plant.
Answer:
The basic parts of a plant are root, stem, leaf, flower, fruit and seed.
Question 2.
Name the types of roots.
Answer:
They are of two main types:
- tap root and
- fibrous root.
Question 3.
Write any two functions of the leaves.
Answer:
- Leaf prepares food for the plant with the help of water, carbondioxide and in the presence of sunlight and chlorophyll.
- The loss of water in the form of gas (water vapour) happens through the tiny pores in the leaves.
![]()
Question 4.
Write the parts of a flower.
Answer:
- Stamen,
- Pistil,
- Petal,
- Sepal
Question 5.
Name the types of plants based on thier habitat
Answer:
- Land (Terrestrial) plants
- Water (aquatic) plants
Question 6.
Write any two adaptations of desert plants.
Answer:
- Leaves are changed to spines to reduce the loss of water.
- The stem is green and fleshy. They store water and produce food.
Question 7.
Write the names of some water plants.
Answer:
- Water hyacinth (Agaya thamarai),
- Pistia.
![]()
IV. Answer the following questions.
Question 1.
Write any two functions of each:
(a) Stem _________, _________
Answer:
Supports, Transports
(b) Root _________, _________
Answer:
Fixation, Absorption
(c) Flower _________, _________
Answer:
Develops, Reproduce
Question 2.
Why is leaf called food factory of the plant?
Answer:
Leaf prepares food for the plant with the help of water, carbon dioxide and in the presence of sunlight and chlorophyll. This process is called photo synthesis. Hence, it is called the food factory of the plant.
![]()
Question 3.
Differentiate between taproot and fibrous root.
Answer:
| S.No | Taproot | Fibrous root |
| 1. | Thick main root that goes deep into the soil. | No main root and the roots do not go deep into the soil. |
| 2. | Side roots are developed from the main root. | Roots are developed from the base of the stem. |
| 3. | Looks like a long tap E.g., Tamarind, Guava. | Looks like a bunch. E.g., Corn, Sugarcane. |
Question 4.
Give two examples of :
(a) Fruit having only one seed
(b) Fruit having many seeds
Answer:
(a) Some fruits have only one seed.
E.g., Apricot, Mango, Coconut and Peach.
(b) Some fruits have many seeds.
E.g., Papaya, Watermelon and Orange.
Question 5.
Name two free floating plants.
Answer:
- Water hyacinth (Agaya thamarai)
- Pistia.
Question 6.
Look at the picture of a water lily.
(a) Which parts of the plant can you see?
(b) Where are the plant’s roots and stem?
Answer:
(a) Flower, Leaf
(b) The plant’s roots and stem are in the bottom of the water bodies.
![]()
In-Text Activity:
Warm-up (Text Book Page No. 58):
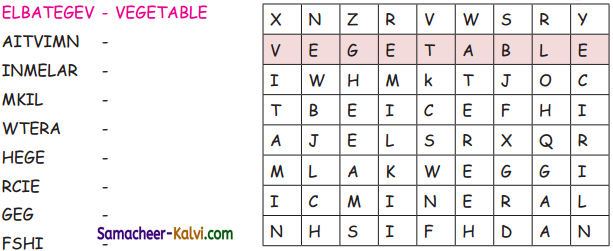
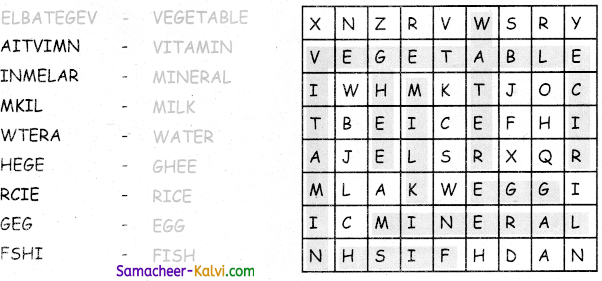
Unscramble the words and label the parts of the plant.
(ETSM, TORO, ELFA, FURTI, LOFEWR, SDEE)
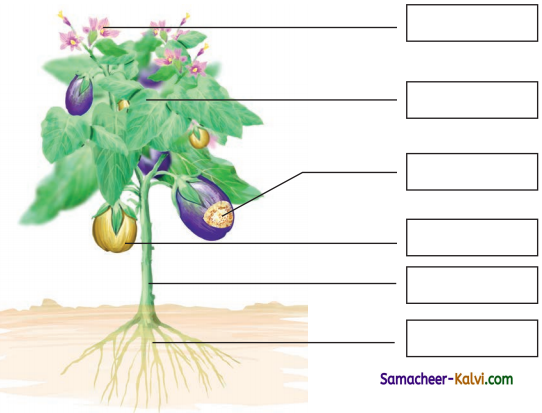
Answer:
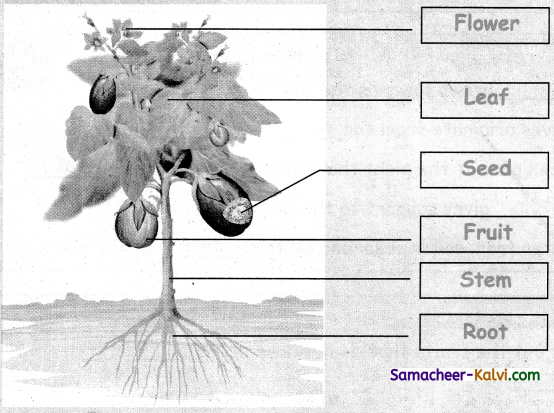
![]()
Let us Do (Text Book Page No. 60):
Take two small potted plants. Cut the root of one of the plants and fix ¡t in the pot. Now water the plants for two to three days. You will observe that the plant without roots will wilt and die. In the absence of roots, plants die.
This activity proves that the function of the roots is to absorb ________ and _______ from the soil.
Answer:
Water, Minerals
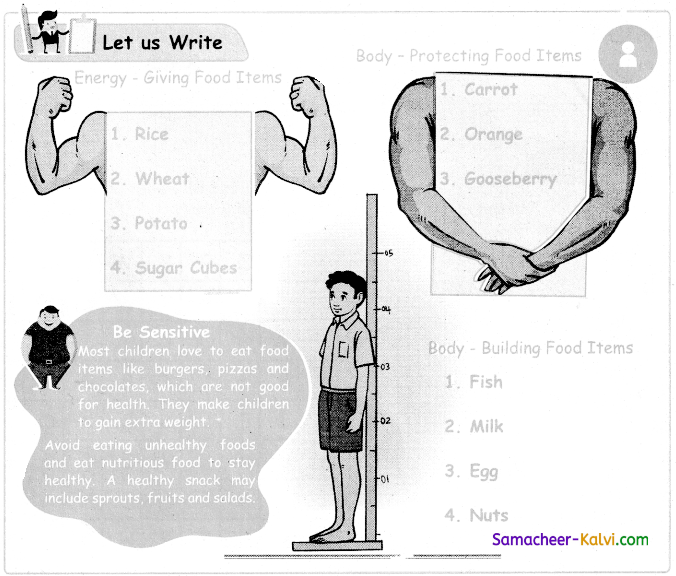
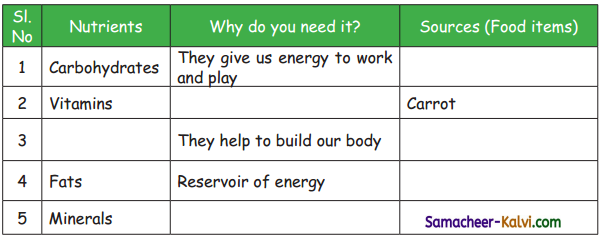
Let us Write (Text Book Page No. 60):
True or False

Question 1.
The roots grow into the soil.
Answer:
True
Question 2.
Fibrous root has a main root.
Answer:
False
Question 3.
Root absorbs water from soil.
Answer:
True
Question 4.
Potato stores food in its root.
Answer:
False
Question 5.
Grass has fibrous roots.
Answer:
True
![]()
Fill in the blanks.
Question 1.
_____________ grows towards the sunlight.
Answer:
Stem
Question 2.
Leaves originate from the _____________ .
Answer:
surface of the stem
Question 3.
Green part of the plant that makes food is called _____________ .
Answer:
leaf
Question 4.
_____________ gives support to the whole plant.
Answer:
Stem
Question 5.
Water from soil is absorbed by the _____________ of the plant.
Answer:
root
![]()
Think and Write
Question 1.
List out the fruits that do not have seeds. _____________ .
Answer:
Pineapple, Banana
Question 2.
Write down the names of the fruit trees that you have never seen0 but have tasted their fruits. ___________________ .
Answer:
Strawnerry tree, Plum tree, Pear tree
![]()
Let us Do
Connect the leaf with its fruit.

Answer:
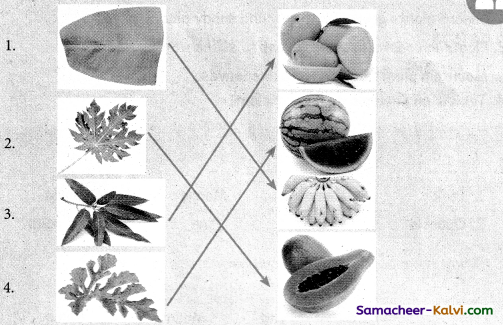
Let us Connect
Match the plants with their living places.
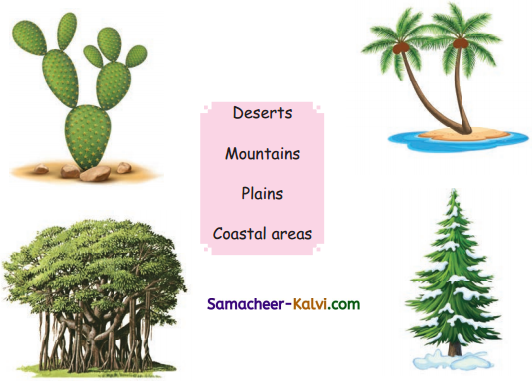
Answer:
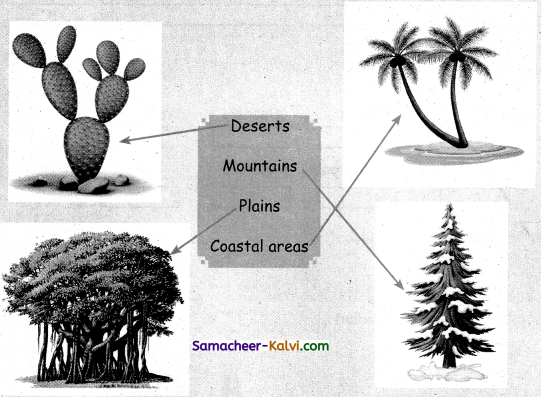
![]()
Let us Try
A. Select the ‘INCORRECT’ statement from the following.
1. Desert plants grow in hot, dry and sandy places.
2. Plants in coastal areas tolerant to saline water.
3. Mountain plants have needle like leaves.
4. Teak is an example of desert plant.
Answer:
4. Teak is an example of desert plant.
B. Tick (✓) the odd one.

Answer:

C. Circle the places which are land habitats.
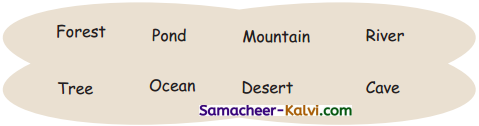
Answer:
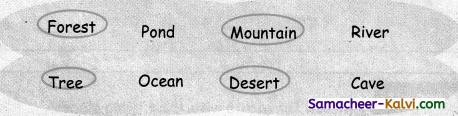
![]()
Let us Try
A. Mark “L” for land plants and W’ for water plants.
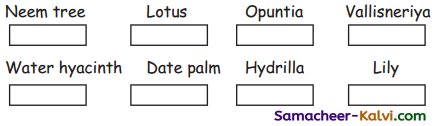
Answer:

B. Colour the water hyacinth plant.
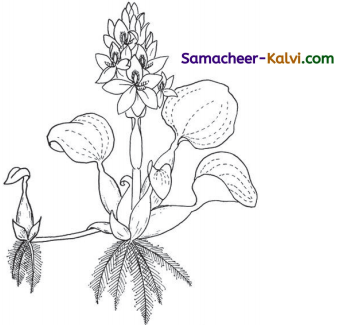
Answer:
Activity to be done by the students
![]()
C. Write true or false.
Question 1.
Fixed rooted plants are present in water bodies.
Answer:
True
Question 2.
Leaves of lotus are submerged in the water.
Answer:
False
Question 3.
Lotus plants are found in many ponds.
Answer:
True
Question 4.
Water hyacinth freely float with the help of spongy body filled with air.
Answer:
True



























































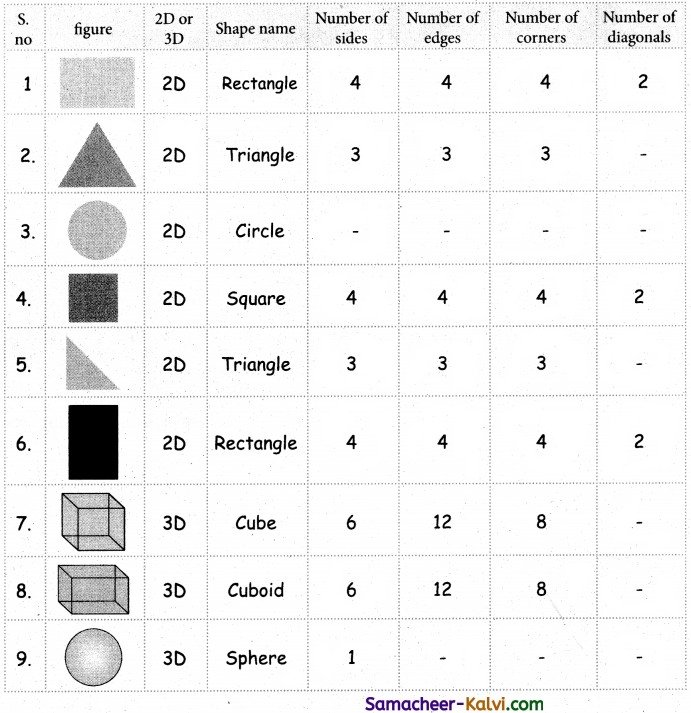
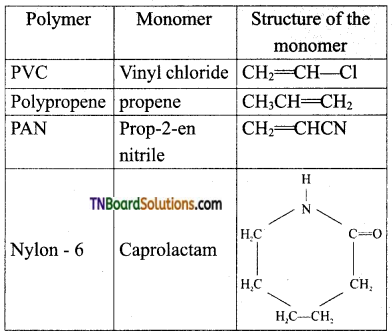
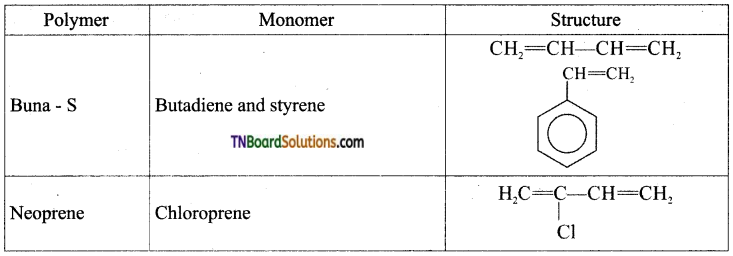
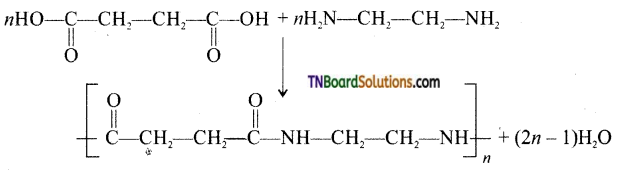
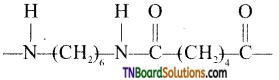
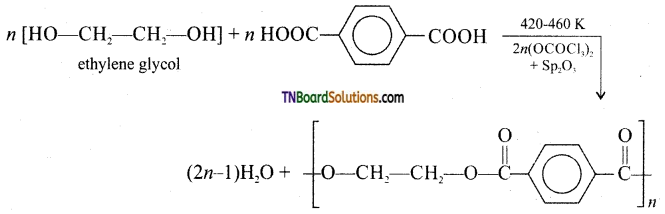
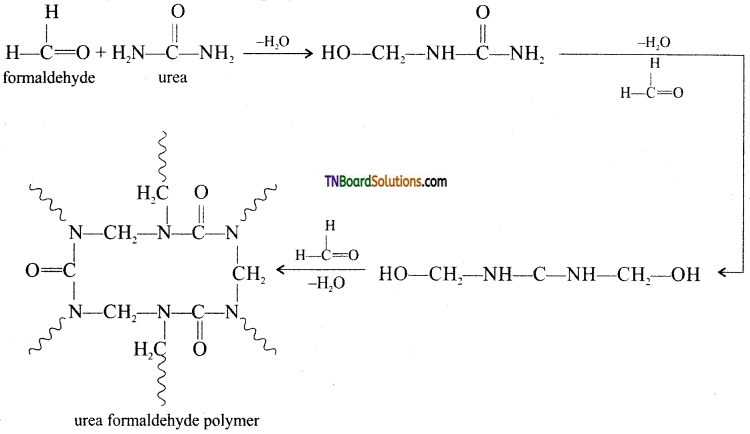
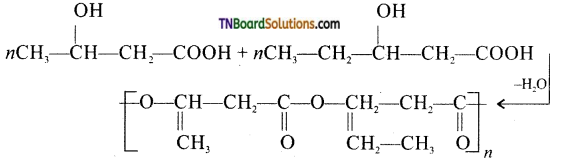
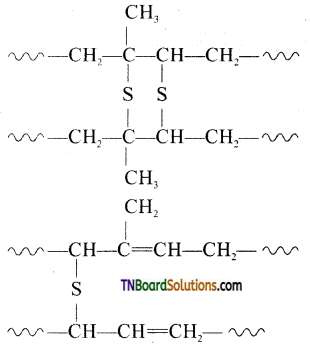
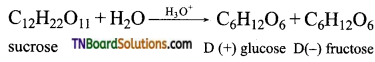
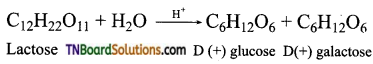
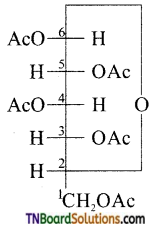
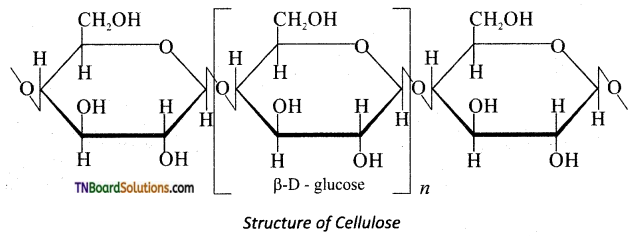
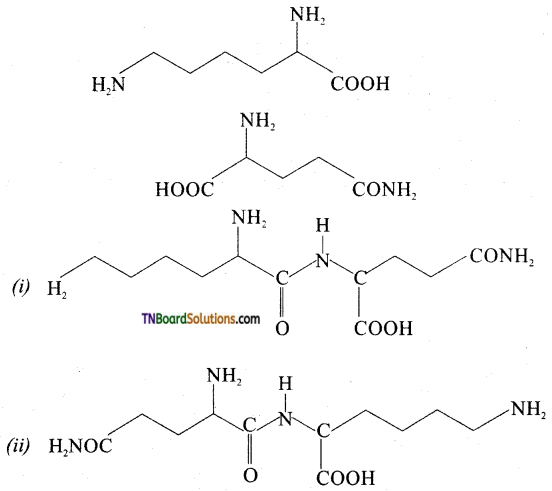
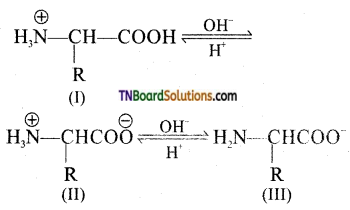
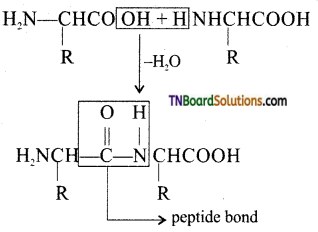
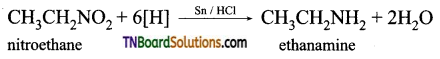
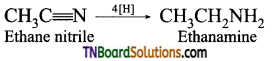
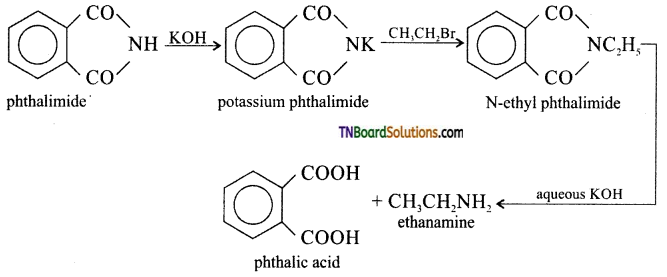
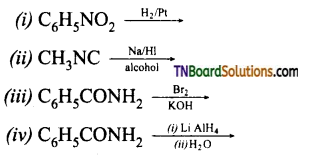
 which is derived from caprolactam.
which is derived from caprolactam.