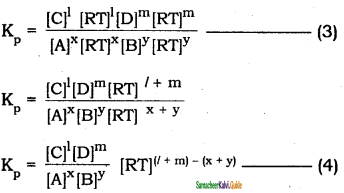
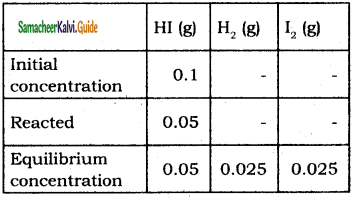
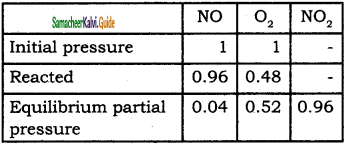
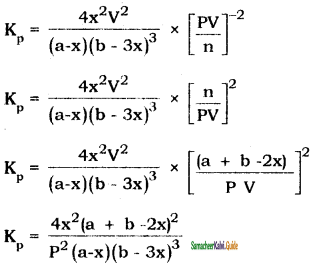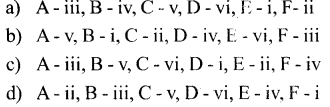Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Bio Zoology Guide Pdf Chapter 6 Respiration Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Bio Zoology Guide Chapter 6 Respiration
11th Bio Zoology Guide Respiration Text Book Back Questions and Answers
Part I
I. Choose The Best Options
Question 1.
Breathing is controlled by
a) cerebrum
b) medulla oblongata
c) cerebellum
d) pons
Answer:
b) medulla oblongata
Question 2.
Intercostal muscles are found between the
a) vertebral column
b) sternum
c) ribs
d) glottis
Answer:
c) ribs
![]()
Question 3.
The respiratory structures of insects are
a) tracheal tubes
b) gills
c) green glands
d) lungs
Answer:
a) tracheal tubes
Question 4.
Asthma is caused due to
a) inflammation of bronchus and bronchioles
b) inflammation of branchiole
c) damage of diaphragm
d) infection of lungs
Answer:
a) inflammation of bronchus and bronchioles
![]()
Question 5.
The Oxygen Dissociation Curve is
a) sigmoid
b) straight line
c) curved
d) rectangular hyperbola
Answer:
a) sigmoid
Question 6.
The Tidal Volume of a normal person is
a) 800 mL
b) 1000-1200 mL
c) 500 mL
d) 1100-1200 mL
Answer:
c) 500 mL
![]()
Question 7.
During inspiration, the diaphragm
a) expands
b) unchanged
c) relaxes to become domed-shaped
d) contracts and flattens
Answer:
d) contracts and flattens
Question 8.
CO2 is transported through blood to lungs as
a) carbonic acid
b) oxyhaemoglobin
c) carbamino haemoglobin
d) carboxy haemoglobin
Answer:
c) carbamino haemoglobin
![]()
Question 9.
When 1500 mL air is in the lungs, it is called
a) vital capacity
b) tidal volume
c) residual volume
d) inspiratory reserve volume
Answer:
c) residual volume
Question 10.
Vital capacity is
a) TV + IRV
b) TV + ERV
c) RV + ERV
d) TV + IRV + ERV
Answer:
d) TV + IRV + ERV
![]()
Question 11.
After a long deep breath, we do not respire for some seconds due to
a) more CO2 in the blood
b) more O2 in the blood
c) less CO2 in the blood
d) less O2 in the blood
Answer:
b) more O2 in the blood
Question 12.
Which of the following substances in tobacco smoke damage the gas exchange system?
a) carbon monoxide and carcinogens
b) carbon monoxide and nicotine
c) carcinogens and tar
d) nicotine and tar
Answer:
b) carbon monoxide and nicotine
![]()
Question 13.
Column I represents diseases and column II represents their symptoms Choose the correctly paired option.
| Column I | Column II |
| P. Asthma | i) Recurring of bronchitis |
| Q. Emphysema | ii) Accumulation of W.B.C in alveolus |
| R. Pneumonia | iii) Allergy |
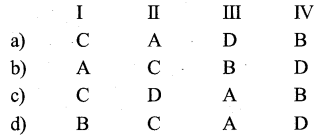
a) P – iii, Q – ii, R – i
b) P – iii, Q – i, R – ii
c) P – ii, Q – iii, R – i
d) P – ii, Q – i, R -iii
Answer:
a) P – iii, Q – ii, R – i
Question 14.
Which of the following best describes the process of gas exchange in the lungs?
a) Air moves in and out of the alveoli during breathing.
b) Carbon dioxide diffuses from deoxygenated blood in capillaries into the alveolar air
c) Oxygen and carbon dioxide diffuse down their concentration gradients between blood and alveolar air
d) Oxygen diffuses from alveolar air into deoxygenated blood.
Answer:
c/d
![]()
Question 15.
Make the correct pairs.
| Column I | Column II |
| PIC | i) maximum volume of air breathe in after forced |
| QEC | ii) Volume of air present after expiration in lungs |
| RVC | iii) Volume of air inhaled after expiration |
| SFRC | iv) Volume of air exhaled after inspiration |
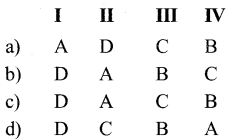
a) P – i, Q – ii, R – iii, S – iv
b) P – ii, Q – iii R – iv, S – i
c) P – ii, Q – iii, R – i, S – iv
d) P – iii, Q – iv, R – i, S – ii
Answer:
d) P – iii, Q – iv, R – i, S – ii
![]()
Question 16.
Make the correct pairs.
| Column I | Column II |
| P. Tidal volume | i) 1000 to 1100 ml |
| Q. Residual volume | ii) 500 ml |
| R. Expiratory reserve volume | iii) 2500 to 3000 ml |
| S. Inspiratory reserve volume | iv) 1100 to 1200 ml |
a) P – ii, Q – iv, R – i, S – iii
b) P – iii, Q – ii R – iv, S – i
c) P – ii, Q – iv, R – iii, S – i
d) P – iii, Q – iv, R – i, S – ii
Answer:
a) P – ii, Q – iv, R – i, S – iii
Question 17.
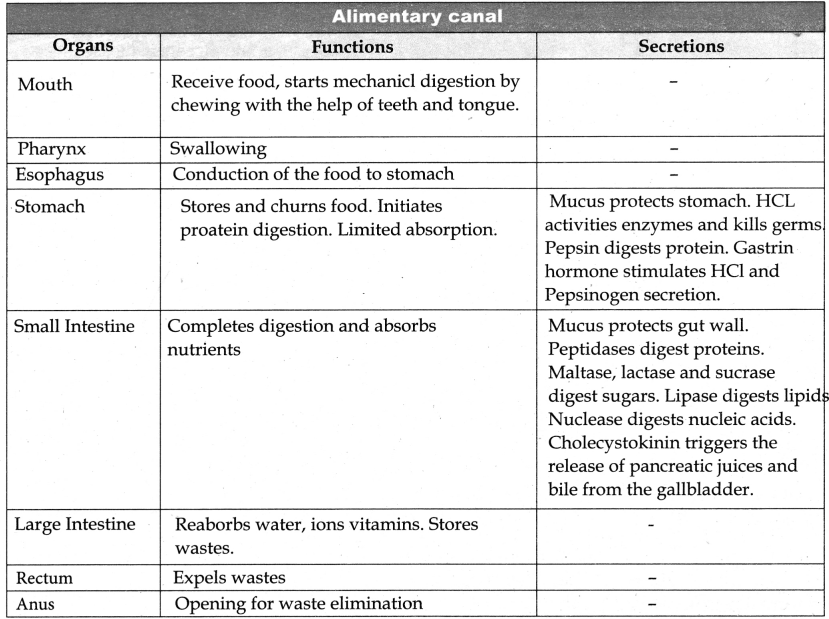
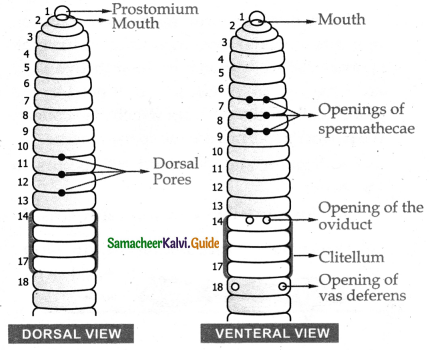
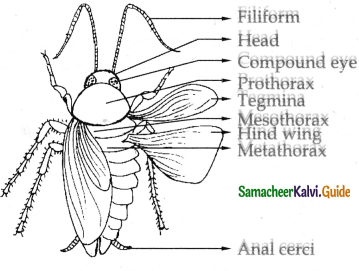
Name the respiratory organs of flatworm earthworm, fish, prawn, cockroach, and cat.
Answer:
Flatworm – Body surface
Earthworm – Moist skin
Fish – Gills
Prawn – Gills
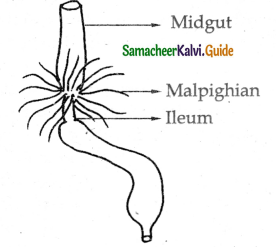
Cockroach – Trachea
Cat – Lungs
Question 18.
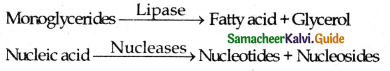
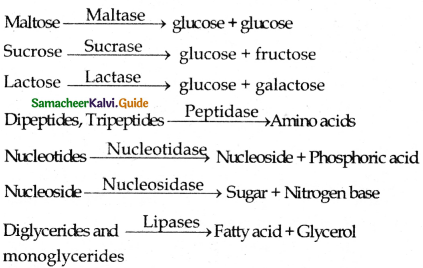
Name the enzyme that catalyses the bicarbonate formation in RBCs.
Answer:
Carbonic anhydrase
![]()
Question 19.
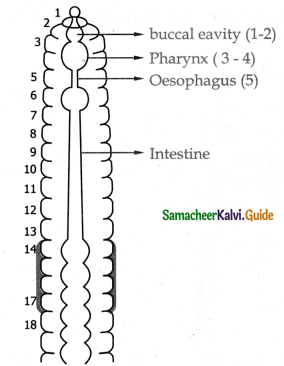
Air moving from the nose to the trachea passes through a number of structures. List in order of the structures.
Answer:
External nostrils, Nasal cavity, pharynx Larynx, trachea, the bronchi bronchioles, and the lungs (alveoli)
Question 20.
Which structures seal the Larynx when we swallow?
Answer:
Epiglottis.
Question 21.
Resistance in the airways is typically low why? Give two reasons.
Answer:
The airway resistance is low because:
- The diameter of most airways is relatively large.
- For smaller airways there are many in parallel, making their combined diameter large.
- Air has a low viscosity.
Question 22.
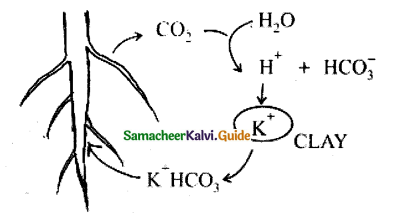
How the body makes long-term adjustments when living in high altitude?
Answer:
- When a person travels from sea level to elevations where the atmospheric pressure and partial pressure of O2 lowered there is a poor binding of O2 with haemoglobin leads to acute mountain sickness.
- When the person lives there for a long time the kidney synthesizes the erythropoietin which stimulates the bone marrow to produce more RBCs
![]()
Question 23.
Why is pneumonia considered a dangerous disease?
Answer:
Inflammation of the lungs due to infection caused by bacteria or viruses is called pneumonia. The symptoms are sputum production, nasal congestion, shortness of breath, sore throat, etc. The alveoli get filled with fluid or pus, making it difficult to breathe (lung abscesses).
Question 24.
Diffusion of gases occurs in the alveolar region and only not in any other part of the respiratory system discuss.
Answer:
- The other parts of the respiratory system do the work of passing the air into the lungs only.
- Real respiration takes place between alveoli and blood capillaries.
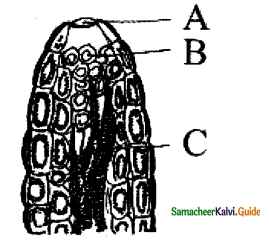
The diffusion membrane of the alveolus is made up of three layers.
- The thin squamous epithelial cells.
- The endothelium of the alveolar capillaries.
- The basement substance found in between them.
The thin squamous epithelial cells of the alveoli provide space for gaseous exchange
![]()
Question 25.
Sketch a flow chart to show the pathway of airflow during respiration.
Answer:
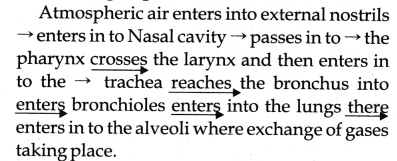
Question 26.
Explain the conditions which create problems in oxygen transport?
Answer:
When a person travels quickly from sea level to elevations above 8000 ft, where the atmospheric pressure and partial pressure of oxygen are lowered, the individual responds with symptoms of acute mountain sickness (AMS)- headache, shortness of breath, nausea, and dizziness due to poor binding of O2 with hemoglobin. When the person moves on a long-term basis to mountains from sea level his body begins to make respiratory and hematopoietic adjustments.
To overcome this situation kidneys accelerate the production of the hormone erythropoietin, which stimulates the bone marrow to produce more RBCs. When a person descends deep into the sea, the pressure in the surrounding water increases which causes the lungs to decrease in volume.
This decrease in volume increases the partial pressure of the gases within the lungs. This effect can be beneficial, because it tends to drive additional oxygen into the circulation, but this benefit also has a risk, the increased pressure can also drive nitrogen gas into the circulation.
This increase in blood nitrogen content can lead to a condition called nitrogen narcosis. When the diver ascends to the surface too quickly a condition called ‘bends’ or decompression sickness occurs and nitrogen comes out of solution while still in the blood-forming bubbles. Small bubbles in the blood are not harmful, but large bubbles can lodge in small capillaries, blocking blood flow or can press on nerve endings.
Decompression sickness is associated with pain in joints and muscles and neurological problems including a stroke. The risk of nitrogen narcosis and bends is common in scuba divers. During carbon-dioxide poisoning, the demand for oxygen increases. As the O2 level in the blood decreases it leads to suffocation and the skin turns bluish-black.
Part II
11th Bio Zoology Guide Respiration Additional Important Questions and Answers
(1 Mark)
I. Choose The Best Options
Question 1.
What are the respiratory organs of the Limulus?
a) Trachea
b) Gills
c) Bookgills
d) Green glands
Answer:
c) Bookgills
Question 2.
The failure of tissues for any reason to receive an adequate supply of oxygen.
a) apnoea
b) Dyspnoea
c) Hypoxia
d) Opnia
Answer:
c) Hypoxia
![]()
Question 3.
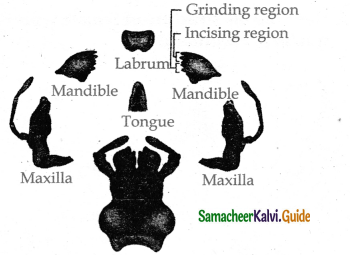
These are mucous-secreting cells.
a) Oxynctic cells
b) Chief cells
c) Goblet cells
d) Parietal cells
Answer:
c) Goblet cells
Question 4.
These are the respiratory surfaces.
a) brunchioles
b) Terminal bronchiole
c) alveoli
d) small bronchi
Answer:
c) alveoli
![]()
Question 5.
What is the average rate of respiration of a healthy man?
a) 12-16 times
b) 8-10 times
c) 5-10 times
d) 15 – 30 times
Answer:
a) 12-16 times
Question 6.
Match:
1) Residual volume i) 6000 ml
2) Expiratory reserve volume ii) 2500 – 3000 ml
3) Inspiratory reserve volume iii) 1000 -1100 ml
4) Total lung capacity iv) 1100 -1200 ml
1) i – iv; 2 – i; 3 – ii; 4 – iii
b) i – i; 2 – ii; 3 – iii; 4 – iv
c) i – iii; 2 – i; 3 – iv; 4 – ii
d) i – iv; 2 – iii; 3 – ii; 4 – i
Answer:
d) i – iv; 2 – iii; 3 – ii; 4 – i
![]()
Question 7.
1) Atmospheric air i) Partial pressure of O2 -104
2) Alveoli ii) Partial pressure of O2 – 40
3) Tissues iii) Partial pressure of O2 – 95
4) Oxygenated iv) Partial pressure of O2 – blood 159
a) i – i; 2 – ii; 3 – iii; 4 – iv
b) i – ii; 2 – iii; 3 – i; 4 – iv
c) i – iv; 2 – iii; 3 – ii; 4 – i
d) i – iv; 2 – i; 3 – ii; 4 – iii
Answer:
d) i – iv; 2 – i; 3 – ii; 4 – iii
Question 8.
Find out the correct statement and assertion:
Assertion: The high partial pressure of CO2 provides essential space for the dissociation of O2 from oxyhemoglobin
Reason: Haemoglobin takes a maximum of 4 molecules of CO2
a) Assertion and reason are correct
b) Assertion wrong reason wrong
c) Assertion correct reason correct. The reason explains the assertion.
d) Assertion correct Reason wrong
Answer:
d) Assertion correct Reason wrong
![]()
Question 9.
Assertion: The CO2 when enters into the blood combines with water to form carbonic acid
Reason: Carbonic anhydrase enzyme acts as a catalyst for this reaction
a) Assertion correct Reason wrong
b) Assertion wrong Reason correct
c) Assertion and reason are wrong
d) Assertion and reason are correct
Answer:
d) Assertion and reason are correct
Question 10.
Find out the causative agent of Tuberculosis.
a) Mycobacterium tuberculate
b) Salmonella tubercular
c) Mycobacterium calaracea.
d) Mycobacterium entries
Answer:
a) Mycobacterium tuberculate
![]()
Question 11.
What is the cause of pleurisy?
a) embolism
b) constriction of airways
c) alveolei is damaged
d) Pleura becomes inflammed
Answer:
d) Pleura becomes inflammed
Question 12.
People workes in the sand grinding mills may have this disease?
a) asbestosis
b) Fibrosis
c) Silicosis
d) Nephrosis
Answer:
c) Silicosis
![]()
Question 13.
What is the cause of the disease bend?
a) Fibrosis
b) Necrosis
c) Narcosis
d) Silicosis
Answer:
c) Narcosis
Question 14.
Find the wrong pair.
| a) Larynx | Epiglottis prevents the food from entering into the larynx |
| b) C-shaped cartilage | Ensures that the air passage does not collapse or burst. |
| c) The rigidity of bronchioles | Prevent them from collapsing |
| d) Fine respiratory brochioler | Terminate into air sacs |
Question 15.
Read the following statement and find whether they are correct or wrong.
1) The thin squamous epithelial cells of the alveoli are composed of Type-I and the gases can diffuse rapidly through them
2) Type-II cells are thin. The gaseous exchange takes place through diffusion.
3) The spirometer is used to find the volume of air
4) A healthy man respires 10-15 times per minute
a) 1 – True; 2 – False; 3 – True; 4 – False
b) 1 – True; 2 – True; 3 – False; 4 – True
c) 1 – False; 2 – True; 3 – False; 4 – True
d) 1 – True; 2 – False; 3 – True; 4 – False
Answer:
a) 1 – True; 2 – False; 3 – True; 4 – False
![]()
Question 16.
Amount of air inspired or expired with each normal breath.
a) 600 ml
b) 500 ml
c) 700 ml
d) 800 ml
Answer:
b) 500 ml
Question 17.
A normal human adult can inspire or expire approximately.
a) 5000-8000 ml
b) 6000 – 7000 ml
c) 6000-8000 ml
d) 5000 – 9000 ml
Answer:
c) 6000-8000 ml
![]()
Question 18.
During vigorous exercise, the tidal volume is about
a) 4 -10 times
b) 4 – 7 times
c) 4 – 6 times
d) 4 – 9 times
Answer:
a) 4 -10 times
Question 19.
Match the following.
i) Inspiratory reserve volume i) 6000 ml
ii) Expiratory reserve volume ii) 1100-1200 ml
iii) Residual volume iii) 1000-31100 ml
iv) Total lung capacity iv) 2500 – 43000 ml
a) i – 2; ii – 3; iii – 4; iv – 2
b) i – 1; ii – 2; iii – 3 ; iv – 4
c) i – 4; ii – 2; iii – 2; iv -1
d) i – 3; ii – 2; iii -1; iv – 4
Answer:
c) i – 4; ii – 2; iii – 2; iv -1
![]()
Question 20.
The amount of air that moves into the respiratory passage in a minute.
a) 7000ml
b) 8000 ml
c) 9000 ml
d) 6000 ml
Answer:
Question 21.
The amount of air that is not involved in gaseous exchange.
a) 200 ml
b) 150 ml
c) 300 ml
d) 250 ml
Answer:
d) 250 ml
![]()
Question 22.
Total lung capacity is ………….
a) VC+RC
b) TV+IRV
c) ERV+TV+IRV
d) TV+ERV
Answer:
a) VC+RC
Question 23.
What is the solubility of carbon dioxide in tissues?
a) 30 – 35 times
b) 20 – 30 times
c) 20 – 25 times
d) 20 – 22 times
Answer:
c) 20 – 25 times
Question 24.
Vital Capacity is:
a) TV+IRV+ERV
b) RV+ERV
c) TV+IRV
d) TV+ERV
Answer:
a) TV+IRV+ERV
![]()
Question 25.
In what form oxygen is transported in blood.
a) HbO4
b) HbO6
c) HbO2
d) HbO3
Answer:
c) HbO2
Question 26.
How many molecules of oxygen are accepted by haemoglobin?
a) 4
b) 3
c) 2
d) 1
Answer:
a) 4
![]()
Question 27.
How much O2 is delivered in 100ml of blood in normal physiological conductions?
a) 6 ml
b) 5 ml
c) 7 ml
d) 8 ml
Answer:
b) 5 ml
Question 28.
Find out the wrong pair.
| a) Dissolved CO2 in blood Plasma | 7- 10% |
| b) The transport of O2 in blood in the dissolved state | 7% |
| c) The dissolved CO2 in haemoglobin | 20-25% |
| d) Emphysema | Smoking |
Answer:
b) The transport of O2 in blood in the dissolved state – 7%
Question 29.
Where is the respiratory regulatory center present in the brain?
a) Pons Varoli
b) Pons
c) Medulla oblongata
d) cerebellum
Answer:
a) Pons Varoli
![]()
Question 30.
In which altitude the symptoms of a cute mountain sickness appear?
a) 1000 feet
b) 9000 feet
c) 8000 feet
d) 7000 feet
Answer:
c) 8000 feet
Question 31.
Find out the wrong pair
| a) Erythropoietin | Increases the red blood cell synthesis |
| b) Nitrogen | narcosis decompression sickness |
| c) Carbonic anhydrase | Synthesis of carbonic acid |
| d) Normal ferrous | Methaemoglobin |
Answer:
d) Normal ferrous – Methaemoglobin
Question 32.
Find the wrong pair.
| a) Level of O2 in the blood is low | Skin turns bluish-black |
| b) Sigmoid curve | Percentage Saturation of haemoglobin |
| c) Haemoglobin | HbO4 |
| d) Emphysema | Smoking |
Answer:
c) Haemoglobin – HbO4
![]()
Question 33.
Whether the following statements are True or False. If so arrange them in order
i) tuberculosis is caused by mycobacterium tubercular
ii) The lungs are affected due to the bacterial infection and pneumonia fever
iii) bronchitis causes mucous accumulation in the lungs
iv) Asthma is caused by virus Sequence
a) i – True, ii – False, iii – True, iv – False
b) i – False, ii – False, iii – True, iv – False
c) i – True, ii – True, iii – True, iv – False
d) i – True, ii – True, iii – False, iv – True
Answer:
a) i – True, ii – False, iii – True, iv – False
Question 34.
Confirm:
Assertion (A): Workers working in grinding industries wear protective masks
Reason (B): People working in grinding industries suffers from silicosis
a) A – True, B – True
b) A-False, B-True
c) The assertion A is wrong
d) assertion A is wrong. The reason B is True
Answer:
a) A – True, B – True
![]()
Question 35.
Confirm:
Part – A: The tar present in the nicotine damages the gaseous exchange.
Part – B: The blood vessels get narrower and the blood pressure increases due to smoking Ans:
a) Part A – False, Part B – Ture
b) Part A- True, Part B-True
c) Part A – True, Part B – is not correct explanation
d) Part A – It is a correct statement. Part B is not a correct statement
Answer:
b) Part A- True, Part B-True
Question 36.
Match and find the correct sequence
i) Pleurisy A) Constriction of alveoli
ii) Atelectasis B) Widening of alveoli
iii) Emphysema c) Accumulation of fluid in the air spaces
iv) Pulmonary edema D) Pleura becomes inflamed
a) I – D, II-A, III – B, IV – C
b) I – A, II – B, III – C, IV – D
c) I – D, II – C, III – B, IV – A
d) I – A, II – C, III-A, IV – B
Answer:
a) I – D, II-A, III – B, IV – C
![]()
Question 37.
Match and find the correct sequence
i) Tuberculosis A) Alveolei will be affected
ii) Pneumonia B) Inflamation of bronchioles
iii) Asthma Q Mycobacterium
iv) Bronchitis D) Mucous secretion
a) I – A, II – B, III – C, IV – D
b) I – C, II-A, III – D, IV – D
c) I – A, II – C, III – B, IV – D
d) I – A, II – B, III – D, IV – C
Answer:
b) I – C, II-A, III – D, IV – D
Question 38.
The world tuberculosis day
a) March 20th
b) March 21st
c) March 23rd
d) March 24th
Answer:
d) March 24th
![]()
Question 39.
What is the surface area of the lungs?
a) 500 square feet
b) 525 square feet
c) 550 square feet
d) 600 square feet
Answer:
b) 525 square feet
Question 40.
What is the speed of sneeze?
a) 165 km/hr
b) 200 km/hr
c) 250 km / hr
d) 225 km/ hr
Answer:
a) 165 km/hr
![]()
Question 41.
The adult respires ………………….. time and newborn child respires …………………. times.
a) 12-16;30-60
b) 12-14;30-50
c) 12-20; 30-70
d) 12-30;30-70
Answer:
a) 12-16;30-60
(2 marks)
II. Very Short Questions
Question 1.
What is excretion?
Answer:
The exchange of oxygen and carbon dioxide between the environment and cells of our body, where organic nutrients are broken down oxygenatically to release energy.
Question 2.
How much air can be respired by a normal human adult?
Answer:
A normal adult can respire approximately 6000 to 8000 ml of air per minute. During vigorous exercise, the tidal volume is about 4-10 times higher.
![]()
Question 3.
The rate of breathing in aquatic animals is faster than the of terrestrial animals. Give reason.
Answer:
The amount of dissolved oxygen is very low in water compared to the amount of oxygen in the air. Hence the rate of breathing in aquatic animals is faster than the terrestrial animals.
Question 4.
What is residual volume?
Answer:
- The volume of air remaining in the lungs after a forceful expiration.
- Ex.: 1100-1200ml.
Question 5.
What is the function of epiglottis?
Answer:
Epiglottis is a thin elastic flap at the junction of the nasopharynx and larynx. It prevents the food from entering into the larynx and avoids choking on food.
Question 6.
What is meant by inspiratory capacity?
Answer:
The total volume of air a person can inhale after normal expiration. It includes tidal volume and inspiratory reserve volume.
IC = TV + IRV
Question 7.
What is expiratory capacity?
Answer:
The total volume of air a person can exhale after a normal inspiration. It includes tidal volume and expiratory reserve volume.
EC = TV + ERV
Question 8.
How are lungs protected?
Answer:
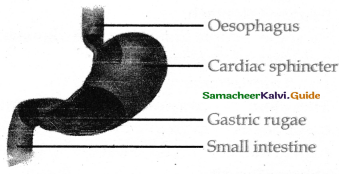
The lungs are light spongy tissues enclosed in the thoracic cavity surrounded by an air-tight space. It is bound dorsally by the vertebral column and ventrally by the sternum, laterally by the ribs, and on the lower side by the dome-shaped diaphragm.
Question 9.
What is meant by minute respiratory volume?
Answer:
- The amount of air that moves into the respiratory passage per minute is called minute respiratory volume.
Normal TV = 500 ml - Normal respiratory rate = 12 times / minute Therefore the minute respiratory volume – 6 litre / minute
Question 10.
What are the characteristic features of the respiratory surface?
Answer:
The surface area of the respiratory surface is large and richly supplied with blood vessels.
- It is extremely thin and kept moist.
- It is in direct contact with the environment.
- It is permeable to respiratory gases.
![]()
Question 11.
Give short notes on a ‘C’ shaped cartilage of bronchi?
Answer:
Bronchi have ‘c’ shaped cartilage plates to ensure that the air passage does not collapse or burst as the air pressure changes during breathing.
Question 12.
What should be the characteristic features of the respiratory surface?
Answer:
- The surface area must be very large and richly supplied with blood vessels.
- Should be extremely thin and kept moist.
- Should be in direct contact with the environment.
- Should be permeable to the respiratory gases.
Question 13.
What is meant by breathing?
Answer:
The movement of air between the atmosphere and the lungs is known as breathing.
![]()
Question 14.
Name the muscle that helps in respiration?
Answer:
- Diaphragm
- Intercostal muscle
- External and internal intercostal muscle.
Question 15.
What is meant by expiratory reserve volume?
Answer:
- The additional volume of air a person can forcefully exhale by forcefully expiration is called expiratory reserve volume.
- The normal value is 1000-1100 ml.
Question 16.
What is the cause for the reduction in the elasticity of the lungs?
Answer:
- Healthy lungs contain large amounts of elastic connective tissue around the alveoli containing elastin which makes the lung tissue elastic.
- People with emphysema and bronchitis have difficulty in exhaling because the enzyme elastase destroys the elastin around the alveoli and reduces the elasticity of the lungs.
Question 17.
Give notes on Asthma.
Answer:
- Allergy is caused by allergens, it may be due to dust, pollens some seafood.
- Allergens provoke an inflammatory response. The allergens affect our respiratory tracts and we immediately start sneezing and coughing.
Question 18.
Why do some people snore?
Answer:
Breathing with a hoarse sound during sleep is caused by the vibration of the soft palate.
Snoring is caused by a partially closed upper airway (nose and throat) which becomes too narrow for enough air to travel through the lungs. This makes the surrounding tissues vibrate and produces the snoring sound.
![]()
Question 19.
Why we should not laugh loudly during eating.
Answer:
- The oesophagus and trachea lies in the pharynx During swallowing a thin elastic flap called epiglottis prevent the food from entering in to the larynx.
- If we talk or laugh during swallowing the closing of trachea becomes disturbed and hence the food may enter in to trachea.
Question 20.
Breathing through the nose is healthy than through the mouth? why?
Answer:
- There are more dust and microbes in the air. If we breathe through the mouth there is a possibility of entering these microbes and dust in to the stomach through oesophagus.
- When we breathe through the nose the dust will be filtered by the bristles. The dust particular is trapped by the mucous membrane of the nasal cavity.
Question 21.
Write the structure of the alveoli.
Answer:
- The diffusion membrane of the alveolus is made up of three layers. The thin squamous epithelial cells.
- The endothelium of the alveolar capillaries
- The basement substance found in between them. The thin requamous epithelial cells of alveoli are composed of Type-I and Type-II cells.
- The Type-I cells are very thin so that gases can diffuse rapidly through them. Type-II cells are thicker synthesize and secrete a substance called surfactant.
Question 22.
Give the passage of breathing.
Answer:
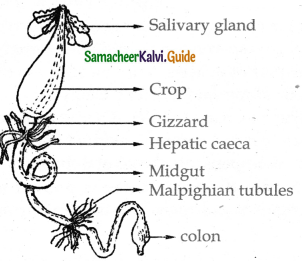
External nostrils → Nasal cavity → Pharynx → Larynx → bronchi → bronchioles → Alveolei → Lungs.
![]()
(3 marks)
III. Short Questions
Question 1.
What is meant by dead space?
Answer:
Some of the inspired air never reaches the gas exchange areas but fill, the respiratory passages where exchange of gases does not occur.
This air in called dead space. This air does not involve in respiration it amounts to 150 ml.
Question 2.
Give an account of the structures of haemoglobin?
Answer:
- Hemoglobin belongs to the class of conjugated protein.
- The iron-containing pigment portion haem constitutes only 4% and the rest colourless protein of the histone class globin.
- The molecular weight of Hb is 68000
- These four Iron atoms can combine with a molecule of oxygen.
Question 3.
What is meant by methaemoglobin?
Answer:
If the iron component of the haem is in ferric state in stead of normal ferrous state it is called methaemoglobin. Methaeglobin does not bind with O2.
![]()
Question 4.
What Are Surfactants?
Answer:
They are the thin non-cellular films made of protein and phospholipids covering the alveolar membrane.
Question 5.
What are the significances of surfactants?
Answer:
The surfactant lowers the surface tension in the alveoli and prevents the lungs from collapsing.
It also prevents pulmonary oedema.
![]()
Question 6.
What is new born respiratory distress syndrome (NRDS)?
Answer:
Premature Babies have low levels of surfactant in the alveoli may develop the new bom respiratory distress syndrome (NRDS) because the synthesis of surfactants begins only afer the 25th week of gestation.
Question 7.
What is the reason for yawning?
Answer:
When there is a shortage of O2, it is sensed by our brain and sends a message to CNS to correct the imbalance for O2 demand and trigger us to yawn. Yawning helps us to breath more oxygen to the lungs.
![]()
Question 8.
Why are hiccups occured?
Answer:
Hiccups are due to eating too fast or having occasional spasms of the diaphragm.
Question 9.
What is the need of respiration?
Answer:
For all the activities of our body energy is needed. This we receive from the food. Oxygen is utilized by the organisms to break down the biomolecules the glucose and to derive energy. Hence Respiration is necessary.
Question 10.
Why the rate of respiration in aquatic animals is high?
Answer:
The amount of dissolved oxygen is very low in water compared to the amount of oxygen in the air. So the rate of breathing in aquatic organisms is much faster than land animals.
![]()
Question 11.
What is the importance of mucus in the respiratory tract?
Answer:
The goblet cells present in the mucus membrane secrete mucus, a slimy material rich in glycoprotein. Microorganisms and dust particles attach to the mucus films and are carried upwards to pass down the gullet during swallowing.
Question 12.
What is dead space?
Answer:
Some of the inspired air never reaches the gas exchange areas but fills the respiratory passages where the exchange of gases does not occur. This air is called dead space. Dead space is not involved in gaseous exchange. It amounts to approximately 150mL.
Question 13.
Why should we avoid breathing with our mouths?
Answer:
Breathing through mouth results in bladder shrinkage and creates an urge to urinate in the middle of the night.
![]()
IV. Competitive Exam Corner
Question 1.
Sarojini’s father has congestion of the lungs. His doctor advised him to take bed rest and prescribed him an inhaler. What disease is he suffering from? List the symptoms of the disease.
Answer:
He is suffering from pneumonia.
Symptoms of pneumonia:
- Sputum Production,
- Nasal congestion,
- Shortness of breath,
- Sore throat
Question 2.
A villager who came to the city was affected by severe respiratory illness due to the inhalation of particulate pollutants. Suggest the reason for his illness and how do particulate pollutants affect him.
Answer:
He is suffering from a dust allergy. As he entered in a polluted area he started sneezing and coughing. The allergens in that place affecting his respiratory tracts and provoked inflammatory response prolonged allergy leads to Asthma.
![]()
Question 3.
Kumar’s mother works in a stone grinding factory. Suddenly she faints and taken to the hospital. The doctor notices fibers in the lungs. What kind of disease is she affected with? How can it be rectified?
Answer:
Long exposure to sand particles can give rise to inflammation leading to fibrosis. She must be hospitalized and have to give medication like anti-coagulation the imatinib. that fight against the disease.
(5 marks)
V. Essay Questions
Question 1.
List the primary functions of the respiratory system?
Answer:
- It helps in exchange of O2 and CO2 between the atmosphere and the blood.
- It maintains homeostatic regulation of body pH.
- It protects us from inhaled pathogens and pollutants.
- It maintains the vocal cords for normal communication.
- It removes the heat produced during cellular respiration through breathing.
Question 2.
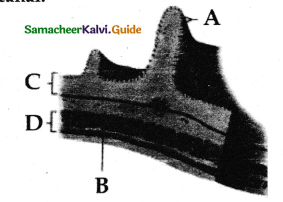
Describe the structure of trachea with a diagram.
Answer:
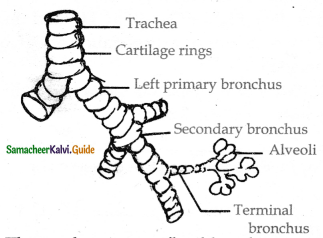
- The trachea is semiflexible tube supported by cartilaginous rings.
- It starts from the pharynx and ends in the lungs there it divides into right and left primary bronchi.
- With in the lungs the bronchi divided repeatedly into secondary and tertiary bronchi.
- That further divides into terminal bronchioles and respiratory bronchioles.
- Bronchi have ‘c’ shaped curved cartilage plates.
- This plate helps in preventing collapsing as the air pressure changes during breathing.
- There is no cartilaginous stingray the brarichioles,
- The rigidity of the bronchioles prevents them from collapsing.
![]()
Question 3.
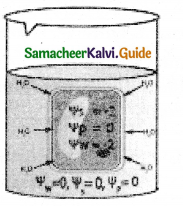
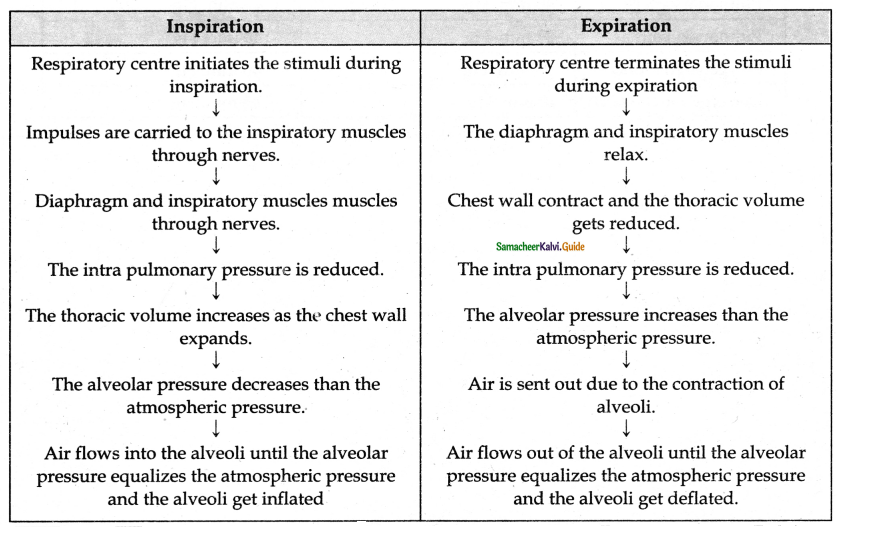
Describe the process of inspiration & expiration with a diagram?
Answer:
Inspiration occurs if the pressure inside the lungs is less than the atmospheric pressure.
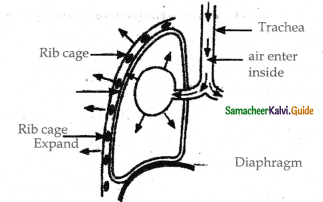
Inspiration:
- There is a contraction of diaphragm muscles and external intercostal muscles which pulls the ribs and sternum upwards and downwards and increases the volume of the thoracic chamber in the dorsoventral axis.
- Hence the pulmonary pressure is less than the atmospheric pressure.
- This forces the fresh air from outside to enter the air passages into the lungs to equalize the pressure.
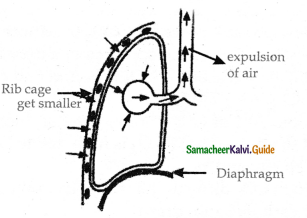
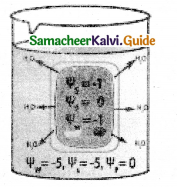
Expiration:
- Expiration takes place when the pressure within the lungs is higher than the atmospheric pressure.
- Relaxation of the diaphragm leads to its original dome-shaped nature.
- The internal intercostal muscles contract pulling the ribs downward reducing the thoracic volume and pulmonary volume.
- Thin results in an increase in the intrapulmonary pressure slightly above the atmospheric pressure causing the expulsion of air from the lungs.
Question 4.
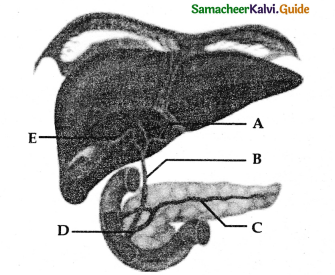
Describe the structure of lung with a diagram.
Answer:
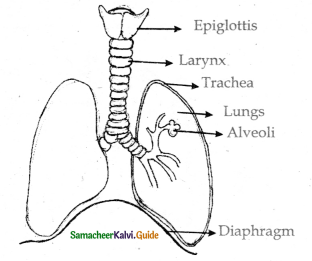
- The lungs are light spongy tissue.
- It is enclosed inthe thoracic cavity surrounded by an air-tight space.
- The thoracic cavity is bound dorsally by the ventral column and ventrally by the sternum. laterally by the ribs and on the lower side by the dome-shaped diaphragm.
- The lungs are covered by a double walled pleural membrane and the plural cavity is filled with pleural fluid which reduces friction.
- The trachea is a semi-flexible tube supported by cartilaginous rings which extends upto the 5th thoracic vertebra.
- It divides into right and left bronchi and enters in to the lungs. There it divides further many times and ends in alveoli.
![]()
Question 5.
Describe the process of transport of oxygen.
Answer:
- Molecular oxygen is carried in blood in two ways. bound to haemoglobin within the red blood cells and dissolved in plasma.
- 3% of O2 is transported in the dissolved form.
- 97% of O2 binds with haemoglobin to form oxyhemoglobin.
- Each haemoglobin carries four molecules of O2
- The high PO2 in the alveoli low PCO2 low temperature and less H+ concentration favours the formation of oxyhaemoglobin.
- The low PO2 high PCO2 high H and high temperature favours the dissociation of O2 from oxyhaemoglobin.
- Every 100ml of oxygenated blood can deliver about 5ml of O2 to the tissues.
Question 6.
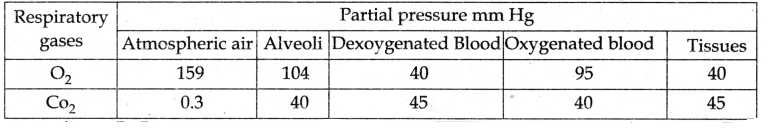
Give the tabulated column of partial pressure of O2 and CO2 in comparison to the gases in the atmosphere.
Answer:
Question 7.
Describe the process of CO2 transport.
Answer:
- 7-10% of CO2 is transported in a dissolved form in the plasma.
- 20-25% of dissolved CO2 is bound and carried in the RBCs as carbamino haemoglobin.
CO2 + H6 ⇌ H6 CO2 - About 70% of CO2 is transported as bicarbonate ions.
- At the tisoues the PCO2 is high due to catabolism and diffuses in the blood to form HCO–3 and H+
- When CO2 diffuses into RBCs it combines with water forming carbonic acid catalyzed by carbonic anhydrase.
- Carbonic acid is dissociated into hydrogen and bicarbonate.
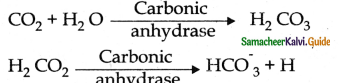
- Every 100 ml of deoxygenated blood delivers 4ml CO2 to the alveoli for elimination.
Question 8.
Describe the process of regulation of respriation.
Answer:
- Medulla oblongata is a repiratory regulation centre.
- The pneumotaxic centre present in the pons varoli is the respiratory rhythm centre.
- The chemosensitive area found close to the rhythm centre is highly sensitive to CO2 and H+
- H+ are eliminated out by respiratory process.
- Receptiors associated with the aortic arch and carotid artery send signals to the rhythm centre for remedial action.
![]()
Question 9.
Write an essay on respiratory disorders.
Answer:
The respiratory system is affected by environmental occupational personal and social factors.
Following are some of the respiratory disorders:
Asthma: It is characterized by narrowing and inflammation of bronchi and bronchioles and difficulty in breathing.
Causes: Allergens like dust drugs pollen grains, certain food items like fish.
Emphysema: It is chronic breathlessness. It is caused by gradual breakdown of the thin wall of the alveoli decreasing the total surface area of a gaseous exchange.
Causes: The widening of the alveoli is called Emphysema.
Cigarette smoking reduces the respiratory surface of the alveolar walls.
Bronchitis: It is the inflammation of the bronchi.
Causes: Pollution smoke ciagratte smoking.
Symptoms: Cough shortness of breath sputum in the lungs.
Pneumonia: It is the inflammation of the lungs.
Causes: Bacteria and virus
Symptoms
- Sputum production Nasal congestion, Shortness of breath sore throat.
- Tuberculosis
Causes
- Tuberculosis is caused by mycobacterium tubercular.
- Infection mainly occurs in the lungs and bones.
Symptoms
Collection of fluid between the lungs and the chest wall is the main complication of this
Question 10.
List the ill effects of smoking
Answer:
- It increases the heart beat rate.
- It narrows the blood vessels results in raised blood pressure and leads to coronary heart diseases.
- Smoking can cause lung diseases by damaging the airways and alveoli and results in emphysema and chronic bronchitis.
Question 11.
Tabulate the organism. respiratary organs and the
Answer:
| ORGANISMS | RESPIRATORY ORGANS |
| 1. Sponges, Coelenterates | Body surface |
| 2. Earth worm | The moist skin |
| 3. Insects | Trachea |
| 4. Aquatic Arthropods mollusca | Gills |
| 5. Fishes | Gills |
| 6. Amphibians, Reptiles Aves mammals | Lungs |
| 7. Frog | Lungs, Moist skin |
Question 12.
What are the steps involved in the respiratory process?
Answer:
Steps involved in respiration are
- The exchange of air between the atmosphere and the lungs.
- The exchange of O2 and CO2 between the lungs and the blood.
- Transport of O2 and CO2 by the blood.
- Exchange of gases between the blood and the cells.
- Intake of O2 by the cells for various activities and release of CO2
![]()
Question 13.
Tabulate the disorders of respiratory system.
Answer:
| Disorders | Symptoms |
| 1. Pulmonary Embolism | Blood clot occurs in the lung |
| 2. Bronchitis | Inflammation of the lining of your bronchial tubes |
| 3. Asthma | Swelling and narrowing of air ways and there is excess secretion of mucus. |
| 4. Lung cancer | Smoking causes cancer |
| 5. Pneumonia | Inflammation of lungs affecting alveoli |
| 6. Pulmonary edema | fluid accumulation of the tissue and air spaces of lung. |
| 7. Emphysema | Shortness of breath due to widening of alveoli |
| 8. Atelectasis | Alveoliand lungs get deflated |
| 9. Tuberculosis | It affects lungs and bones and effasion (fluid accumulation in the lungs) |
| 10. Pleurisy | Pleura becomes inflammed |
Question 14.
List the problems in oxygen transport.
Answer:
- When a person travels from sea level to elevations above 8000 ft there is a poor binding of O2 with haemoglobin.
- There is a symptom of headache shortness of breath nausea and dizziness develop. (Acute mountain sickness)
- To overcome this situation kidneys accelerate the production of the hormone erythropoietin which stimulates the synthesis of RBCs.
II. Inthedeepsea
- When a person descends deep in to the sea the pressure in the water increases which causes the lungs to decrease in volume.
- There is an increased nitrogen level in the blood lead to nitrogen narcosis.
- When the diver ascends to the surface a condition called decompression sickness occurs. As nitrogen comes out of solution while still in the blood-forming bubbles.
- The large bubbles can block the blood flowor can press on the nerve ending. This also causes pain in joints, muscles and causes neurological problems.
![]()
Question 15.
List the toxic substances present in tobacco. What are the ill-effects of smoking.
Answer:
a) Toxic substances present in tobacco.
Nicotine tar, carbon monoxide ammonia, arsenic and sulphur dioxide.
b) ill effects
- Carbon monoxide and Nicotine damaged the cardie vascular system.
- The tar damages the gaseous exchange system.
- Nicotine stimulate the heart to beat faster and narrowing the blood vessels results in raised blood pressure and coronary heart diseases.
- Carbon monoxide reduces O2 Supply.
- Smoking causes lung, stomach, and pancreases and bladder cancer.
- It lowers sperm count in men.
Question 16.
What is meant by chronic obstructive pulmonary disease?
Answer:
- Smoking can cause lung diseases by damaging the airways and alveoli and results in emphysema and chronic bronchitis.
- These two diseases along with asthma are referred to as a chronic obstructive pulmonary disease.
- When a preson smokes 85% of the smoke released is inhaled by the smoker himself and others in the vicinity called passive smokers are also affected indirectly.
![]()
Question 17.
List the events in inspiration and expiration.
Answer:
Question 18.
Describe the relationship between partial pressure of O2 and the nature of O2 dissolving the haemoglobin.
Answer:
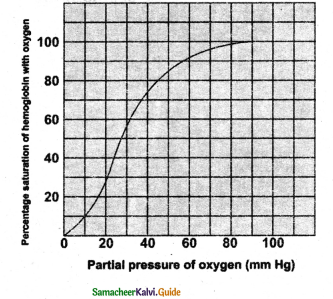
- In the alveoli high PO2 low PCO3 Low temperature and less H+ Concentration favours the formation of oxyhemoglobin wjiere as in the tissues low PO2 high PCO2 high H and high-temperature favoures the dissocation of O2 from oxyhemoglobin.
- A sigmoid curve is obtained when the percentage saturation of haemoglobin with O2 is plotted against PO2.
- This S, Shaped curve has a steep slope for PO2 valuer between 10 and 50 mm Hg and then flattens between 70 and 100 mm Hg.
- Under normal physiological conditions, every 100 ml of oxygenated blood can deliver about 5 ml of O2 to the tissues.
![]()
Question 19.
List the PO2 and PCO2 during inspiration expiration and in lungs and blood vessels.
Answer:
| Location | Partial Pressures of Oxygen PO2 | The partial pressure of CO2 PCO2 |
| Inspiration | 159 mm. Hg | 0.3 mm. Hg |
| Expiration | 120 mm. Hg | 127 mm. Hg |
| Alveoli | 104 mm. Hg | 40 mm. Hg |
| Pulmonary artery | 40 mm. Hg | 45 mm.Hg |
| Pulmonary vein | 95 mm. Hg | 40 mm. Hg |
| Oxygenated blood | 95 mm. Hg | 40 mm. Hg |
| Deoxygenated blood | 40 mm. Hg | 45 mm. Hg |