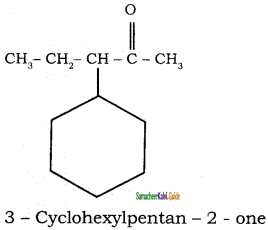
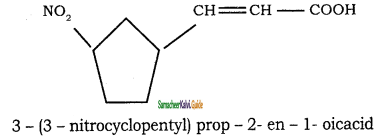
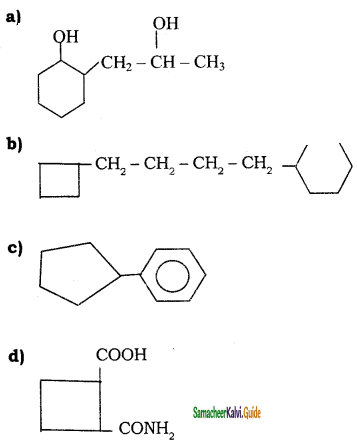
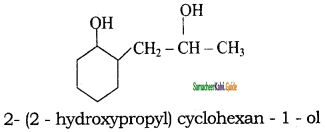
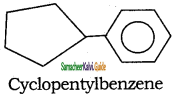
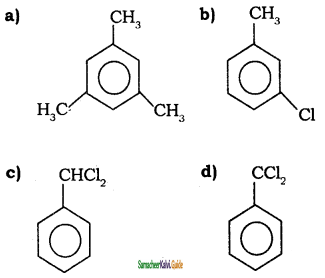
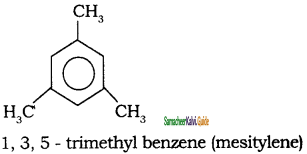
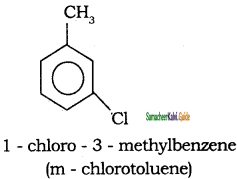
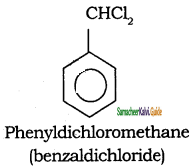
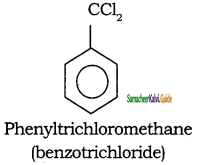
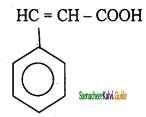
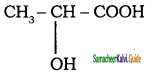
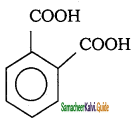
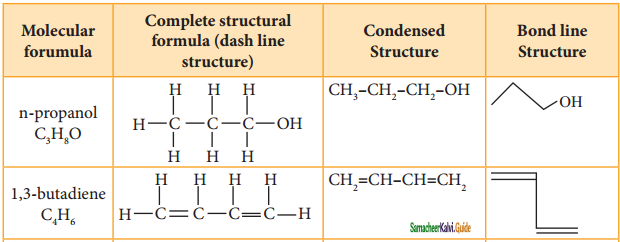
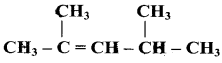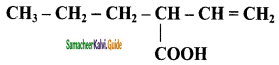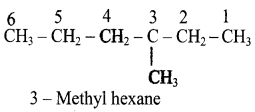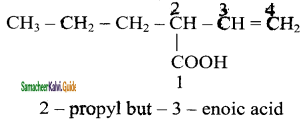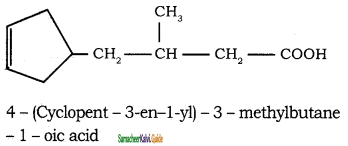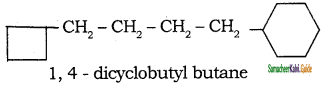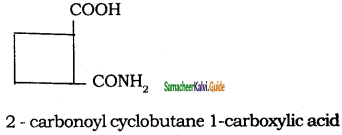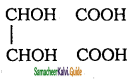Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Chemistry Guide Pdf Chapter 13 Hydrocarbons Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Chemistry Solutions Chapter 13 Hydrocarbons
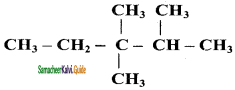
11th Chemistry Guide Hydrocarbons Text Book Back Questions and Answers
Textbook Evaluation:
I. Choose the best answer:
Question 1.
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is
a) The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain.
b) The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
c) The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain.
d) The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has no torsional strain.
Answer:
b) The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain.
Question 2.
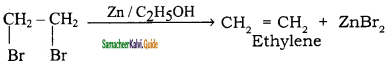
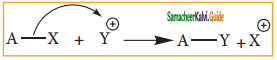
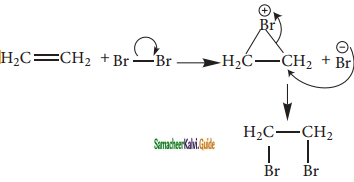
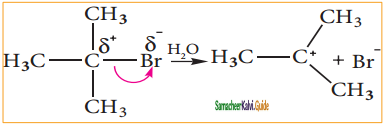
C2H5 Br + 2Na ![]() C4H10 + 2NaBr.
C4H10 + 2NaBr.
The above reaction is an example of which of the following
a) Reimer Tiemann reaction
b) Wurtz reaction
c) Aldol condensation
d) Hoffmann reaction
Answer:
b) Wurtz reaction
Question 3.
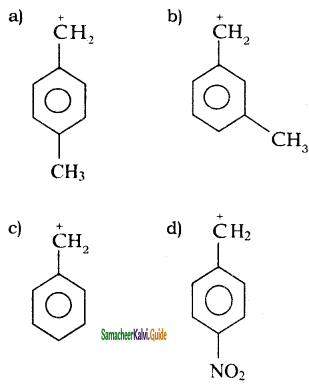
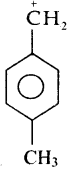
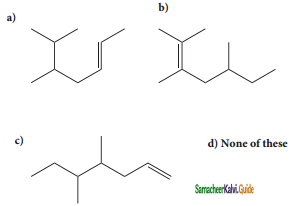
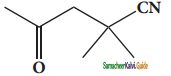
An alkyl bromide (A) reacts with sodium in ether to form 4, 5 – diethyloctane, the compound (A) is
a) CH3(CH2)3Br
b) CH3(CH2)5Br
c) CH3(CH2)3 CH(Br)CH3
d) 
Answer:
d) 
Question 4.
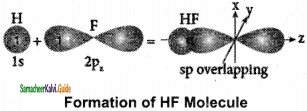
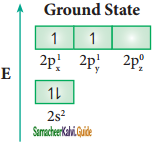
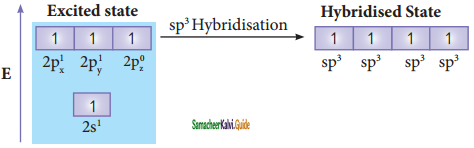
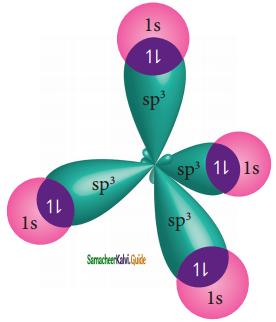
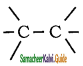
The C – H bond and C – C bond in ethane are formed by which of the following types of overlap
a) sp3 – s and sp3 – sp3
b) sp2 – s and sp2 – sp2
c) sp – sp and sp – sp
d) p – s and p – p
Answer:
a) sp3 – s and sp3 – sp3
Question 5.
In the following reaction,
Answer:
c) 
![]()
Question 6.
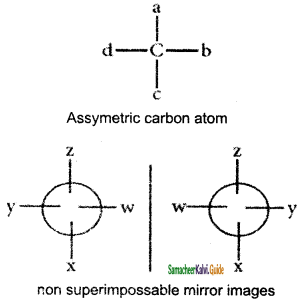
Which of the following is optically active
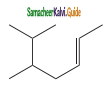
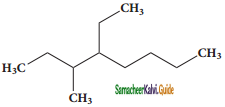
a) 2 – methyl pentane
b) citric acid
c) Glycerol
d) none of these
Answer:
a) 2 – methyl pentane
Question 7.
The compound formed at anode in the electrolysis of an aqueous solution of potassium acetate are
a) CH4 and H2
b) CH4 and CO2
c) C2H6 and CO2
d) C2H4 and Cl2
Answer:
c) C2H6 and CO2
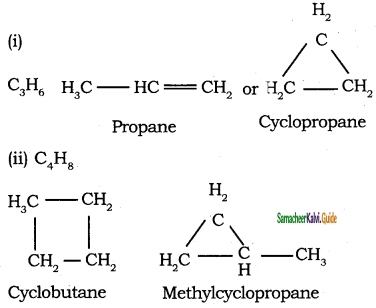
Question 8.
The general formula for cyclo alkanes
a) CnHn
b) CnH2n
C) CnH2n-2
d) CnH2n + 2
Answer:
b) CnH2n
Question 9.
The compound that will react most readily with gaseous bromine has the formula
a) C3H6
b) C2H2
c) C4H10
d) C2H4
Answer:
a) C3H6
Question 10.
Which of the following compounds shall not produce propene by reaction with HBr followed by elimination (or) only direct elimination reaction
a) ![]()
b) CH3 – CH2 – CH2 – OH
c) H2C = C = O
d) CH3 – CH2 – CH2Br
Answer:
c) H2C = C = O
![]()
Question 11.
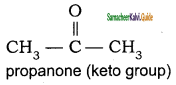
Which among the following alkenes on reductive ozonolysis produces only propanone?
a) 2 – Methyl propene
b) 2 – Methyl but – 2 – ene
c) 2, 3 – Dimethyl but – 1- ene
d) 2, 3 – Dimethyl but – 2 – ene
Answer:
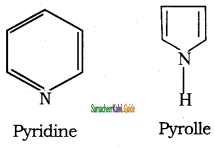
d) 2, 3 – Dimethyl but – 2 – ene
Question 12.
The major product formed when 2 – bromo – 2 – methyl butane is refluxed with ethanolic KOH is
a) 2 – methylbut – 2- ene
b) 2 – methyl butan – 1 – ol
c) 2 – methyl but – 1 – ene
d) 2 – methyl butan – 2- ol
Answer:
a) 2 – methylbut – 2- ene
Question 13.
Major product of the below mentioned reaction is, (CH3)2 C = CH2 ![]()
a) 2 – chloro – 1 – iode – 2 – methyl propane
b) 1 – chloro – 2 – iodo – 2 – methyl propane
c) 1, 2 – dichloro – 2 – methyl propane
d) 1, 2 – diiodo – 2 – methyl propane
Answer:
a) 2 – chloro – 1 – iode – 2 – methyl propane
Question 14.
The IUPAC name of the following compound is
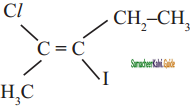
a) trans – 2- chloro – 3- iodo – 2- pentane
b) cis – 3- iodo – 4 chloro – 3 – pentane
c) trans – 3 – iodo – 4 – chloro – 3 – pentene
d) cis – 2 – chloro – 3 iodo – 2 – pentene
Answer:
a) trans – 2- chloro – 3- iodo – 2- pentane
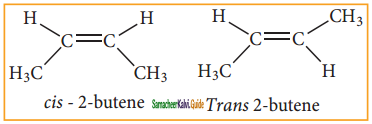
Question 15.
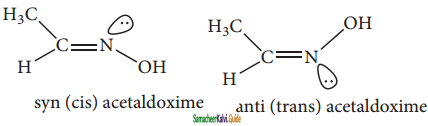
Cis – 2- butene and trans – 2 – butene are
a) conformational isomers
b) structural isomers
c) configurational isomers
d) optical isomers
Answer:
c) configurational isomers
![]()
Question 16.
Identify the compound (A) in the following reaction
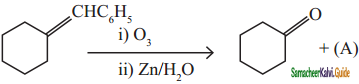
Answer:
a) 
Question 17.
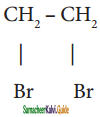
![]() CH ≡ CH , where A is,
CH ≡ CH , where A is,
a) Zn
b) conc. H2SO4
c) alc. KOH
d) dil. H2SO4
Answer:
c) alc. KOH
Question 18.
Consider the nitration of benzene using mixed con H2SO4 and HNO3 if a large quantity of KHSO4 is added to the mixture, the rate of nitration will be
a) unchanged
b) doubled
c) faster
d) slower
Answer:
d) slower
Question 19.
In which of the following molecules, all atoms are co-planar
a) 
b) 
c) 
d) both (a) and (b)
Answer:
d) both (a) and (b)
Question 20.
Propyne on passing through red hot iron tube gives
a) 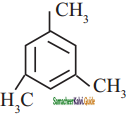
b) 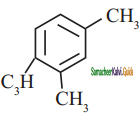
c) 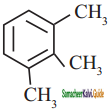
d) none of these
Answer:
a) 
![]()
Question 21.
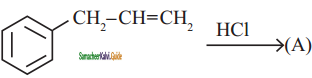 is
is
a) 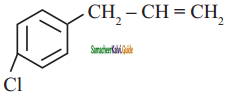
b) 
c) both (a) and (b)
d) 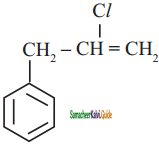
Answer:
d) 
Question 22.
Which one of the following is non aromatic?
a) 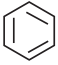
b) 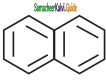
c) 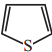
d) ![]()
Answer:
d) ![]()
Question 23.
Which of the following compounds will not undergo Friedal – crafts reaction easily?
a) Nitro benzene
b) Toluene
c) Cumene
d) Xylene
Answer:
a) Nitro benzene
Question 24.
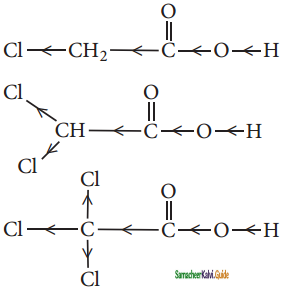
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
a) – COOH
b) – NO2
c) -C ≡ N
d) -SO3H
Answer:
b) – NO2
Question 25.
Which of the following can be used as the halide component for friedal – crafts reaction?
a) Chloro benzene
b) Bromo benzene
c) Chloro ethene
d) Isopropyl chloride
Answer:
d) Isopropyl chloride
![]()
Question 26.
An alkane isobtainedbydecarboxylation of sodium propionate. Same alkane can be prepared by
a) Catalytic hydrogenation of propene
b) action of sodium metal on iodomethane
c) reduction of 1 – chloro propane
d) reduction of bromomethane
Answer:
b) action of sodium metal on iodomethane
Question 27.
Which of the following is aliphatic saturated hydrocarbon
a) C8H18
b) C9H18
c) C8H14
d) All of these
Answer:
a) C8H18
Question 28.
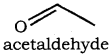
Identify the compound ‘Z’ in the following reaction
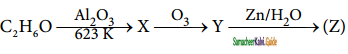
a) Formaldehyde
b) Acetaldehyde
c) Formic acid
d) none of these
Answer:
a) Formaldehyde
Question 29.
Peroxide effect (Kharasch effect) can be studied in case of
a) Oct – 4 – ene
b) hex – 3 – ene
c) pent – 1 – ene
d) but – 2 – ene
Answer:
c) pent – 1 – ene
Question 30.
2 – butyne on chlorination gives
a) 1 – chloro butane
b) 1, 2 – dichloro butane
c) 1, 1, 2, 2 – tetrachlorobutane
d) 2, 2, 3, 3 – tetra chloro butane
Answer:
d) 2, 2, 3, 3 – tetra chloro butane
![]()
II. Write brief answer to the following questions:
Question 31.
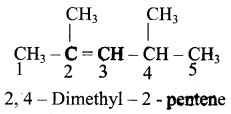
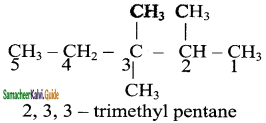
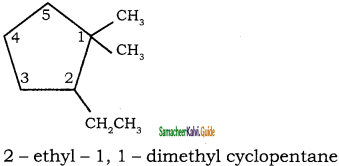
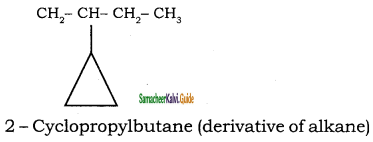
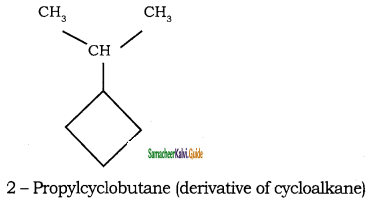
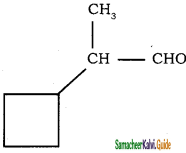
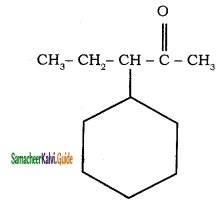
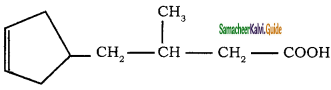
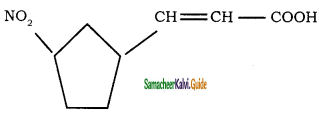
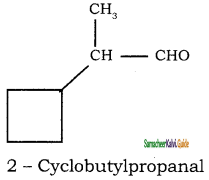
Give IUPAC names for the following compounds
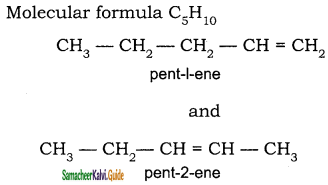
1) CH3 – CH = CH – CH = CH – C ≡ C – CH3
2) 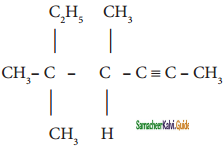
3) (CH3)3 C – C ≡ C – CH(CH3)2
4) ethyl isopropyl acetylene
5) CH ≡ C – C ≡ C – C ≡ CH
Answer:
1) 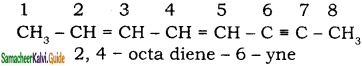
2) 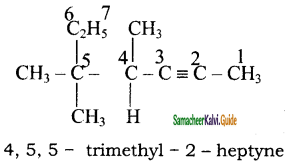
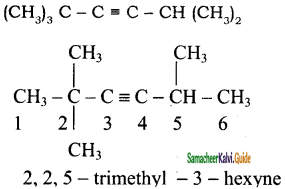
3) 
4) ethyl isopropyl acetylene
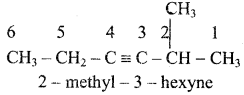
5) 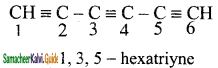
Question 32.
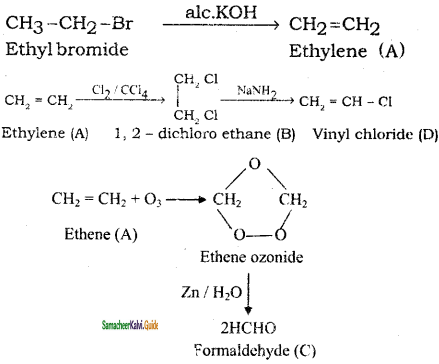
Identify the compound A, B, C and D in the following series of reactions
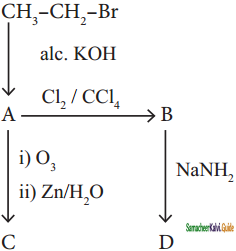
Answer:
Question 33.
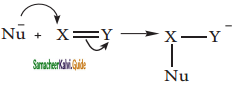
Write short notes on ortho, para directors in aromatic electrophilic substitution reactions.
Answer:
All the activating groups are ‘ortho-para’ directors.
Example: – OH, – NH2, -NHR, -NHCOCH3, -OCH3 -CH3 – C2H5 etc.
Let us consider the directive influences of phenolic (-OH) group. Phenol is the resonance hybrid of following structures.
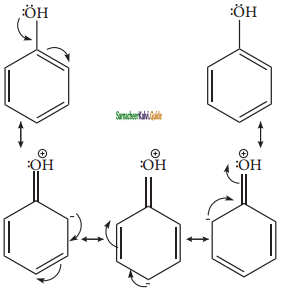
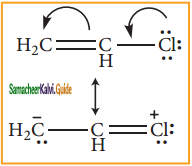
In these resonance structures, the (-) charge residue is present on ortho and para position of ring structure. It is quite evident that the lone pair of electron on the atom which is attached to the ring involves in resonance and makes the ring more electron rich than benzene. The electron density at ortho and para positions increases as compared to the meta position. Therefore phenolic group activates the benzene ring for electrophilic attack at ‘ortho’ and ‘para positions and hence -OH group is an ortho-para director and activator.
In aryl halides, the strong -I effect of the halogens (electron withdrawing tendency) decreases the electron density of benzene ring, thereby deactivating for electrophilic attack. However the presence of lone pair on halogens involved in the resonance with pi electrons of benzene ring, increases electron density at ortho and para position. Hence the halogen group is an ortho-para director and deactivator.
![]()
Question 34.
How is propyne prepared from an alkene dihalide?
Answer:
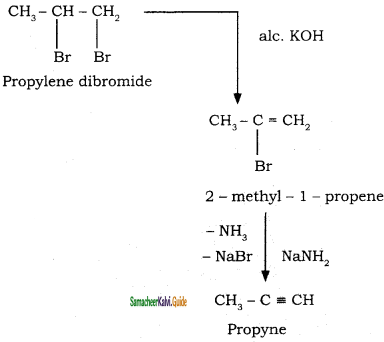
Question 35.
An alkylhalide with molecular formula C6H13Br on dehydro halogenation gave two isomeric alkenes X and Y with molecular formula C6H12. On reductive ozonolysis, X and Y gave four compounds CH3COCH3, CH3CHO, CH3CH,CHO and (CH3)2 CHCHO. Find the alkyihalide.
Answer:
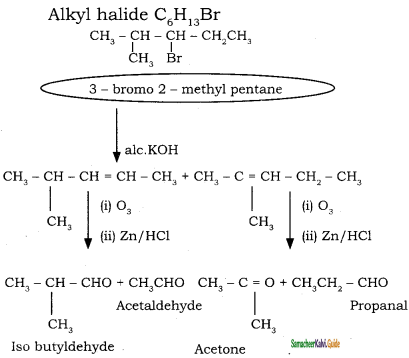
Question 36.
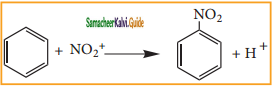
Describe the mechanism of Nitration of benzene.
Answer:
It is prepared by the action of a mixture of con. HNO3 and con. H2SO4 (nitrating mixture) on benzene maintaining the temperature below 333 K.
Nitration:
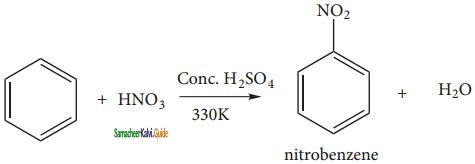
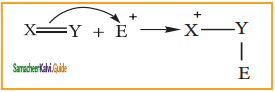
Sulphuric acid generates the electrophile – NO2+, nitronium ion-from nitric acid. This is an example of aromatic electrophilic substitution reaction.
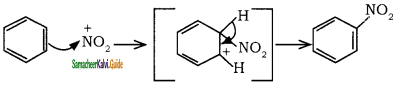
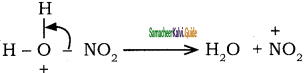
The generation of nitronium ion
H2SO4 + HONO2 → 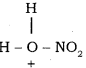 + HSO4–
+ HSO4–
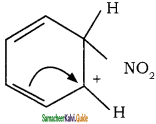
To the nitronium ion (being an electron deficient species) the π bond of benzene, donates a pair of electrons forming a 6-bond. A species with a + ve charge is formed as an intermediate. This is called ‘arenium ion’ and is stabilised by Resonance.
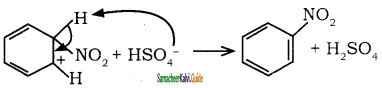
In the last step, the hydrogen atom attached to the carbon carrying the nitro group is pulled out as a proton, by the Lewis base HSO4–, so that stable aromatic system is formed.
Question 37.
How does Huckel rule help to decide the aromatic character of a compound.
Answer:
In 1865, August Kekule suggested that benzene consists of a cyclic planar structure of six carbon with alternate single and double bonds.
There were two objections:
Benzene forms only one ortho disubstituted products whereas the Kekule’s structure predicts two o-di substituted products as shown below.
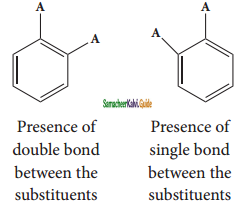
Kekule’s structure failed to explain why benzene with three double bonds did not give addition reactions like other alkenes.To overcome this objection, Kekule suggested that benzene was mixture of two forms (1 and 2) which are in rapid equilibrium.
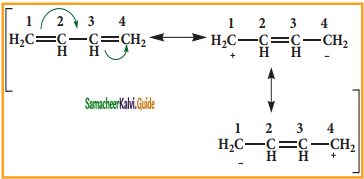
Resonance description of benzene:
The phenomenon in which two or more structures can be written for a substance which has identical position of atoms is called resonance. The actual structure of the molecule is said to be resonance hybrid of various possible alternative structures. In benzene, Kekule’s structures I & II represented the resonance structure, and structure III is the resonance hybrid of structure I & II.
The structures 1 and 2 exist only in theory. The actual structure of benzene is the hybrid of two hypothetical resonance structures.
Spectroscopic measurements:
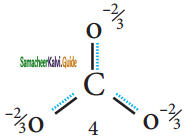
Spectroscopic measurements show that benzene is planar and all of its carbon- carbon bonds are of equal length 1.40 Å. This value lies between carbon-carbon single bond length 1.54 Å and carbon- carbon double bond length 1.34 Å.
Molecular orbital structure:
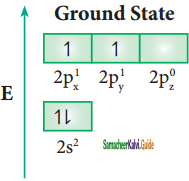
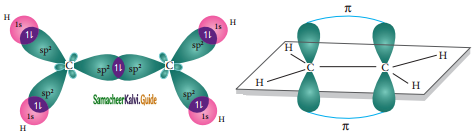
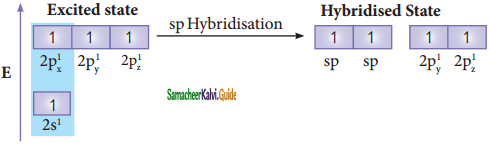
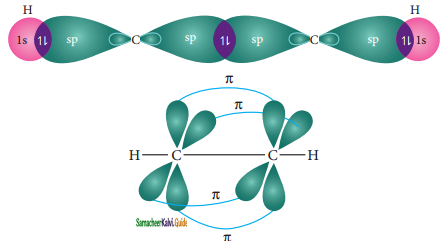
The structure of benzene is best de¬scribed in terms of the molecular orbital theory. All the six carbon atoms of benzene are sp2 hybridized. Six sp2 hybrid orbitals of carbon linearly overlap with six 1 s orbitals of hydrogen atoms to form six C – H sigma bonds. Overlap between the remaining sp2 hybrid orbitals of carbon forms six C – C sigma bonds.
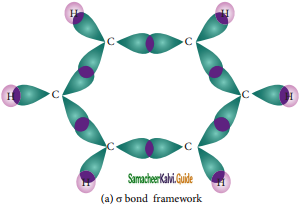
Formation of Sigma bond in benzene
All the σ bonds in benzene lie in one plane with bond angle 120°. Each carbon atom in benzene possess an un hybridized p-orbital containing one electron. The lateral overlap of their p-orbital produces 3 π- bond. The six electrons of the p-orbitals cover all the six carbon atoms and are said to be delocalised. Due to delocalization, strong π-bond is formed which makes the molecule stable. Hence unlike alkenes and alkynes benzene undergoes substitution reactions rather addition reactions under normal conditions.
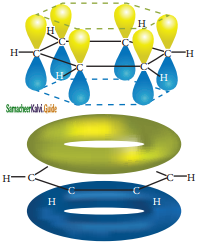
All carbon atoms have the delocalized π MO is formed by p-orbitais the overlap of six p-orbitals.
Representation of benzene:
Hence, there are three ways in which benzene can be represented.
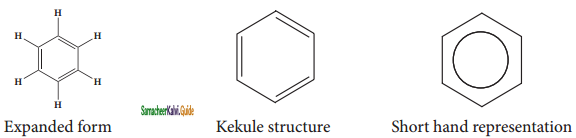
![]()
Question 38.
Suggest the route for the preparation of the following from benzene.
1) 3 – chloro nitrobenzene
2) 4 – chlorotoluene
3) Bromo benzene
4) m – dinitro benzene
Answer:
1) 3 – chloro nitrobenzene
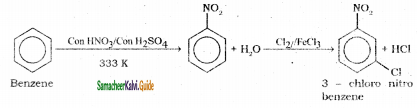
2) 4 – chlorotoluene
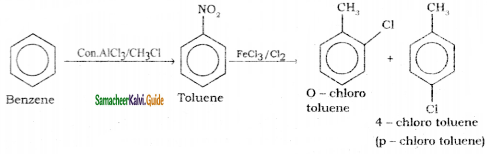
3) Bromo benzene
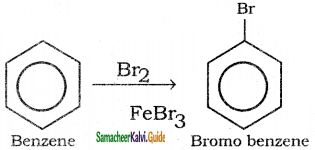
4) m – dinitro benzene
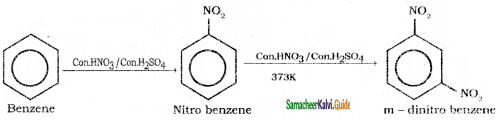
Question 39.
Suggest a simple chemical test to distinguish propane and propene.
Answer:
Propene decolourises Br2/H2O it forms dibromo compound but propane does not react with Br2/ H2O.
Question 40.
What happens when Isobutylene is treated with acidified potassium permanganate?
Answer:
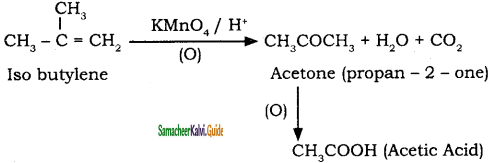
Question 41.
How will you convert ethyl chloride into
(i) ethane
(ii) n – butane
Answer:
(i) ethane
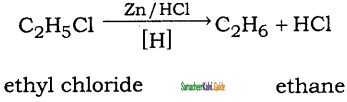
(ii) n – butane
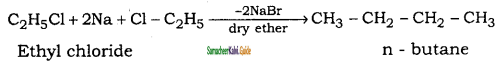
![]()
Question 42.
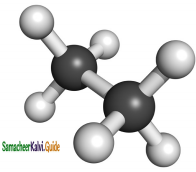
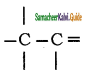
Describe the conformers of n – butane.
Answer:
Conformations of n-Butane:
n-Butane may be considered as a derivative of ethane, as one hydrogen on each carbon is replaced by a methyl group
Eclipsed conformation:
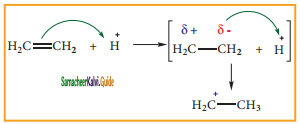
In this conformation, the distance between the two methyl group is minimum. So there is maximum repulsion between them and it is the least stable conformer.
Anti or staggered form:
In this conformation, the distance between the two methyl groups is maximum and so there is minimum repulsion between them. And it is the most stable conformer.
The following potentially energy diagram shows the relative stabilities of various conformers of n-butane.
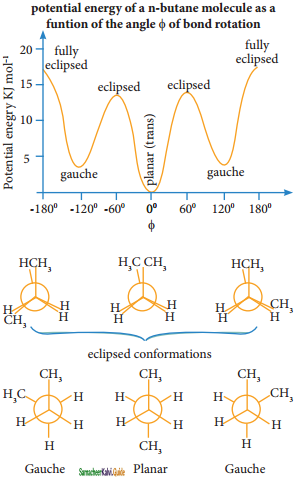
Question 43.
Write the chemical equations for combustion of propane.
Answer:
C3H8(g) + 5O2(g) ) → 3CO2(g) + 4H2O(l)
propane
Question 44.
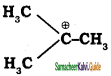
Explain Markovnikoff’s rule with suitable example.
Answer:
Markovnikoff ‘s rule:
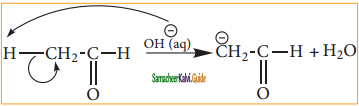
“When an unsymmetrical alkene reacts with hydrogen halide, the hydrogen adds to the carbon that has more number of hydrogen and halogen add to the carbon having fewer hydrogen”. This rule can also be stated as in the addition reaction of alkene/alkyne, the most electro negative part of the reagent adds on to the least hydrogen attached doubly bonded carbon.
Addition of water: (Hydration of alkenes)
Normally, water does not react with alkenes. In the presence of concentrated sulphuric acid, alkenes react with water to form alcohols. This reaction follows carbocation mechanism and Markovnikoff’s rule.
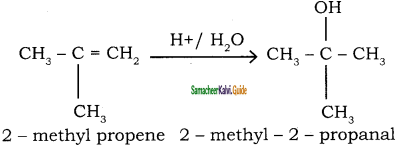
Question 45.
What happens when ethylene is passed ‘ through cold dilute alkaline potassium permanganate.
Answer:
CH2 = CH + H2O + (O) ![]() CH2OH – CH2OH
CH2OH – CH2OH
Ethylene Ethylene glycol
![]()
Question 46.
Write the structures of following alkanes.
1) 2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane
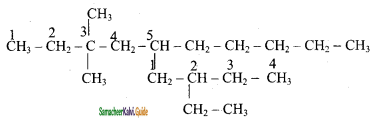
2) 5 – (2 – Ethyl butyl ) – 3, 3, – dimethyldecane
3) 5 – (1, 2 – Dimethyl propyl) – 2 – methylnonane
Answer:
1) 2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane
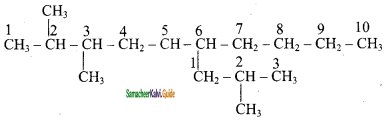
2) 5 – (2 – Ethyl butyl ) – 3, 3, – dimethyldecane
3) 5 – (1, 2 – Dimethyl propyl) – 2 – methylnonane
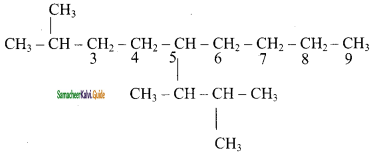
Question 47.
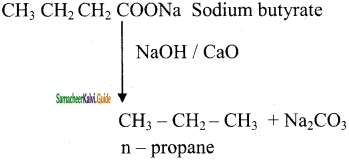
How will you prepare propane from a sodium salt of fatty acid?
Answer:
Question 48.
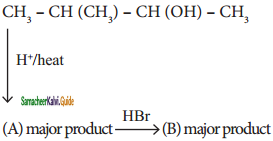
Identify A and B
Answer:
A) 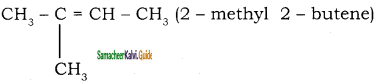
b) 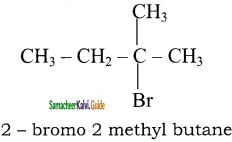
Question 49.
Complete the following :
i) 2 – butyne ![]()
ii) CH2 = CH2 ![]()
iii) 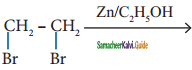
iv) CaC2 ![]()
Answer:
i) 2 – butyne ![]()
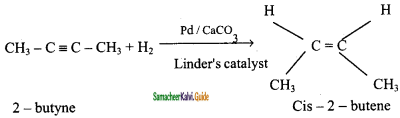
ii) CH2 = CH2 ![]()

iii) 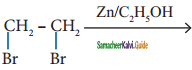
iv) CaC2 ![]()
CaC2 + 2H2O → Ca(OH)2 + C2H2↑
Calcium Carbide Acetylene
Question 50.
How will distinguish 1 – butyne and 2 – butyne?
Answer:
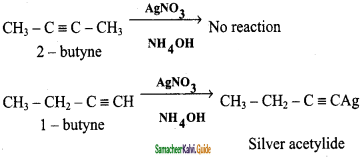
1 – butyne reacts with ammoniacal AgNO3 solution it forms white precipitate of silver acetylide but, 2 – butyne doesnot reacts with ammoniacal AgNO3 solution.
![]()
11th Chemistry Guide Hydrocarbons Additional Questions and Answers
I. Choose the best answer:
Question 1.
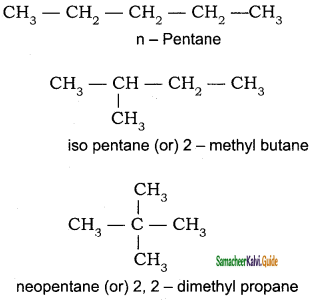
The IUPAC name of neopentane is
a) 2 – methyl butane
b) 2, 2 – dimethyl propane
c) 2 – methyl propane
d) 2, 2- dimethyl butane
Answer:
b) 2, 2 – dimethyl propane
Question 2.
Alkanes are also known as
a) olefins
b) unsaturated aliphatic hydrocarbons
c) saturated aromatic hydrocarbon
d) paraffins
Answer:
d) paraffins
Question 3.
The compressed gas available in cooking gas cylinders is a mixture of:
a) C6H6 + C6H5CH3
b) C2H4 + C2H2
c) C2H4 + CH4
d) C4H10 + C3H8
Answer:
d) C4H10 + C3H8
Question 4.
The gas supplied in cylinders for cooking is
a) marsh gas
b) LPG
c) mixture CH4 and C2H6
d) mixture of ethane and propane
Answer:
d) mixture of ethane and propane
Question 5.
Adam’s catalyst is:
a) platinum metal
b) palladium
c) nickel metal
d) PtO2
Answer:
d) PtO2
![]()
Question 6.
Soda lime is
a) NaOH
b) NaOH + CaO
c) CaO
d) Na2CO3
Answer:
b) NaOH + CaO
Question 7.
Methyl bromide is converted into ethane by heating it in ether medium with
a) Al
b) Mg
c) Na
d) Cu
Answer:
c) Na
Question 8.
Hydrocarbon which is liquid at room temperature is
a) Pentane
b) Butane
c) Propane
d) Ethane
Answer:
a) Pentane
Question 9.
Pyrolysis of Methane and respectively are
a) Exothermic & Endothermic
b) Endothermic & Exothermic
c) Both are endothermic
d) Both are exothermic
Answer:
c) Both are endothermic
Question 10.
Select the correct statement about alkanes
a) they are polar in nature
b) they are soluble in water
c) they are non – combustible
d) their dipole moment is zero
Answer:
d) their dipole moment is zero
![]()
Question 11.
Final products of complete oxidation of hydrocarbon is
a) Acid
b) Dihydric alcohol
c) Aldehyde
d) H2O + CO2
Answer:
d) H2O + CO2
Question 12.
Isomerisation in alkane can be brought by using
a) Al2O3
b) Fe2O3
c) Anh.AlCl3/ HCl at 200°C
d) Cone. H2SO4
Answer:
c) Anh.AlCl3/ HCl at 200°C
Question 13.
In aromatization of n – hexane, the catalyst used is
a) Cr2O3
b) V2O5
c) Mo2O3
d) All
Answer:
d) All
Question 14.
The most oxidized form of ethane is
a) CO2
b) HCHO
c) HCOOH
d) CH3COOH
Answer:
a) CO2
Question 15.
The following substance is used as anti knocking compound
a) TEL
b) Lead tetrachioride
c) Lead acetate
d) C2H2PbCl
Answer:
a) TEL
![]()
Question 16.
Conformational isomers are due to
a) Free rotation about C – C single bond
b) Frozen rotation about C – C single bond
e) Frozen rotation about C – C double bond
d) Restricted rotation about C – C single bond
Answer:
a) Free rotation about C – C single bond
Question 17.
The most stable conformation of ethane is
a) Eclipsed
b) Skew
c) Staggered
d) All are equally stable
Answer:
c) Staggered
Question 18.
IUPAC name of the following compound
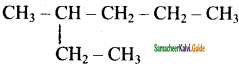
a) 3 – methyl hexane
b) 3 – Ethyl pentane
c) 2, 3 – dimethyl pentane
d) 2, 2 – dimethyl pentane
Answer:
a) 3 – methyl hexane
Question 19.
The number of sigma bonds formed in ethane by the overlapping of sp3 – sp3 orbitals
a) 7
b) 5
c) 1
d) 4
Answer:
c) 1
Question 20.
How many types of carbon atoms are present in 2, 2, 3 – trimethyl pentane
a) one
b) Two
c) Three
d) Four
Answer:
d) Four
![]()
Question 21.
The correct IUPAC name of the following alkane is
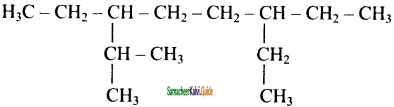
a) 3, 6 – diethyl – 2- methyloctane
b) 5 – isopropyl – 3- ethyloctane
c) 3 – ethyl – 5- isopropyloctane
d) 3 – isopropyl – 6 – ethyloctane
Answer:
a) 3, 6 – diethyl – 2- methyloctane
Question 22.
In Wurtz reaction, n – hexane is obtained from
a) n – propyl chloride
b) n – butyl chloride
c) Ethyl chloride
d) isopropyl chloride
Answer:
a) n – propyl chloride
Question 23.
When sodium acetate is heated with sodalime the reaction is called
a) Dehydration
b) Decarboxylation
c) Dehydrogenation
d) Dehydrohalogenation
Answer:
b) Decarboxylation
Question 24.
The following substance reacts with water to give ethane
a) CH4
b) C2H5MgBr
c) C2H4OH
d) C2H5OC2H5
Answer:
b) C2H5MgBr
Question 25.
Reaction of ROH with R’ MgX produces
a) RH
b) R’H
c) R – R
d) R’ – R”
Answer:
b) R’H
![]()
Question 26.
The solvent used in Wurtz reacton is
a) C2H5OH(aq)
b) CH3COOH
c) H2O
d) C2H5OC2H5(dry)
Answer:
d) C2H5OC2H5(dry)
Question 27.
Arrange the following in the decreasing order of their boiling points
i) n – butane
ii) 2 – methylbutane
iii) n – pentane
iv) 2 – methylbutane
a) i > ii > iii > iv
b) ii > iii > iv > i
c) iv > iii > ii > i
d) iii > ii > iv > i
Answer:
d) iii > ii > iv > i
Question 28.
The compound with the highest boiling point is
a) n – Hexane
b) n – Pentane
c) 2, 2 – dimethyl propane
d) 2 – methyl butane
Answer:
a) n – Hexane
Question 29.
The increasing order of reduction of alkyl halides with zinc and dilute HCl is
a) R – Cl < R – I < R – Br
b) R – Cl < R – Br < R – I
c) R – I < R – Br < R – Cl
d) R – Br < R – I < R – Cl
Answer:
b) R – Cl < R – Br < R – I
Question 30.
The volume of oxygen required for the complete combustion of 4 lit of ethane is
a) 4 lit
b) 8 lit
c) 12 lit
d) 14 lit
Answer:
d) 14 lit
![]()
Question 31.
The dihedral angle between the hydrogen atoms of 2 methyl groups in staggered conformation of ethane is
a) 0°
b) 60°
c) 120°
d) 240°
Answer:
b) 60°
Question 32.
The distances between the hydrogen nuclei in staggered and eclipsed form in ethane respectively are
a) 2.55 Å & 2.29 Å
b) 1.54 Å & 1.34 Å
c) 3.5 Å & 2.5 Å
d) 2.29 Å & 2.55 Å
Answer:
a) 2.55 Å & 2.29 Å
Question 33.
Energy barrier between staggered and eclipsed form in ethane is
a) 0.6 kcal / mole
b) 2.9 kcal / mole
c) 12 kcal / mole
d) 14 cal / mole
Answer:
b) 2.9 kcal / mole
Question 34.
IUPAC name of the following compound
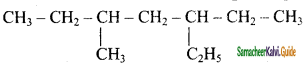
a) 3 – Ethyl, 5 – methyl heptane
b) 5 – Ethyl, 3 – methyl heptane
c) 2 – Ethyl, 5 – methyl heptane
d) 4 – Ethyl, 5 – methyl heptane
Answer:
a) 3 – Ethyl, 5 – methyl heptane
Question 35.
Ethylene is converted to ethane in the presence of Ni at 300°C. In this reaction the hybridization of carbon changes from
a) sp to sp2
b) sp2 to sp3
c) sp3 to sp
d) sp to sp3
Answer:
b) sp2 to sp3
![]()
Question 36.
In which of the following reactions in the preparation of ethane a new C – C bond is formed
a) Sabatier – Senderen’s reaction
b) Reduction of ethyl iodide
c) Decarboxylation
d) Kolbe’s electrolysis
Answer:
d) Kolbe’s electrolysis
Question 37.
Select the correct statements
a) eclipsed and staggered ethanes give different products on reaction with chlorine in presence of light
b) the conformational isomers can be isolated at room temperature
c) torsional strain is minimum in ethane at dihedral angles 60°, 180° and 300°
d) steric strain is minimum in gauche form of n – butane
Answer:
c) torsional strain is minimum in ethane at dihedral angles 60°, 180° and 300°
Question 38.
The fully eclipsed conformation of n – butane is least stable due to the presence of
a) bond opposition strain only
b) steric strain only
c) bond opposition strain as well as steric strain
d) no strain is present in the molecule
Answer:
c) bond opposition strain as well as steric strain
Question 39.
Vinyl group among the following is
a) (CH3)2 CH –
b) HC ≡ C –
c) H2C = CH – CH2 –
d) CH2 = CH –
Answer:
d) CH2 = CH –
Question 40.
The alkene that exhibits geometrical isomerism is
a) propene
b) 2 – methyl propene
c) 2 – butene
d) 2 – methyl – 2 – butene
Answer:
c) 2 – butene
![]()
Question 41.
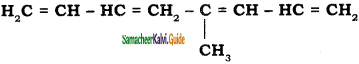
a) 5 – methylocta – 1, 3, 5, 7 – tetraene
b) 4 – methylocta – 1, 3, 5, 7 – tetraene
c) 4 – butenylocta -1, 3- diene
d) octa – 1, 5 – diene
Answer:
b) 4 – methylocta – 1, 3, 5, 7 – tetraene
Question 42.
CH2 = C (CH2CH2CH3)2
a) 2 – Propyl pent – 1 – ene
b) 2 – Propyl pent – 2- ene
c) 2 – Propyl pent – 3 – ene
d) 3 – Propyl pent – 1- ene
Answer:
a) 2 – Propyl pent – 1 – ene
Question 43.
The number of sigma (σ) and pi (π) bonds in the following structure are
a) σ bonds – 33 π bonds – 2
b) σ bonds – 22 π bonds – 2
c) σ bonds – 42 π bonds – 2
d) σ bonds – 40 π bonds – 3
Answer:
a) σ bonds – 33 π bonds – 2
Question 44.
In dehydrohalogenation, hydrogen and halogen are removed from
a) the same carbon atom
b) from adjacent carbon atoms
c) from isolate carbon atoms
d) from any two carbon atoms
Answer:
b) from adjacent carbon atoms
Question 45.
Ethylene readily undergoes the following type of reaction.
a) Elimination
b) Addition
c) Rearrangement
d) Substitution
Answer:
b) Addition
![]()
Question 46.
Baeyer’s reagent is
a) Aqueous bromine solution
b) Neutral permanganate solution
c) Acidified permanganate solution
d) Alkaline potassium permanganate solution
Answer:
d) Alkaline potassium permanganate solution
Question 47.
Baeyer’s reagent oxidizes ethylene to
a) Ethylene chlorohydrin
b) Ethyl alcohol
c) CO2 and H2O
d) Ethane – 1, 2 – diol
Answer:
d) Ethane – 1, 2 – diol
Question 48.
On reductive ozonolysis ethylene gives
a) Aldehyde
b) Ketone
c) Carboxylic acid
d) Ether
Answer:
a) Aldehyde
Question 49.
Polythene is obtained by the polymerization of
a) Styrene
b) A mixture of ethylene and styrene
c) Acetylene
d) Ethene
Answer:
c) Acetylene
Question 50.
Polytetrafluoroethylene is commercially known as
a) Teflon
b) Freon
c) Lewisite
d) Westron
Answer:
a) Teflon
![]()
Question 51.
Polythens is
a) (- H2C = CH2 -)n
b) (- HC = CH – )n
c) (- H3C – CH3 – )n
d) (- H2C – CH2 -)n
Answer:
d) (- H2C – CH2 -)n
Question 52.
When ethanol vapours are passed over alumina heated at 350°C, the main product obtained is
a) C2H6
b) C2H4
c) C2H2
d) C2H5OC2H5
Answer:
b) C2H4
Question 53.
Dehydrohalogenation of ethyl chloride in presence of ale. KOH produces the following
a) HC ≡ CH + KCl + H2O
b) CH4 + KCl + H2O
c) CH2 = CH2 + KCl + H2O
d) C2H4 + HCl
Answer:
c) CH2 = CH2 + KCl + H2O
Question 54.
Ethylene is prepared by
a) Dehalogenation of chloroform
b) Pyrolysis of ethane at 450°C
c) Dehydration of methanol with Al2O3 / 350°C
d) Methyl chloride on reduction
Answer:
b) Pyrolysis of ethane at 450°C
Question 55.
The peroxide effect involves
a) Ionic mechanism
b) Free – radical mechanism
c) Heterolytic fission of double bond
d) Homolytic fission of double bond
Answer:
b) Free – radical mechanism
![]()
Question 56.
In which of the following will Kharasch effect operate?
a) CH3 – CH2 – CH = CH2 + HCl
b) CH3 – CH2 – CH = CH2 + HBr
c) CH3 – CH = CH – CH3 + HBr
d) CH3 – CH2 – CH = CH2 + HI
Answer:
b) CH3 – CH2 – CH = CH2 + HBr
Question 57.
Anti Markownikoff addition of HBr is not observed in
a) Propene
b) Butene – 1
c) Butene – 2
d) pentene – 2
Answer:
c) Butene – 2
Question 58.
Conditions used for the formation of ethylene glycol from ethylene
a) bromine water
b) cold alkaline KMnO4
c) dil H2SO4, 60°C
d) Ag / 200°C
Answer:
b) cold alkaline KMnO4
Question 59.
The olefin which on ozonolysis gives CH3CH2CHO and CH3CHO is
a) 1 – butene
b) 2 – butene
c) 1 – pentene
d) 2 – pentene
Answer:
d) 2 – pentene
Question 60.
In the following reaction, A and B respectively are,
![]()
a) C2H4 and alcoholic KOH / ∆
b) C2H5Cl and aqueous KOH / ∆
c) C2H5OH and aq KOH / ∆
d) C2H2 and Br2
Answer:
a) C2H4 and alcoholic KOH / ∆
![]()
Question 61.
The number of possible alkynes with molecular formula C5H8 is
a) 3
b) 4
c) 5
d) 6
Answer:
a) 3
Question 62.
The number of open chain structural isomers possible with molecular formula C5H8 is
a) 7
b) 6
c) 5
d) 4
Answer:
a) 7
Question 63.
Alkynes exhibit.
a) Chain isomerism
b) Position isomerism
c) Functional isomerism
d) All the above
Answer:
d) All the above
Question 64.
The IUPAC name of the compound having the formula CH ≡ C – CH = CH2
a) Butene – 2 – ye
b) But – 2- yne – 3 – ene
c) 3 – butane 1 – ene
d) But – 1 – ene – 3 – yne
Answer:
d) But – 1 – ene – 3 – yne
Question 65.
Acetylene can be obtained by the electrolysis of the following compound
a) Potassium fumerate
b) Potassium succinate
c) Potassium acetate
d) Potassium formate
Answer:
a) Potassium fumerate
![]()
Question 66.
The gas obtained when ethylene chloride reacts with alcoholic potash and sodamide is
a) C2H4
b) C2H6
c) C2H2
d) C2H5Cl
Answer:
c) C2H2
Question 67.
PVC is the polymer of the following
a) Ethyl chloride
b) Vinyl Chloride
c) Allyl Chloride
d) Ethynyl chloride
Answer:
b) Vinyl Chloride
Question 68.
Which of the following possess acidic hydrogen
a) C2H6
b) C2H4
c) C2H2
d) CH4
Answer:
c) C2H2
Question 69.
Hydrocarbon which gives oxyacetylene flame
a) ethane
b) ethene
c) ethyne
d) ethanol
Answer:
c) ethyne
Question 70.
The isomer of propyne
a) Allene
b) Propene
c) Cyclo propane
d) Propane
Answer:
a) Allene
![]()
Question 71.
Bond angle between C – C in alkyne
a) 109°.28′
b) 120°
c) 180°
d) 60°
Answer:
c) 180°
Question 72.
The molecule having linear structure is
a) Methane
b) Ethylene
c) Acetylene
d) Water
Answer:
c) Acetylene
Question 73.
The C – C bond length is shortest in
a) C2H6
b) C3H8
c) C6H6
d) C2H4
Answer:
b) C3H8
Question 74.
The hydrolysis of Mg2C3 produces
a) acetylene
b) propyne
c) butyne
d) ethylene
Answer:
b) propyne
Question 75.
1 – pentyne and 2 – pentyne can be distinguished by
a) Silver mirror test
b) Iodoform test
c) Addition of H2
d) Baeyers test
Answer:
a) Silver mirror test
![]()
Question 76.
Acetylene on reaction with silver nitrate shows
a) Oxidizing property
b) Reducing property
c) Basic nature
d) Acidic nature
Answer:
d) Acidic nature
Question 77.
The acidic nature of hydrogens in acetylene cannot be explained by the reaction with
a) Sodium metal
b) Ammonical cuprous chloride solution
c) Ammonical silver nitrate solution
d) HCN
Answer:
d) HCN
Question 78.
Westron is the solvent obtained by the reaction of chlorine with
a) Ethylene
b) Ethyne
c) Ethane
d) Methane
Answer:
b) Ethyne
Question 79.
CH ≡ CH![]() is
is
a) CH2 = CH – CH = CH2
b) HC ≡ C – CH3
c) CH2 = CH – CH3
d) CH3 – CH2 – CH3
Answer:
b) HC ≡ C – CH3
Question 80.
Hydration of ethyne to ethanal takes place through the formation of
a) CH3CH(OH)2
b) CH2 = CHOH
c) CH2 = CHO–
d) CH ≡ C–
Answer:
b) CH2 = CHOH
![]()
Question 81.
![]() B is
B is
a) Acetylene
b) Acetaldehyde
c) Acetone
d) Acetic acid
Answer:
b) Acetaldehyde
Question 82.
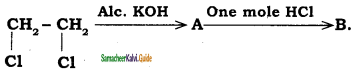 is
is
a) Ethyl chloride
b) 1, 2 dichloro ethene
c) Vinyl chloride
d) Ethylidine chloride
Answer:
c) Vinyl chloride
Question 83.
![]() The Polymer ‘B’ is
The Polymer ‘B’ is
a) orlon
b) PVC
c) nylon
d) Teflon
Answer:
b) PVC
Question 84.
Benzene is ______ molecule.
a) Tetrahedral
b) Planar
c) Trigonal
d) Square planar
Answer:
b) Planar
Question 85.
Bond length of C – C in benzene.
a) 1.34 Å
b) 1.39 Å
c) 1.54 Å
d) 1.20 Å
Answer:
c) 1.54 Å
![]()
Question 86.
The resonance energy Benzene is
a) 36 kcal / mol
b) 85.8 kJ / mole
c) 150.48 kJ / mole
d) Both (a) and (c)
Answer:
d) Both (a) and (c)
Question 87.
In Huckel’s (4n + 2) π rule for aromaticity, ‘n’ represents
a) Number of carbon atoms
b) Number of rings
c) Whole number
d) Fractional number (or) integer (or) zero
Answer:
c) Whole number
Question 88.
Coal tar is obtained as a by product during
a) Destructive distillation of wood
b) Destructive distillation of coal
c) Destructive distillation of bones
d) steam distillation of light oil
Answer:
b) Destructive distillation of coal
Question 89.
Gammaxene is ________ isomer of benzene hexa chloride.
a) α
b) β
c) γ
d) δ
Answer:
c) γ
Question 90.
The empirical formula of benzene and acetylene is/are
a) CH2, CH
b) CH2, CH2
c) CH, CH
d) CH3, CH3
Answer:
c) CH, CH
![]()
Question 91.
Chemical name of the insecticide gammaxene is
a) DDT
b) Benzene hexa chloride
c) Chloral
d) Hexa chloro ethane
Answer:
b) Benzene hexa chloride
Question 92.
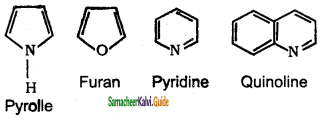
Which is non benzenoidal aromatic compound?
a) Benzene
b) Pyridine
c) Toluene
d) Phenol
Answer:
b) Pyridine
Question 93.
Benzene is purified by
a) distillation
b) fractional distillation
c) Evaporation
d) sublimation
Answer:
b) fractional distillation
Question 94.
Preparation of benzene from phenol is
a) Reduction
b) Oxidation
c) Addition
d) Dehydrogenation
Answer:
a) Reduction
Question 95.
The true statement about benzene is
a) Because of unsaturation benzene easily undergoes addition reactions
b) There are two types C – C bonds in benzene molecule
c) There is a cyclic delocalization of pi – electrons in benzene
d) Mono substitution of benzene gives three isomeric products
Answer:
c) There is a cyclic delocalization of pi – electrons in benzene
![]()
Question 96.
– COOH group in electrophilic substitution directs the incoming group to
a) o – position
b) p – position
c) m – position
d) o – and p – position
Answer:
c) m – position
Question 97.
Which of the following is not meta directing group?
a) -SO3H
b) – NO2
c) – CN
d) – NH2
Answer:
d) – NH2
Question 98.
Which among the following is very strong o – , p – directing group?
a) – Cl
b) – OR
c) – NH2
d) – NHR
Answer:
d) – NHR
Question 99.
Cyclo butadiene is said to be
a) Aromatic
b) Aliphatic
c) anti aromatic
d) heterocyclic
Answer:
c) anti aromatic
Question 100.
In the reaction 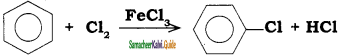
the attacking species is
a) Cl2
b) Cl+
c) Cl–
d) FeCl4–
Answer:
b) Cl+
![]()
Question 101.
The electrophile in Acetylation of Benzene

Answer:
b) ![]()
Question 102.
The ratio of sigma and pi bonds in benzene is
a) 4 : 1
b) 2 : 3
c) 6 : 1
d) 1 : 1
Answer:
a) 4 : 1
Question 103.
Benzene reacts with _______ to yield acetophenone.
a) CH3COCl + AlCl3
b) C6H5COCl + AlCl3
c) RCOCl + AlCl3
d) C2H5COCl + AlCl3
Answer:
a) CH3COCl + AlCl3
Question 104.
The order of activites of the various O and P director is
a) – O– > – OH > – OCOCH3 > – COCH3
b) – OH > – O– > – OCOCH3 > – COCH3
c) – OH > – O– > – COCH3 > – OCOCH3
d) -O– > – COCH3 > -OCOCH3 > -OH
Answer:
a) – O– > – OH > – OCOCH3 > – COCH3
![]()
II. Very short question and answers (2 Marks):
Question 1.
What are alkanes? Give example.
Answer:
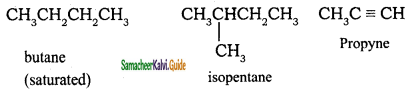
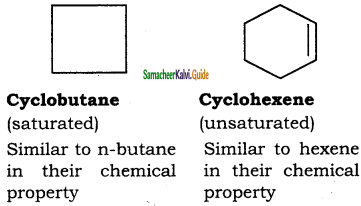
Alkanes are saturated hydrocarbons represented by the general formula CnH2n + 2 where ‘n’ is the number of carbon atoms in the molecule. Methane CH4, is- the first member of alkane family. The successive members are ethane C2H6, propane C3H8,butane C4H10, pentane C5H12 and so on. It is evident that each member differs from its proceeding or succeeding member by a -CH2 group.
Question 2.
Give the IUPAC name of the following alkane.
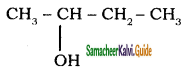
a) 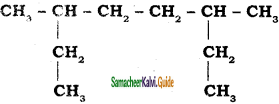
b) 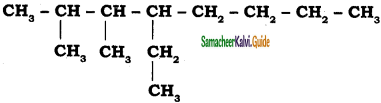
Answer:
a) 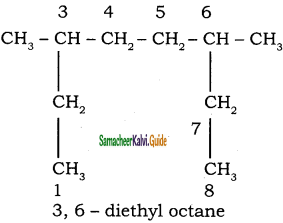
b) 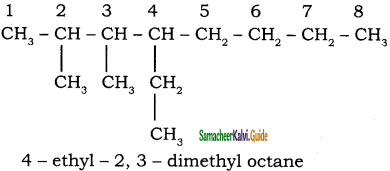
Question 3.
Draw the structural formula for 4,5 – diethyl – 3, 4, 5 – trimethyl octane.
Answer:
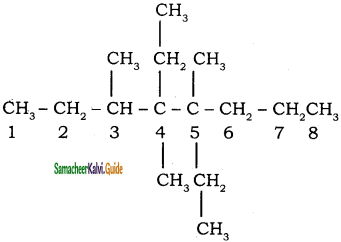
Question 4.
How is methane prepared from sodium acetate?
Answer:
When a mixture of sodium salt of carboxylic acid and soda lime (sodium hydroxide + calcium oxide) is heated, alkane is formed. The alkane formed has one carbon atom less than carboxylic acid. This process of eliminating carboxylic
group is known as decarboxylation.
CH3COONa + NaOH ![]() CH4 + Na2CO3
CH4 + Na2CO3
Sodium acetate Methane
![]()
Question 5.
Write a short note on Kolbe’s Electrolytic method.
Answer:
When sodium or potassium salt of carboxylic acid is electrolyzed, a higher alkane is formed. The decarboxylative dimerization of two carboxylic acid occurs. This method is suitable for preparing symmetrical alkanes(R-R).
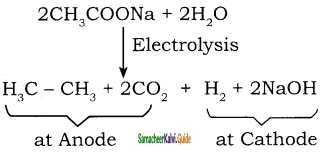
Question 6.
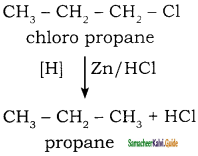
How will you convert chloro propane into propane?
Answer:
When chloro propane is reduced with Zn / HCl it gives propane.
Example:
Question 7.
Explain the combustion reaction of alkane with suitable example.
Answer:
A combustion reaction is a chemical reaction between a substances and oxygen with evolution of heat and light (usually as a flame). In the presence of sufficient oxygen, alkanes undergoes combustion when ignited and produces carbondioxide and water.
The combustion reaction is expressed as follows.
Example:
CH4 + 2O2 → CO2 + 2H2O
∆H° = -890.4 KJ
When alkanes burn in insufficient supply of oxygen, they form carbonmonoxide and carbon black.
2CH4 + 3O2 ![]() 2CO + 4H2O
2CO + 4H2O
CH4 + O2 → C + 2H2O
Question 8.
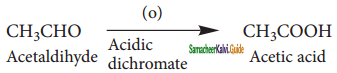
How are the following compound prepared by Aromatisation method?
(i) Benzene
(ii) Toluene
Answer:
(i) Benzene
n-Hexane passed over Cr2O3 supported on alumina at 873 k gives benzene.
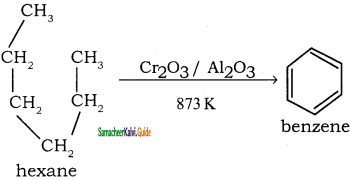
(ii) Toluene
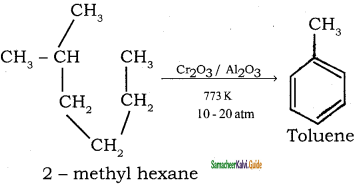
![]()
![]()
Question 9.
What happens when methane reacts with steam?
Answer:
Methane reacts with steam at 1273K in the presence of Nickel and decomposes to form carbon monoxide and hydrogen gas.
CH4(g) + H2O(g) ![]() CO(g) + 3H2
CO(g) + 3H2
Production of H2 gas from methane is known as steam reforming process and it is a well-established industrial process for the production of H2 gas from hydrocarbons.
Question 10.
Explain the Isomerisation reaction with suitable example.
Answer:
Isomerisation is a chemical process by which a compound is transformed into any its isomeric forms. Normal alkanes can be converted into branched alkanes in the presence of AlCl3 and HCl at 298 k.
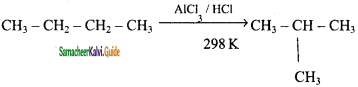
This process is of great industrial importance. The quality of gasoline is improved by isomerising its components.
Question 11.
What are alkenes? Give example.
Answer:
Alkenes are unsaturated hydrocarbons that contain carbon-carbon double bond. They are represented by the general formulae CnH2n where ‘n’ stands for number of carbon atoms in the molecule. Alkenes are also known as olefins (in Latin – oil maker) because the first member ethene combines with chlorine gas to form an oily liquid as a product.
Example:
Ethylene (C2H4)
Question 12.
Explain the Geometrical isomerism of alkene with suitable example.
Answer:
It is a type of stereoisomerism and it is also called cis-trans isomerism. Such type of isomerism results due to the restricted rotation of doubly bounded carbon atoms.
If the similar groups lie on the same side, then the geometrical isomers are called Cis-isomers. When the similar groups lie on the opposite side, it is called a Trans isomer.
Example:
The geometrical isomers of 2-Butene is expressed as follows
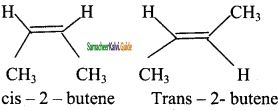
![]()
Question 13.
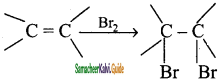
Write the test for Alkene.
Answer:
Bromine in water is reddish brown colour. When small about of bromine water is added to an alkene, the solution is decolourised as it forms dibromo compound. So, this is the characteristic test for unsaturated compounds.
Example:
Question 14.
Mention uses of Alkenes.
Answer:
- Alkenes find many diverse applications in industry. They are used as starting materials in the synthesis of alcohols, plastics, liquors, detergents and fuels
- Ethene is the most important organic feed stock in the polymer industry. E.g,’ PVC, Sarans and polyethylene. These polymer are used in the manufacture of floor tiles, shoe soles, synthetic fibres,raincoats, pipes etc.
Question 15.
How is propyne prepared from propylidine chloride?
Answer:
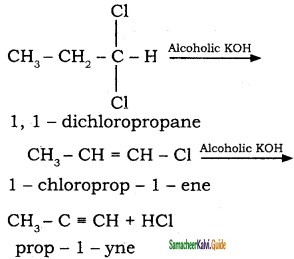
Question 16.
How is ethyne prepared from CaC2?
Answer:
Ethyne can be manufactured in large scale
by action of calcium carbide with water.
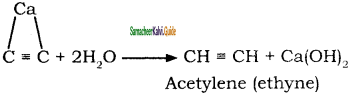
Calcium carbide needed for this reaction is prepared by heating quick lime and coke in an electric furance at 3273 K
CaO + 3C ![]() CaC2 + CO
CaC2 + CO
![]()
Question 17.
Write the reaction of propyne using Br2.
Answer:
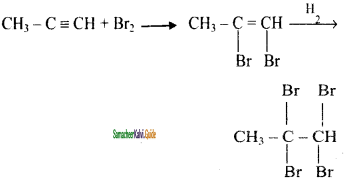
When Br2 in CCl4 (Reddishbrown) is added to an aikyne, the colour is decolounsed. This is the test for unsaturation.
Question 18.
Mention the uses of alkynes.
Answer:
- Acetylene is used in oxy acetylene torch used for welding and cutting metals.
- It is used for manufacture of PVC, polyvinyl acetate, polyvinyl ether, orlon and neoprene rubbers.
![]()
III. Short question and answers (3 Marks):
Question 1.
How are the following conversions carried out?
(i) propene → propane
(ii) ethene → ethane
(iii) prop – 1 – yne → propane
Answer:
(i) propene → propane
CH3 – CH = CH2 + H2 ![]() CH3 – CH2 – CH3
CH3 – CH2 – CH3
propene propane
(ii) ethene → ethane
CH2 = CH2 + H2 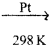 CH3 – CH3
CH3 – CH3
ethene ethane
(iii) prop – 1 – yne → propane
CH3 – C ≡ CH + 2H2 ![]() CH3 – CH2 – CH3
CH3 – CH2 – CH3
prop – 1 – yne propane
Question 2.
write the IUPAC name of the following compounds.
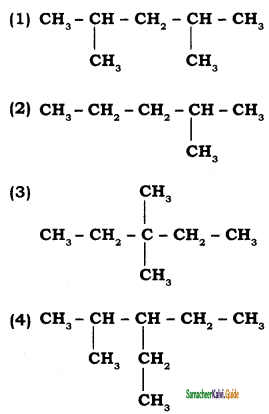
Answer:
![]()
Question 3.
Write the structural formula for the following compounds.
(i) 3 – Ethyl – 4, 5 – dipropyl octane
(ii) 2, 3 – Dimethyl pentane
(iii) 4 – Ethyl – 2,7 – Dimethyl octane
Answer:
(i) 3 – Ethyl – 4, 5 – dipropyl octane
(ii) 2, 3 – Dimethyl pentane
(iii) 4 – Ethyl – 2,7 – Dimethyl octane
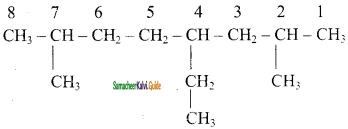
Question 4.
Write a short note on
(i) Wurtz reaction
(ii) Corey – House Mechanism
Answer:
(i) Wurtz reaction:
When a solution of halo alkanes in dry ether is treated with sodium metal, higher alkanes are produced. This reaction is used to prepare higher alkanes with even number of carbon atoms.
Example:
CH3 – Br + 2Na + Br – CH3 ![]() CH3 – CH3 + NaBr
CH3 – CH3 + NaBr
methyl bromide ethane
(ii)Corey-House Mechanism:
An alkyl halide and lithium di alkyl cuprate are reacted to give higher alkane.
Example:
CH3CH2Br + (CH3)2LiCu → CH3CH2CH3 + CH3Cu + LiBr
Ethyl bromide
![]()
Question 5.
How is methane prepared from Grignard reagent?
Answer:
Methyl chloride reacts with magnesium in presence of dry ether gives methyl magnesium chloride. Then methyl magnesium chloride reacts with water to give methane.
CH3 – Cl + Mg ![]() CH3MgCl
CH3MgCl
chloromethane methyl magnesium chloride
CH3MgCl + H2O → CH4 + Mg(OH)Cl
methane
Question 6.
What is meant by pyrolysis? Explain the pyrolysis reaction of Ethane and propane.
Answer:
Pyrolysis is defined as the thermal decomposition of organic compound into smaller fragments in the absence of air through the application of heat. ‘Pyro’ means ‘fire’ and ‘lysis’ means ‘separating’. Pyrolysis of alkanes also named as cracking.
In the absence of air, when alkane vapours are passed through red-hot metal it breaks down into simpler hydrocarbons.
1) 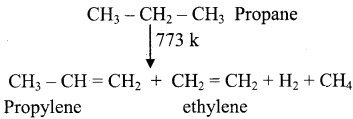
2) 2CH3 – CH3  CH2 = CH2 + 2CH4
CH2 = CH2 + 2CH4
Ethane Ethylene
Question 7.
Mention the uses of alkanes.
Answer:
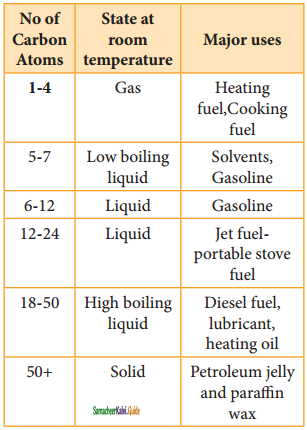
The exothermic nature of alkane combustion reaction explains the extensive use of alkanes as fuels. Methane present in natural gas is used in home heating. Mixture of propane and butane are known as LPG gas which is used for domestic cooking purpose. GASOLINE is a complex mixture of many hydrocarbons used as a fuel for internal combustion engines.
Carbon black is used in the manufacture of ink, printer ink and black pigments. It is also used s fillers.
Question 8.
Write all possible structural isomers with the molecular formula C4H10 and name them.
Answer:
(i) CH3 – CH = CH – CH3 2 – butene
(ii) CH2 = CH – CH2 – CH3 1 – butene
(iii) 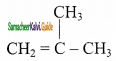 2 – mehyl – 1 – propene
2 – mehyl – 1 – propene
structures (i) 8s (ii) are position isomers, structures (i) & (iii), (ii) 8s (iii) are chain isomers.
![]()
Question 9.
How is ethene prepared by Kolbe’s electrolytic method?
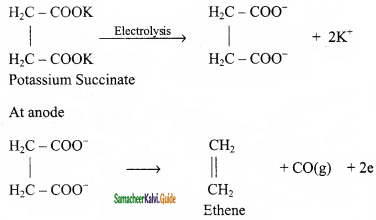
Answer:
When an aqueous solution of potassium succinate is electrolyzed between two platinum electrodes, ethene is produced at the anode.
Question 10.
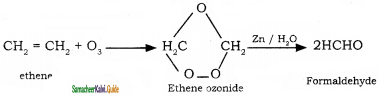
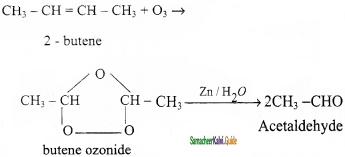
How are the following compounds prepared by ozonolysis method?
(i) Formaldehyde
(ii) Acetaldehyde
Answer:
(i) Formaldehyde:
(ii) Acetaldehyde:
Question 11.
How ozone reacts with 2 – methyl propene?
Answer:
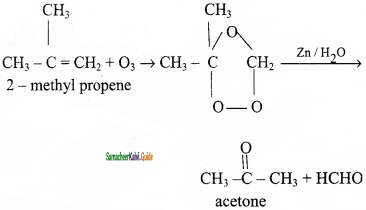
When 2 – methyl propene reacts with ozone to give ozonoid. Ozonide is treated with Zn/H2O gives acetone.
Question 12.
How is acetylene prepared from ethylene?
Answer:
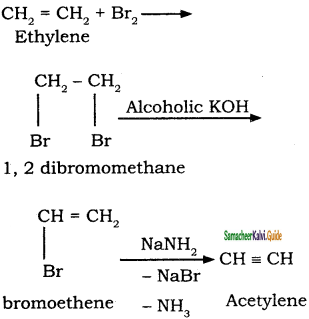
This process involves two steps:
(i) Halogenation of alkenes to form vicinal dihalides
(ii) Dehalogenation of vicinal dihalides to form alkynes.
![]()
Question 13.
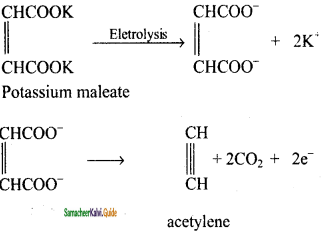
How is acetylene prepared by Kolbe’s electrolytic method?
Answer:
Electrolysis of sodium or potassium salt of maleic or fumaric acid yields alkynes.
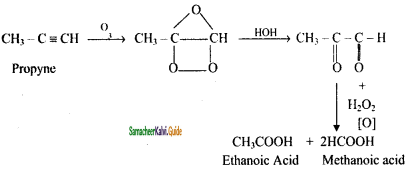
Question 14.
Write Ozonolysis reaction of Propyne?
Answer:
Question 15.
How is BHC prepared? Give its uses.
Answer:
Chlorination of Benzene:
Benzene reacts with three molecules of Cl2 in the presence of sun light or UV light to yield Benzene Hexa Chloride (BHC) C6H6Cl6. This is known as gammaxane or Lindane which is a powerful insecticide.

Question 16.
How propane is prepared form 1, 2 – dichloro propane?
Answer:
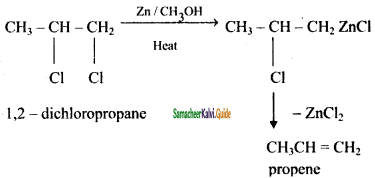
![]()
Question 17.
Explain the polymerization reaction of alkenes.
Answer:
A polymer is a large molecule formed by the combination of larger number of small molecules. The process is known as polymensation. Alkenes undergo polymerisation at high temperature and pressure, in the presence of a catalyst.
Example:
red hot
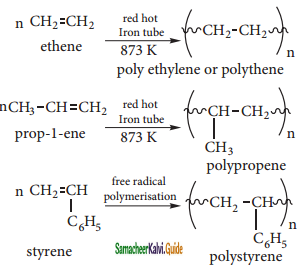
Question 18.
Write a note on acidic nature of Alkynes.
Answer:
An alkyne shows acidic nature only if it contains terminal hydrogen. This can be explained by considering sp hybrid orbitals of carbon atom in alkynes. The percentage of S-character of sp hybrid orbital (50%) is more than sp2 hybrid orbital of alkene (33%) and sp3 hybrid orbital of alkane (25%). Because of this, Carbon becomes more electronegative facilitating donation of H+ ions to bases. So hydrogen attached to triply bonded carbon atoms is acidic but not the others.
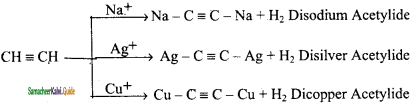
Question 19.
Write a short note on
(i) Wurtz – Fittig reactions
(ii) Friedel Craft’s reaction
Answer:
(i) Wurtz – Fittig reactions:
When a solution of bromo benzene and iodo methane in dry ether is treated with metallic sodium, toluene is formed.
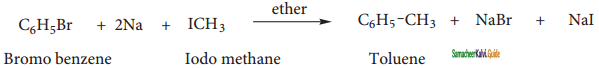
(ii) Friedel Craft’s reaction:
When benzene is treated with methyl chloride in the presence of anhydrous aluminium chloride, toluene is formed.

![]()
IV. Long Question and answers (5 Marks):
Question 1.
How to draw structural formula for given IUPAC name with suitable example.
Answer:
After you learn the rules for naming alkanes, it is relatively easy to reverse the procedure and translate the name of an alkane into a structural formula. The example below show how this is done.
Let us draw the structural formula for
3 – ethyl – 2, 3 – dimethyl pentane
Step 1:
The parent hydrocarbon is pentane. Draw the chain of five carbon atoms and number it. .
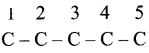
Step 2:
Complete the carbon skeleton by attaching the alkyl group as they are specified in the name. An ethyl group is attached to carbon 3 and two methyl groups are attached to carbon 2 and 3.
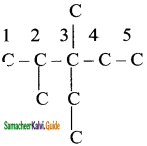
Step 3:
Add hydrogen atoms to the carbon skeleton so that each carbon atoms has four bonds.
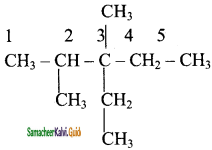
Question 2.
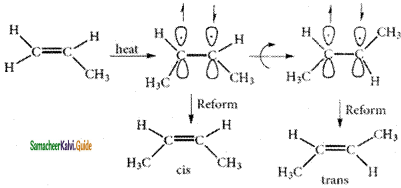
Explain the conformations of ethane.
Answer:
The two tetrahedral methyl groups can rotate about the carbon – carbon bond axis yielding several arrangements called conformers. The extreme conformations are staggered and eclipsed conformation. There can be number of other arrangements between staggered and eclipsed forms and their arrangements are known as skew forms.
Eclipsed conformation:
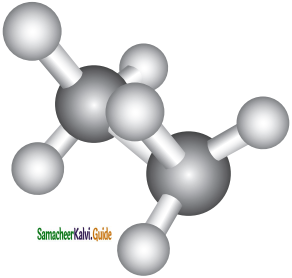
In this conformation, the hydrogen’s of one carbon are directly behind those of the other. The repulsion between the atoms is maximum and it is the least stable conformer.
Staggered conformation:
In this conformation, the hydrogen’s of both the carbon atoms are far apart from each other. The repulsion between the atoms is minimum and it is the most stable conformation.
Skew conformation:
The infinite numbers of possible intermediate conformations between the two extreme conformations are referred as skew conformations. The stabilities of various conformations of ethane are:
Staggered > Skew > Eclipsed
The potential energy difference between the staggered and eclipsed conformation of ethane is around 12.5 kJmol-1. The various conformations can be represented by new man projection formula.
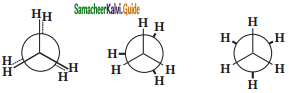
Eclipsed Skew Staggered newmann projection formula for Ethane.
![]()
Question 3.
Explain the steps involved in the mechanism of halogenations of alkane.
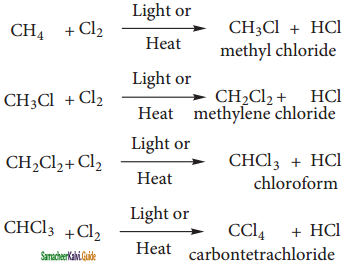
Halogenation:
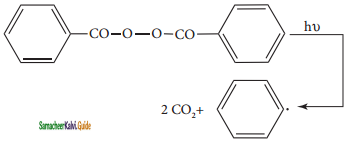
A Halogenation reaction is the chemical reaction between an alkane and halogen in which one or more hydrogen atoms are substituted by the halogens.
Chlorination and Bromination are two widely used halogenation reactions. Fluorination is too quick and iodination too slow. Methane reacts with chlorine in the presence of light or when heated as follows.
Mechanism:
The reaction proceeds through the free radical chain mechanism. This mechanism is characterized by three steps initiation, propagation and termination.
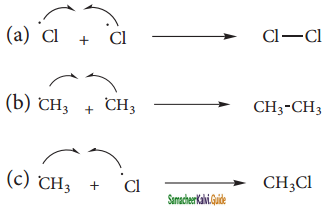
(i) Chain Initiation:
The chain is initiated by UV light leading to homolytic fission of chlorine molecules into free radicals (chlorine atoms).
![]()
Here we choose Cl – Cl bond for fission because C – C & C – H bonds are stronger than Cl – Cl.
ii) Propagation:
It proceeds as follows
a) Chlorine free radial attacks the methane molecule and breaks the C – H bond resulting in the generation of methyl free radical
![]()
b) The methyl free radical thus obtained attacks the second molecule of chlorine to give chloromethane (CH3Cl) and a chlorine free radical as follows.
![]()
c) This chlorine free radical then cycles back to step a) and both step a) and b) are repeated many times and thus chain of reaction is set up.
iii) Chain termination:
After sometimes, the reactions stops due to consumption of reactant and the chain is terminated by the combination of free radicals.
Question 4.
Write the IUPAC name of the following compounds.
1) CH3 – CH = CH2
2) CH3 – CH = CH – CH3
3) 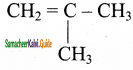
4) 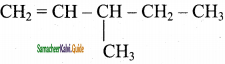
Answer:
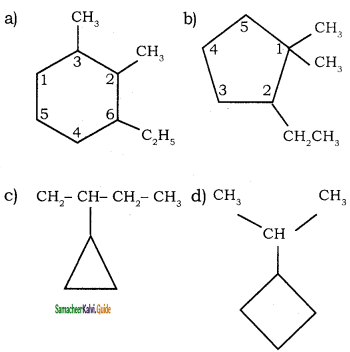
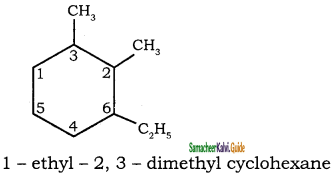
![]()
Question 5.
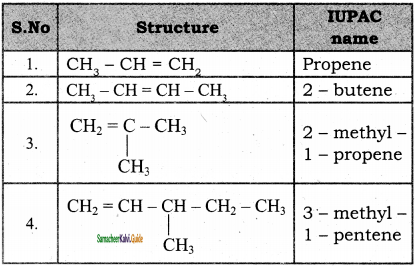
Write the IUPAC name of the following compounds.
Answer:
1) 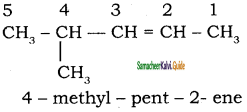
2) 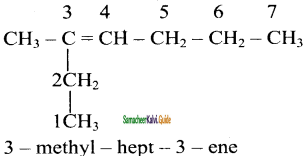
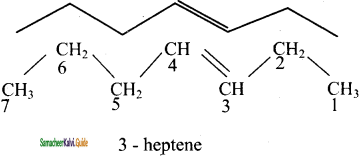
3) 
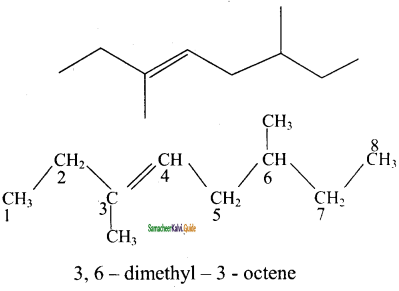
4) 
Question 6.
Draw the structures for the following alkenes.
(i) 6 – Bromo – 2, 3 – dimethyl – 2 – hexene
(ii) 5 – Bromo – 4 – chloro – 1 -heptene
(iii) 2, 5 – dimethyl – 4 – octene
(iv) 4 – Methyl – 2 – pentene
Answer:
(i) 6- Bromo – 2, 3-dimethyl – 2 -hexene
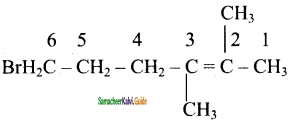
(ii) 5 – Bromo – 4 – chloro – 1 -heptene
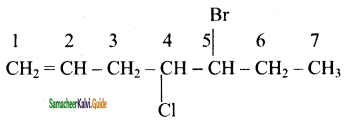
(iii) 2, 5 – dimethyl – 4 – octene
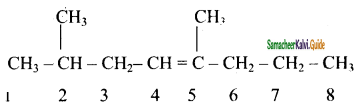
(iv) 4 – Methyl – 2 – pentene

![]()
Question 7.
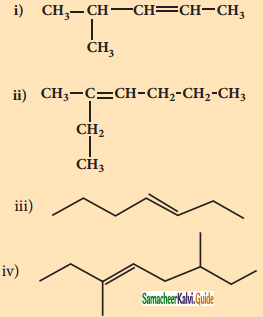
Draw the structure and write down the IUPAC name for the isomerism exhibited by the molecular formulae:
(i) C5H12 – Pentene (3 isomers)
(ii) C6H14 – Hexene (5 isomers)
(iii) C5H12 – Pentene (3 isomers)
Answer:
(i) C5H12 – Pentene
a) CH3 – CH2 – CH2 – CH = CH2
1 – Pentene
b) 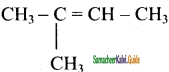
2 – methyl – 2 – butene
c) CH3CH = CH – CH2CH3
2 – pentene
(ii) C6H14 – Hexene
a) CH3 – CH2 – CH2 – CH2 – CH = CH2
1 – hexane
b) CH3 – CH2 – CH2 – CH = CH – CH3
2 – hexane
c) CH3 – CH2 – CH = CH – CH3 – CH2
3 – hexane
d) 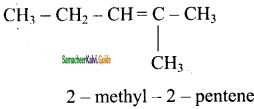
e) 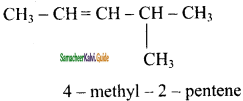
Question 8.
How are the following conversions carried out?
(i) ethanol → ethene
(ii) 2- butyne cis → 2 – butene and trans – 2 – butane
(iii) 1 – bromopropane → prop – 1 – ene
(iv) 1 – 2 – dibromoethane – ethene
Answer:
1) ethanol → ethene
C2H5OH ![]() H2C = H2C
H2C = H2C
ethanol ethane
(ii) 2 – butyne → cis – 2 – butene
H3C – C ≡ C – CH3 + H2 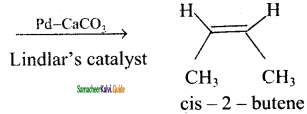
2 – butane → trans – 2 – butane
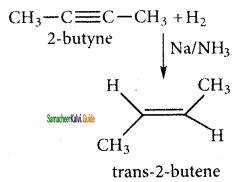
(iii) 1 – bromopropane → prop – 1 – ene
H3C – CH2 – CH2 – Br ![]() H3C – CH = CH2 + KBr + H2O
H3C – CH = CH2 + KBr + H2O
1 – bromopropane → prop – 1 – ene
(iv) 1 – 2 – dibromoethane – ethene
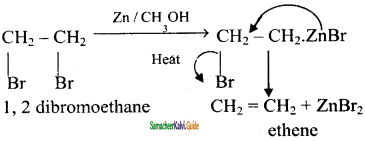
1 – 2 – dibromoethane – ethene
![]()
Question 9.
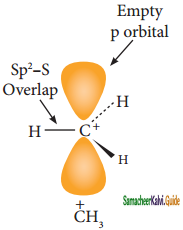
Write the mechanism involved in the addition of HBr to alkene in the presence of organic peroxide.
Answer:
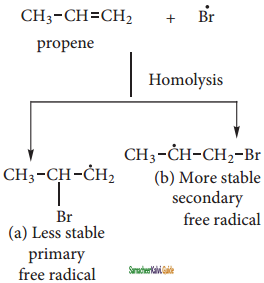
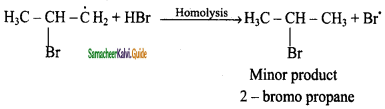
Anti-Markovnikoff’s Rule (Or) Peroxide Effect (Or) KharaschAddition:
The addition of HBr to an alkene in the presence of organic peroxide takes place in the direction opposite to that predicted by Markovnikoff’s rule.
CH3 – CH = CH2 + HBr ![]() CH3 – CH2 – CH3 – CH2 – CH2 – Br
CH3 – CH2 – CH3 – CH2 – CH2 – Br
prop – 1 – ene 1 – bromopropane
Mechanism:
The reaction proceeds via free radical mechanism.
Step 1:
The week O – O single bond linkages of peroxides undergoes homolytic cleavage to generate free radical.
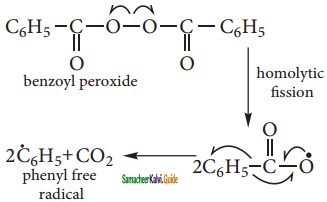
Step 2:
The radicals abstract H from HBr thus generating bromine radical.
![]()
Step 3:
The Bromine radical adds to the double bond in the way to form more stable alkyl free radical.
Step 4:
Addition of HBr to secondary free radical
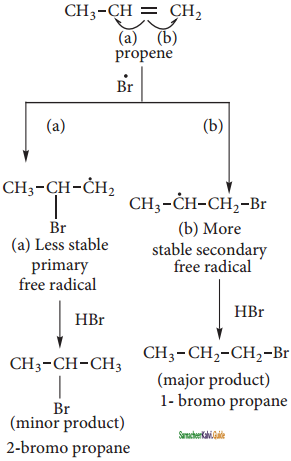
Step 5:
Addition of HBr to primary free radical
Question 10.
How are the following conversions carried out?
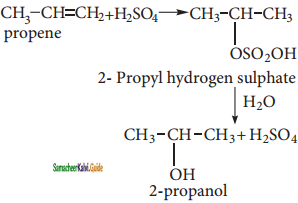
Answer:
(i) Propene → 2 – Propanol
(ii) ethene → ethylene glycol
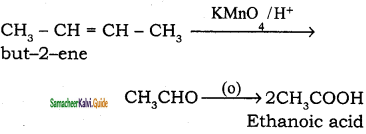
(iii) 2 – butene → Acetic acid
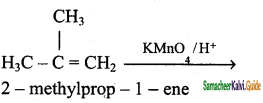
(iv) 2 – methyl – 1 – propene → Acetone
Answer:
(i) Propene → 2 – Propanol
(ii) ethene → ethylene glycol

(iii) 2 – butene → Acetic acid
(iv) 2 – methyl – 1 – propene → Acetone
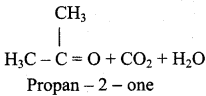
![]()
Question 11.
An organic compound (A) C2H4 decolourises bromine water. (A) on reaction with chlorine gives (B) A reacts with HBr to give (C). Identity (A), (B), (C). Explain th. reactions.
Answer:
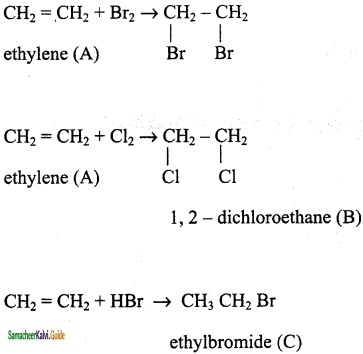
Result:
(A) – ethylene
(B) – 1, 2 – dichloroethane
(C) – ethyl bromide
Question 12.
How are the following conversions carried out?
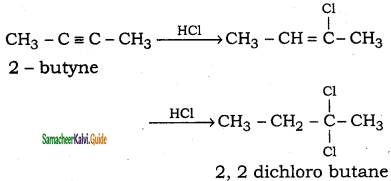
(i) 2 – butyne → 2, 2 – dichlorobutane
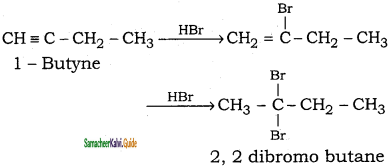
(ii) 1 – butyne → 2, 2 – dibromobutane
(iii) ethyne → acetaldehyde
(iv) Propyne → propan – 2 – one
Answer:
(i) 2 – butyne → 2, 2 – dichlorobutane
(ii) 1 – butyne → 2, 2 – dibromobutane
(iii) ethyne → acetaldehyde
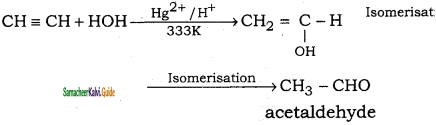
(iv) Propyne → propan – 2 – one
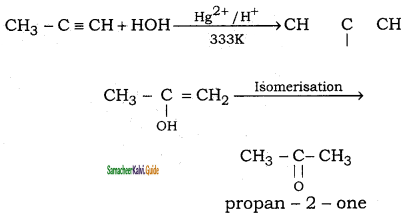
Question 13.
How are the following compounds prepared from ethyne?
(i) Formic acid
(ii) Vinyl ethylene
(iii) Benzene
Answer:
(i) Formic acid
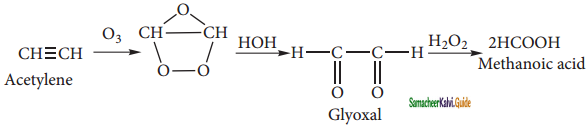
(ii) Vinyl ethylene
2CH ≡ CH ![]() CH2 = CH – C ≡ CH (Vinyl ethylene)
CH2 = CH – C ≡ CH (Vinyl ethylene)
(iii) Benzene
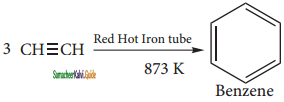
Question 14.
Explain the Huckel’s rule of Aromaticity.
Answer:
Huckel proposed that aromaticity is a function of electronic structure. A compound may be aromatic, if it obeys the following rules (Huckel’s rule)
i) The molecule must be co-planar
ii) Complete delocalization of n electron in the ring
iii) Presence of (4n+2) n electrons in the ring where n is an integer (n = 0,1,2….)
This is known as Huckle’s rule.
Some of the examples which obey Huckel rule.
1. The benzene ring is planar with delocalized π electrons (6)
It obeys Huckel’s rule because 4n + 2 = (4 × 1) + 2 = 6π electrons.
Hence, it is an aromatic compound

2. It has planar ring structure with delocalized π electrons (10).
Applying Huckle’s rule (4 × 2) + 2 = 10π electrons.
Hence it is an aromatic compound
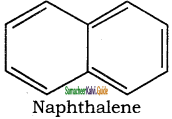
Naphthalene
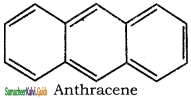
3. It has a planar ring structure with delocalized π electrons(14).
Applying Huckle’s rule (4 × 3) + 2 = 14π electrons.
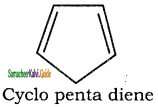
4. It has planar ring structure but the π electrons are not delocalized.
Applying Huckle’s rule (4 × 1) + 2 = 6π electrons.
But it has only 4electrons. So it is not an aromatic compound
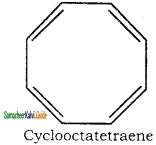
5. It has a tub shaped ring structure. It does not obey Huckel’s rule.
Applying Huckle’s rule 4n + 2 = 8π electrons if n = 2
So it is not an aromatic compound.
![]()
Question 15.
Explain the Kekule’s structure of benzene.
Answer:
In 1865, August Kekule suggested that benzene consists of a cyclic planar structure of six carbon with alternate single and double bonds.
There were two objections:
(i) Benzene forms only one orthodisubstituted products whereas the Kekule’s structure predicts two o-di substituted products as shown below.
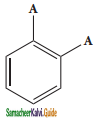
Presence of double bond between the substituents
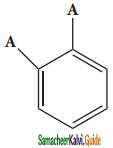
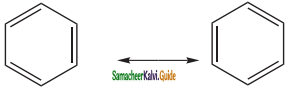
Presence of single bond between the substituents
(ii) Kekule’s structure failed to explain why benzene with three double bonds did not give addition reactions like other alkenes.To overcome this objection, Kekule suggested that benzene was mixture of two forms (1 and 2) in rapid equilibrium.
Question 16.
Explain the resonance in benzene.
Answer:
The phenomenon in which two or more structures can be written for a substance which has identical portions of atoms in called resonance. The actual structure of the molecule is said to be resonance hybrid of various possible alter nature structures. In benzene, Kekule’s structures I & II represented the resonance structure, and structure III is the resonance hybrid of structure I & II.
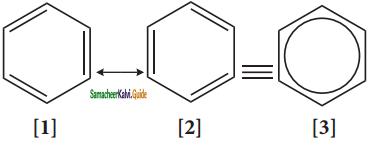
The structures 1 and 2 exist only in theory. The actual structure of benzene is the hybrid of two hypothetical resonance structures.
![]()
Question 17.
Write the industrial preparation of benzene from coal tar.
Answer:
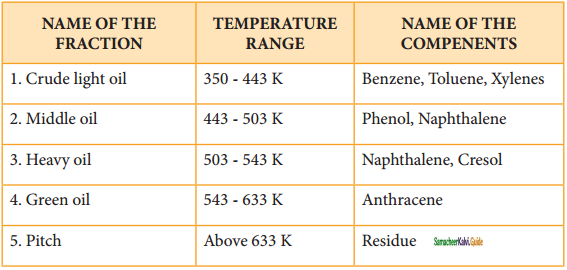
Coal tar is a viscous liquid obtained by the pyrolysis of coal. During fractional distillation, coal tar is heated and distills away its volatile compounds namely benzene, toluene, xylene in the temperature range of 350 to 443 K. These vapours are collected at the upper part of the fractionating column
Question 18.
How is benzene prepared from
(i) Acetylene
(ii) Decarboxylation
(iii) Phenol
Answer:
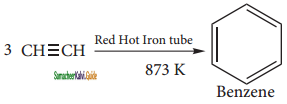
(i) From acetylene
Acetylene on passing through a red -hot tube trimerises to give benzene. We havealready studied this concept in polymerization of alkynes.
(ii) Decarboxylation of aromatic acid.
When sodium benzonate is heated with sodalime, benzene vapours distil over.
C6H5COONa + NaOH ![]() C6H6 + Na2CO3
C6H6 + Na2CO3
(iii) Preparation of benzene from Phenol
When phenol vapours are passed over zinc dust, then it is reduced to benzene.
C6H6OH + Zn ![]() C6H6 + ZnO
C6H6 + ZnO
Question 19.
Explain Electrophilic substitution reaction of benzene.
Answer:
(i) nitration:
When benzene is heated at 330K with a nitrating mixture (Con. HNO3 + Con. H2SO4), nitro benzene is formed by replacing of hydrogen atom by nitronium ion NO2+ (electrophile).
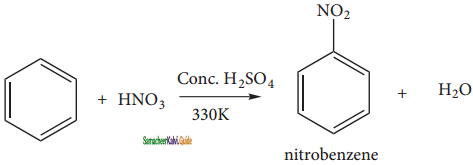
Concentrated H2SO4 is added to produce nitronium ion NO2+.
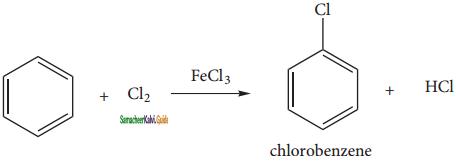
(ii) Halogenation:
Benzene reacts with halogens (X2 = Cl2, Br2,) in the presence of Lewis acid such as FeCl3, FeBr3 orAlCl3 and give corresponding halo benzene.
(iii) Sulphonation:
Benzene reacts with fuming sulphuric acid (Con. H2SO4 + SO3) and gives benzene sulphonic acid. The electrophile SO3 is a molecule.
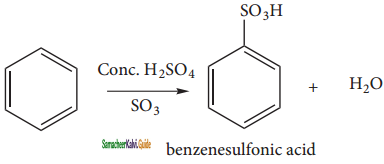
(iv) Friedel craft’s alkylation: (methylation)
When benzene is treated with a alkyl halide in the presence of only AlCl3, alkyl benzene is formed.
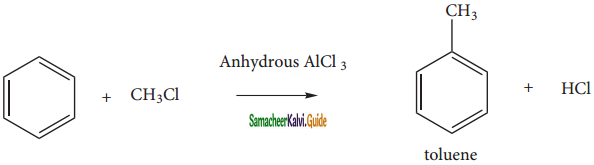
(v) Friedel craft’s acylatlon: (Acetylation)
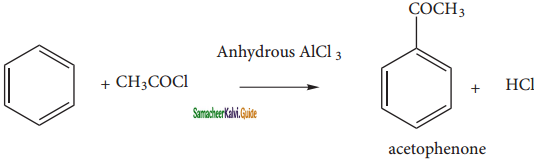
When benzene is treated with acetyichioride in the presence of AlCl3, acyl benzene is formed.
![]()
Question 20.
How is benzene converted into
a) Cyclo hezane
b) maleic anhydride
c) 1, 4 cyclo hexadiene
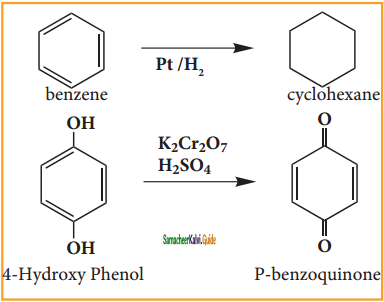
Answer:
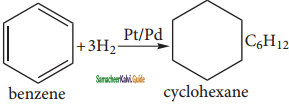
a) Cyclo hexane:
Benzene reacts with hydrogen in the presence of Platinum or Palladium to yield Cyclohexane. This is known as hydrogenation.
b) maleic anhydride:
Although benzene is very stable to strong oxidizing agents, it quickly undergoes vapour phase oxidation by passing its vapour mixed with oxygen over V2O5 at 773k. The ring breaks to give maleic anhydride.
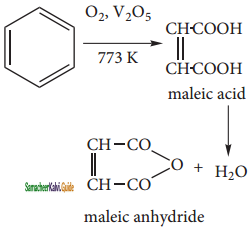
c) 1, 4 cyclo hexadiene:
Benzene can be reduced to non- conjugated dienes 1, 4 – cyclohexadiene by treatment with Na or Li in a mixture of liquid ammonia and alcohol. It is the convenient method to prepare cyclicdienes.
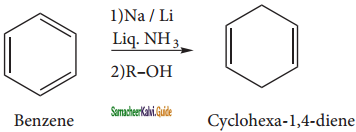

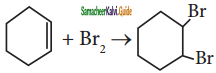
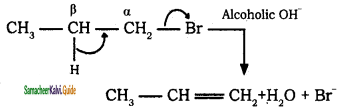
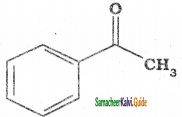
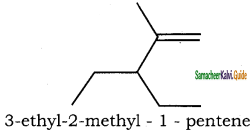
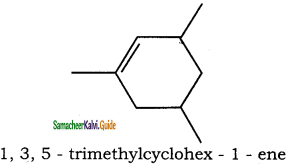
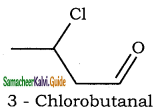
 is
is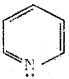
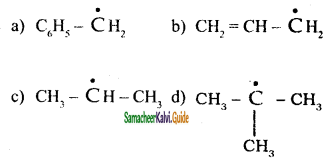
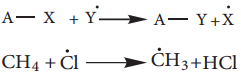
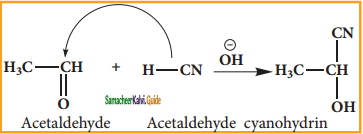
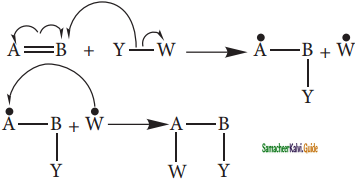
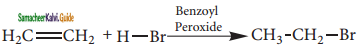
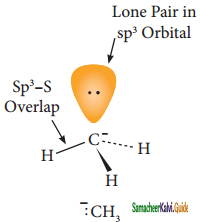
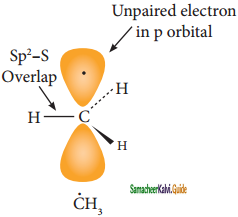
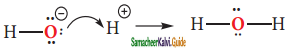
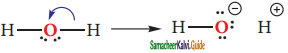
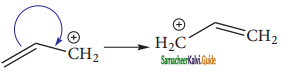
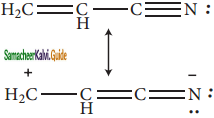






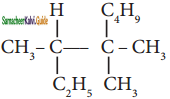 is
is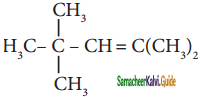 is
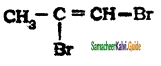
is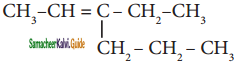 is
is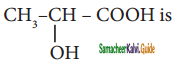 is
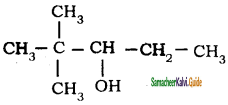
is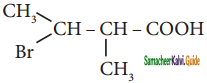 is
is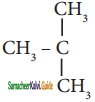
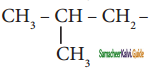
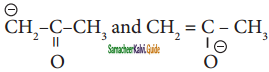 are
are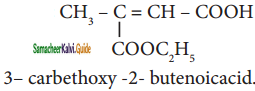
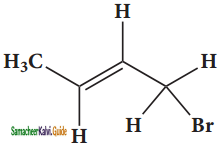
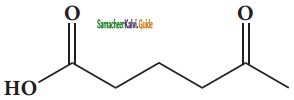
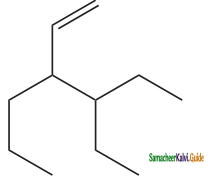
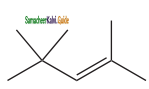


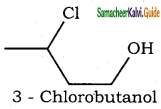
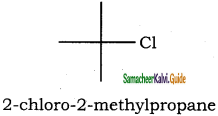
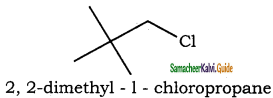
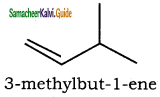
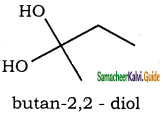
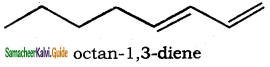
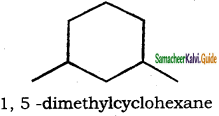
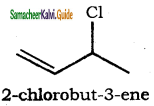

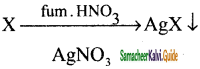
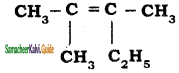 is
is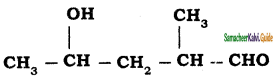 is
is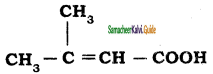 is
is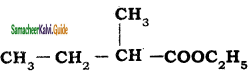 is
is is
is is
is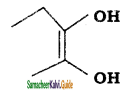 is
is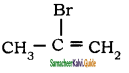
 is
is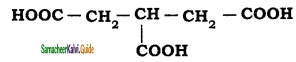 is
is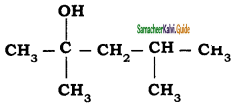 is
is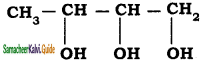 is
is is
is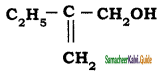 is
is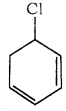 is
is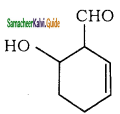 is
is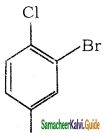
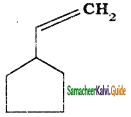
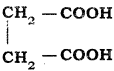 is
is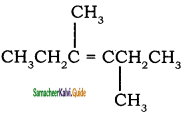
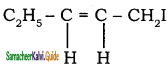
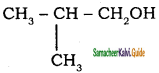
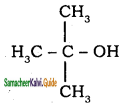
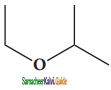
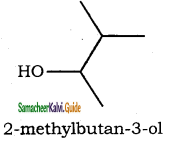
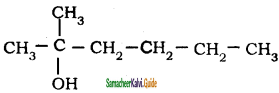
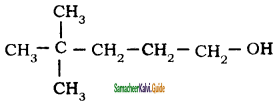
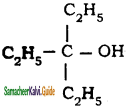
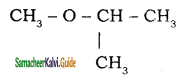 – methyl iso – propyl ether 2 – methoxypropane.
– methyl iso – propyl ether 2 – methoxypropane.