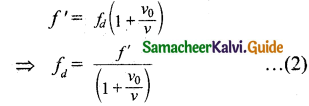
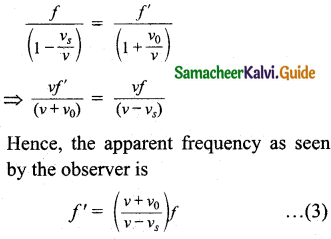
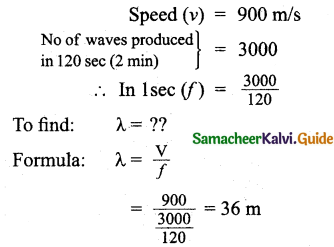
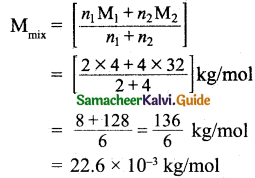
Tamilnadu State Board New Syllabus Samacheer Kalvi 11th Bio Botany Guide Pdf Chapter 10 Secondary Growth Text Book Back Questions and Answers, Notes.
Tamilnadu Samacheer Kalvi 11th Bio Botany Solutions Chapter 10 Secondary Growth
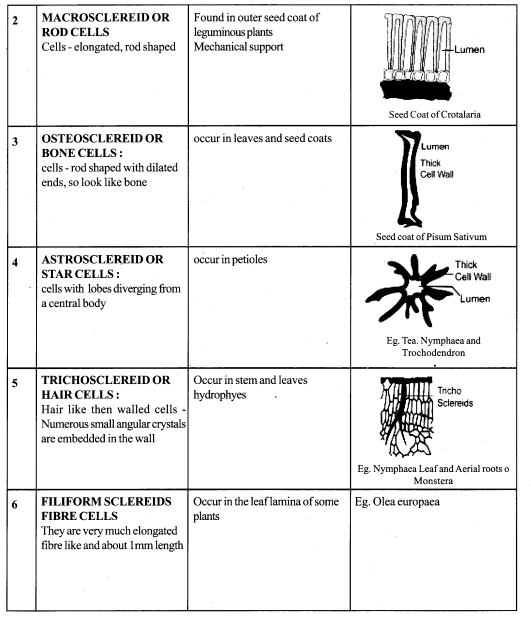
11th Bio Botany Guide Secondary Growth Text Book Back Questions and Answers
Part -I
I. Consider the following statements.
Question 1.
In spring vascular cambium
i) is less active
ii) Produces a large number of xylary elements
iii) forms vessels with wide cavities of these,
a) (i) is correct but (ii) and (iii) are not correct
b) (i) is not correct but (ii) and (iii) are correct
c) (i) and (ii) are correct but (iii) is not correct
d) (i) and (ii) are not correct but (iii) is correct
Answer:
b) (i) is not correct but (ii) and (iii) are correct
Question 2.
Usually, the monocotyledons do not increase their girth, because
a) They possess actively dividing cambium
b) They do not possess actively dividing cambium
c) Ceases activity of cambium
d) All are correct
Answer:
b) They do not possess actively dividing cambium
![]()
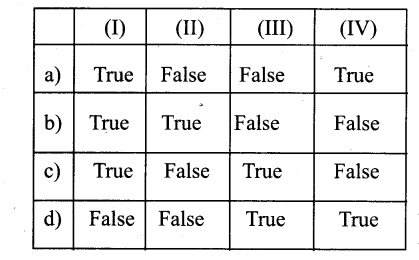
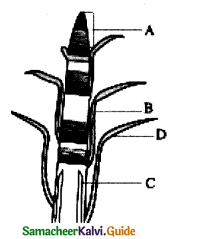
Question 3.
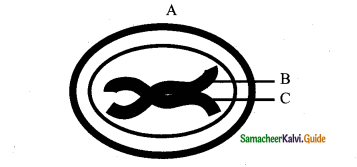
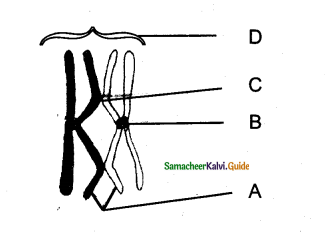
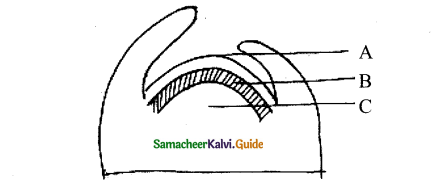
In the diagram of lenticel identify the parts marked as A, B, C, D
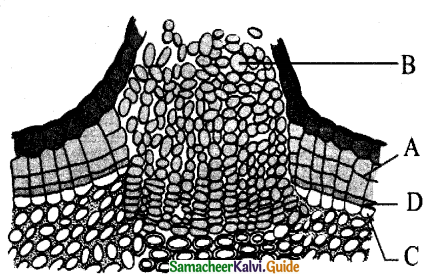
a) A. phellem, B. Complementary tissue, C. Phelloderm, D. Phellogen.
b) A. Complementary tissue, B.Phellem, C. Phellogcn,
c) A. Phellogen, B.Phellem, C. Phelloderm, D. Complementary tissue
d) A. Phelloderm, B. Phellem, C. Complementary tissue, D. Phellogen
Answer:
a) A. phellem, B. Complementary tissue, C. Phelloderm, D. Phellogen.
Question 4.
The common bottle cork is a product of
a) Dermatogen
b) Phellogen
c) Xylem
d) Vascular cambium
Answer:
b) Phellogen
![]()
Question 5.
What is the fate of primary xylem in a dicot root showing extensive secondary growth?
a) It is retained in the center of the axis
b) It gets crushed
c) May or may not get crushed
d) It gets surrounded by primary phloem
Answer:
b) It gets crushed
Question 6.
In a forest, if the bark of a tree is damaged by the horn of a deer, How will the plant overcome the damage?
Answer:
When the bark is damaged, the phellogen forms a complete cylinder around the stem and it gives rise to ring barks.
![]()
Question 7.
In which season the vessels of angiosperms are larger in size, why?
Ans:
In spring season the vessels are larger in size, because the cambium cells are very active during spring season.
Question 8.
Continuous state of dividing tissue is called meristem. In connection to this, what is the role is lateral meristem?
Answer:
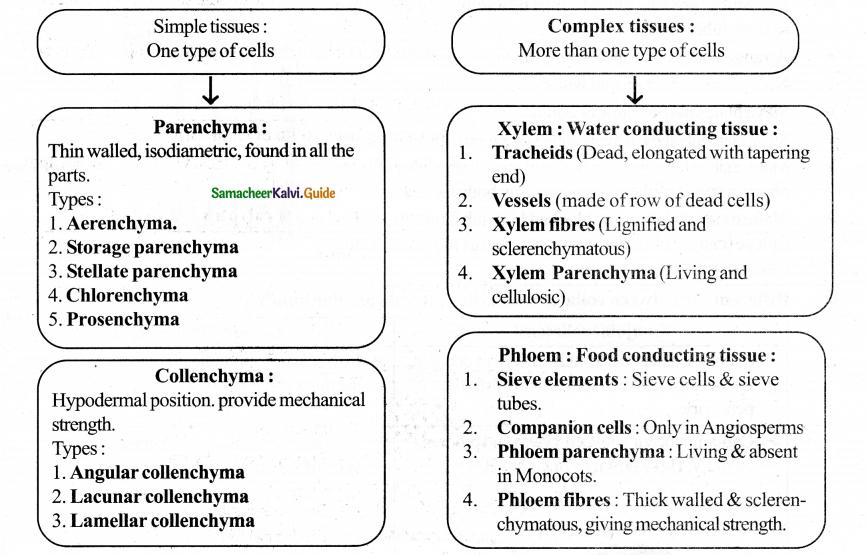
The secondary growth in dicots and gymnosperms is brought about by two lateral meristems.
- Vascular cambium and
- Cork cambium
1. Vascular cambium:
The vascular cambium is the lateral meristem that produces the secondary vascular tissues, i.e.. secondary xylem and secondary phloem.
Origin and Formation of Vascular Cambium:
- A strip of vascular cambium originate from the procambium is present between xylem and phloem of the vascular bundle. This cambial strip is known as intrafascicular or fascicular cambium.
- In between the vascular bundles, a few parenchymatous cells of the medullary rays that are in line with the fascicular cambium become meristematic and form strips of vascular cambium. It is called interfascicular cambium.
A. Organization of Vascular cambium:
- The active vascular cambium possesses cells with large central vacuole (or vacuoles) surrounded by a thin, layers of dense cytoplasm.
- The most important character of the vascular cambium is the presence of two kinds of initials, namely fusiform initials and ray initials.
Fusiform Initials:
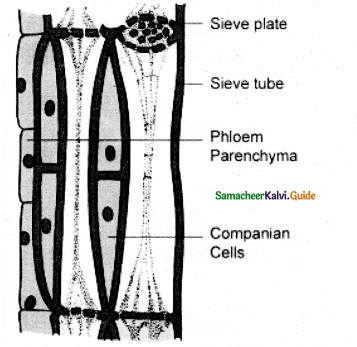
- These are vertically elongated cells. They give rise to the longitudinal or axial system of the secondary xylem (tracheary elements, fibres, and Axia? parenchyma) and pholem (sieve, elements, fibres, and axial parenchyma).
- Based on the arrangement of the fusiform initials two types of vascular cambium are recognized.
Stoned (Stratified cambium) and Non – storied (Non – stratified cambium)
- If the fusiform initials are arranged in horizontal tiers, with the end of the cells of one tier appearing at approximately the same level, as seen in tangential longitudinal section (TLS) it is called storied (stratified) cambium. It is the characteristic of the plants with short fùsiform initials.
- In plants with long fusiform initials, they strongly overlap at the ends, and this type of cambium is called non – storied (non stratified) cambium.
Ray Initials:
These are horizontally elongated cells. They give rise to the ray cells and form the elements of the radial system of secondary xylem and pholem.
Activity of Vascular Cambium:
- The vascular cambial ring, when active, cuts off new cells both towards the inner and outer side. The cells which are produced outward form secondary phloem and inward secondary xylem.
- Due to the continued formation of secondary xylem and phloem through vascular cambial activity, both the primary xylem and phloem get gradually crushed.
B. Phellogen (Cork Cambium)
- It is a secondary lateral meristem. It comprises homogenous meristematic cells unlike vascular cambium. It arises from epidermis, cortex, pholem or pericycle (extrastelar in origin). Its cells divide periclinally and produce radially arranged files of cells.
- The cells towards the outer side differentiate into phellem (cork) and those towards the inside as phelloderm (secondary cortex).
![]()
Question 9.
A timer merchant bought 2 logs of wood from, a forest & named them A & B, The log A was 50 year old & B was 20 years old. Which log of wood will last longer for the merchant? Why?
Answer:
- In wood, the older it is, the stronger it becomes.
- Log A – Which was 50 years old is stronger and it will last longer.
- In a tree the central part of the wood will be darker in colour, dead in nature known as Heartwood or Duramen, and the outer sad wood is lighter in colour, living and conducting water.
- In the central Heartwood the conduction is blocked by the formation of tyloses from the nearby parenchyma cells, and dead.
- In the fully developed tyloses, starch crystals, resins, gums, oils tannins and coloured substances are found and it becomes very hard and durable.
- It is more resistant to the attack of microbes and insects like termites.
- Older woods have more heartwood than sapwood.
- Here log ‘A’ is older, has more heartwood and it is stronger and will last longer.
Question 10.
A transverse section of the trunk of a tree shows concentric rings which are known as growth rings. How are these rings formed? What are the significance of these rings?
Answer:
Growth (or) Annual Rings:
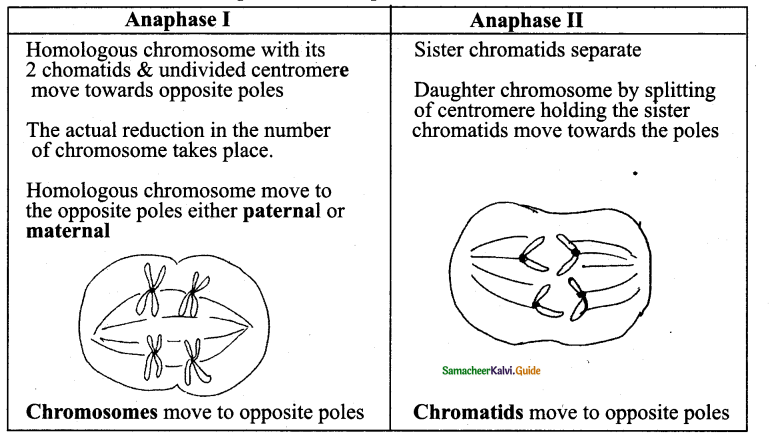
1. In the spring season cambium is very active and produces large number of xylary elements called Earlywood or Springwood. In the Winter season – cambium is less active and form few xylary elements – Latewood or Autumn Wood.
2. The springwood is lighter in color and has a lower density whereas the autumn wood is darker and has a higher density. The annual ring denotes the combination of earlywood and latewood and the ring becomes evident to our eye due to the high density of latewood. Sometimes annual rings are called growth rings
3. Pseudo – Annual Rings:
Additional growth rings are developed within a year due to adverse natural calamities like drought, frost defoliation, flood, mechanical, injury and biotic factors. Such rings arc called pseudo – or false – annual rings
4. Dendrochronology:
Each annual ring corresponds to one year’s growth and on the basis of these rings, the age of a particular plant can easily be calculated. The determination of the age of a tree by counting the annual rings is called dendrochronology.
Part – II.
11th Bio Botany Guide Secondary Growth Additional Important Questions and Answers
I. Choose The Correct Answer.
Question 1.
The roots and stems grow in length with the help of:
(a) cambium
(b) secondary growth
(c) apical meristem
(d) vascular parenchyma
Answer:
(c) apical meristem
Question 2.
The Gymnosperm in which vessel is present
a) Pinus
b) Cýcas
c) Ginkgo
d) Gnetum
Answer:
d. Gnetum
![]()
Question 3.
The secondary vascular tissues include:
(a) secondary xylem and secondary phloem
(b) secondary xylem, cambium strip and secondary phloem
(c) secondary phloem and fascicular cambium
(d) secondary xylem and primary phloem
Answer:
(a) secondary xylem and secondary phloem
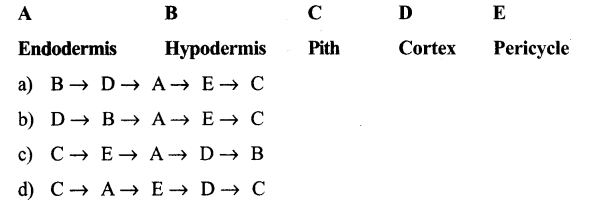
Question 4.
In a dicotyledonous stem, the sequence of tissues from the outside to the inside of
a) Phellem, Pericycle, Endodermis, phloem
b) Phellem phloem, Endodermis, Pericycle
c) Phellem, Endodermis, Pericycle, phloem
d) Pericycle, Phellem, Endodermis, Phloem
Answer:
c. Phellem, Endodermis, Pericycle, Phloem
![]()
Question 5.
For a critical study of secondary growth in plants which one of the following pairs is suitable?
a) Sugarcane and sunflower
b) Teak and pine
c) Bamboo and Fem
d) Wheat and Fem
Answer:
b. Teak and pine
Question 6.
The axial system of the secondary xylem includes:
(a) treachery elements, sieve elements, fibers and axial parenchyma
(b) treachery elements, fibers and axial parenchyma
(c) treachery elements and fibers
(d) sieve elements and axial parenchyma
Answer:
(b) treachery elements, fibers and axial parenchyma
![]()
Question 7.
Tissues considered in an annual ring is/are
a) Secondary xylem and phloem
b) Primary xylem and phloem
c) Secondary xylem only
d) Primary phloem and secondary xylem
Answer:
c. Secondary xylem only
Question 8.
Ray cells are present between:
(a) primary xylem and phloem
(b) primary xylem and secondary xylem
(c) secondary xylem and phloem
(d) secondary phloem and cambium
Answer:
(c) secondary xylem and phloem
![]()
Question 9.
Interfascicular cambium is a
a) Primary meristematic tissue
b) Primordial meristem
c) Type of protoderm
d) Secondary meristematic tissue
Answer:
d. Secondary meristematic tissue
Question 10.
Interfascicular cambium develops from the cells of
a) Xylem parenchyma
b) endodermis
c) Pericycle
d) Medullary rays
Answer:
d. Medullary rays
![]()
Question 11.
Which of the statement is not correct?
(a) In temperate regions, the cambium is very active in the winter season.
(b) In temperate regions, the cambium is very active in the spring season.
(c) In temperate regions, cambium is less active in the winter season.
(d) In temperate regions earlywood is formed in the spring season.
Answer:
(a) In temperate regions, the cambium is very active in the winter season.
Question 12.
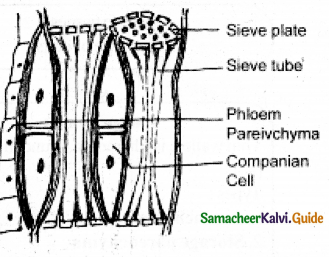
At maturity, the sieve plates become impregnated with
a) Cellulose
b) Pectin
c) Suberin
d) Callose
Answer:
d. Callose
![]()
Question 13.
You are given a fairly old piece of dicot stem and a dicot root, which of the following anatomical structures will you distinguish between the two?
a) Secondary xylem
b) Secondary phloem
c) Protoxylem
d) Cortical cells
Answer:
c. Protoxylem
Question 14.
determination of the age of a tree by counting the annual rings is called:
(a) chronology
(b) dendrochronology
(c) palaeology
(d) histology
Answer:
(c) palaeology
![]()
Question 15.
Which one of the following is dead and works efficiently?
a) Sieve tube
b) Companian cells
c) Vessels
d) Both (b) and (c)
Answer:
c. Vessels
Question 16.
Which one of the following pairs is an example for meristematic tissue
a) Phellogen and phelloderm
b) Phellogen and Fascicular cambium
c) Procambium and phelloderm
d) Interfascicular cambium and phellem
Answer:
b. Phellogen and Fascicular cambium
![]()
Question 17.
In fully developed tyloses:
(a) only starchy crystals are present
(b) resin and gums only are present
(c) oil and tannins are present
(d) starchy crystals, resins, gums, oils, tannins, or colored substances are present
Answer:
(d) starchy crystals, resins, gums, oils, tannins, or colored substances are present
Question 18.
Which wood is also known as Non-porous
a) Softwood
b) Heartwood
c) Hardwood
d) Sapwood
Answer:
a. Softwood
![]()
Question 19.
Which of the statement is not correct?
(a) Sapwood and heartwood can be distinguished in the secondary xylem
(b) Sapwood is paler in colour
(c) Heartwood is darker in colour
(d) The sapwood conducts minerals, while the heartwood conduct water
Answer:
(d) The sapwood conducts minerals, while the heartwood conduct water
Question 20.
Removal of a ring of wood tissue outside the vascular cambium from the tree trunk kills it because
a) Water cannot move up
b) Food does not travel down and root become starved
c) Shoot apex become starved
d) Annual rings are not produced
Answer:
food does not travel down root become starved
![]()
Question 21.
The trees growing in the desert will
a) Show alternate rings of xylem and sclerenchyma
b) Have only conjunctive tissue and phloem formed by the activity of cambium
c) do show distinct annual rings
d) do not show distinct annual rings
Answer:
d. do not show distinct annual rings.
Question 22.
Canada balsam is produced from:
(a) Pisum sativum
(b) the resin of Arjuna plant
(c) Abies balsamea
(d) the root of Vinca rosea
Answer:
(c) Abies balsamea
![]()
Question 23.
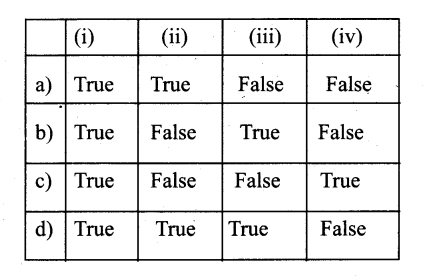
Choose the living cells from the given
I) Phellem
II) Phloem
III) Phellogen
IV) Xylem parenchyma
a) (I) (II) & (III)
b) (II) (III) & (IV)
c) (I) (III) & (IV)
d) (I) (II) & (IV)
Answer:
b) (II) (III) & (IV)
Question 24.
When we peel the skin of a potato tuber we remove
a) Periderm
b) Epidermis
c) Cuticle
d) Sapwood
Answer:
a) Periderm
![]()
Question 25.
Phelloderm is otherwise called as:
(a) primary cortex
(b) corkwood
(c) secondary cortex
(d) rhytidome
Answer:
(c) secondary cortex
Question 26.
The waxy substances associated with cell walls of cork cells are impervious to water because of the presence of ………………. which gets deposited on the cork cells
a) Cutin
b) Suberin
c) Eignin
d) Hemicellulose
Answer:
b) Suberin
![]()
Question 27.
The antimalarial compound quinine is, extracted from:
(a) seeds of cinchona
(b) bark of cinchona
(c) leaves of cinchona
(d) flowers of cinchona
Answer:
(b) bark of cinchona
Question 28.
Annual rings are distinct in plants growing in
a) Tropical regions
b) Arctic regions
c) Grasslands
d) Temperate region
Answer:
d) Temperate region
![]()
Question 29.
The bark does include three except one from the options.
I) Cortex
II) Periderm
III) Pith
IV) Secondary phloem
a) (I) (II) & (Ill)
b) (II) (III) & (IV)
c) (I) (III) & (IV)
d) (I) (II) & (IV)
Answer:
d) (I) (II) & (IV)
Question 30.
Rubber is obtained from:
(a) Bombax mori
(b) Hevea brasiliensis
(c) Quercus suber
(d) Morus rubra
Answer:
(b) Hevea brasiliensis
![]()
Question 31.
Wood actually means
a) Primary xylem
b) Secondary xylem
c) Primary phloem
d) Secondary phloem
Answer:
b) Secondary xylem
Question 32.
When a plant is wounded, the wound is healed by the formation of new cells, by the cavity of
a) Primary meristem
b) Apical meristem
c) Secondary meristem
d) Intercalary meristem
Answer:
c) Secondary meristem
![]()
Question 33.
Commercial cork is obtained from
a) Oak
b) Silver oak
c) Pine
d) Ficus
Answer:
a) Oak
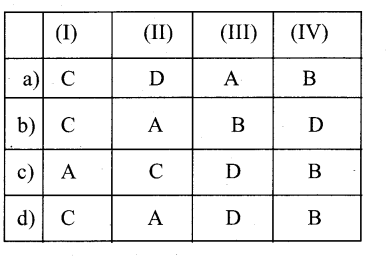
II. Match Correctly And Choose The Right Answer
Question 1.
I) Spicy bark – A Turpentine
II) Ornamental Antique – B Quinine
III) Active drug – C Cinnamon
IV) Thinner and solvent – D Amber

Answer:
d) C-D- B-A
Question 2.
I) Phellogen – A. Cork
II) Phelloderm – B. Cork cambium
III) Phellem – C. Lack suberin
IV) Phelloids – D. Secondary cortex

Answer:
a) B-D- A-C
![]()
Question 3.
I) Sapwood – A. Softwood
II) Heartwood – B. Hardwood
III) Porous wood – C. Albumum
IV) Nonporous wood – D. Duramen

Answer:
c) C-D-B-A
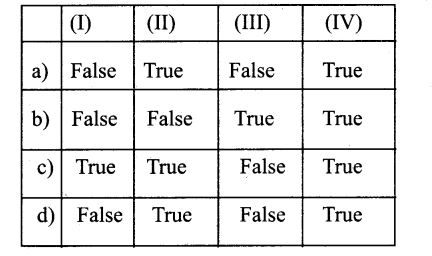
III Identify True Or False And From The Given Option Choose The Right Answer:
Question 1.
A) In pinus wood is Non-porous
B) In Morns wood is porous
C) In Quercus the wood is Diffuse porous
D) In Acer the wood is Ring porous
a) A&DTrue B&C False
b) A & B True C & D False
c) A & C True B & D False
d) A&BFalseC &DTrue
Answer:
b) A & B True C & D False
Question 2.
Arrange the given plants in order from more distinct Annual rings to least distinct Annual rings.
a) Seashore plants, Desert plants, Tropical plants & Temperature plants
b) Temperature plants, Tropical plants, Desert plants, Seashore plants
c) Tropical plants, Desert plants, Temperature plants, Seashore plants
d) Temperature plants, Seashore plants, Tropical plants, Desert plants
Answer:
b) Temperature plants, Tropical plants, Desert plants, Seashore plants.
![]()
Question 3.
In wood, the Annual rings become clearly evident to our eyes due to
a) The high density and dark coloured latewood or Autumn wood
b) The low density and light coloured early wood or springwood
c) The high density and dark coloured early wood or springwood
d) The low density and light coloured latewood or Autumn wood
Answer:
a) The high density and dark coloured of latewood or Autumn wood
Question 4.
Column I and Column II – Match them correctly and Find out the right option.
Answer:
| Column I | Column II |
| A. Springwood or earlywood B. Autumn wood or Latewood |
1. Lighter in colour 2. Density high 3. Density low 4. Darker in colour 5. Larger number of xylem elements 6. Vessels with wider cavity 7. Lesser number of xylem elements 8. Vessels with a small cavity |
Which of the following combination is correct?
a) A – 2, 4, 7, 8 B- 1,3, 5,6
b) A- 1,2, 7,8 B-3,4, 5, 6
c) A – 1,3, 5,6 B – 2,4, 7, 8
d) A- 1,3,7, 8 B – 2, 4, 5, 6
Answer:
b) A – 1, 2, 7,8 B-3,4,5, 6
![]()
IV. Assertion And Reason
Question 1.
ASSERTION: – A All tissues lying inside the Vascular Cambium are called as Bark
REASON -R: Bark is made up of Phellogen. Phellem and Phelloderm Cortex, primary and secondary phloem
a) Both A and R are true and R is the correct explanation of A
b) Both A and R are true, but R is not the correct explanation of A
c) A is true but ‘R’ is false
d) A is false and ‘R’ is true
e) Both A and ‘R’ are false
Answer:
d) ‘A’ is false and ‘R’ is true
Question 2.
ASSERTION: -A In angiosperms, the conduction of water is more efficient because their xylem has vessels
REASON – R: Conduction of water by vessel element is an active process in which energy is supplied by xylem parenchyma with a large number of Mitochondria
Answer:
a) Both A and R – are true and ‘R’ is the correct explanation of A
![]()
Question 3.
ASSERTION: -A. All the endodermal cells of the root do not contain Casparian thickenings on their radial and transverse walls.
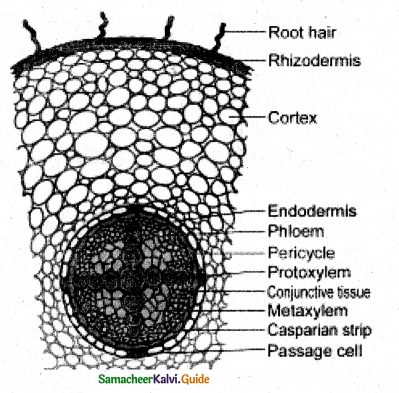
REASON-R: Passage cells are found in the root endodermis, which conducts water in to the xylem
Answer:
a) Both A and R are true and ‘R’ is the correct explanation of A
Question 4.
ASSERTION:-A Cambium is a lateral meristem and causes growth in width
REASON-R Cambium is made up of fusiform and ray initials in stem
Answer:
b) Both A and R are true, but R is not the correct explanation of A
Question 5.
ASSERTION: – A The lenticel is meant for gaseous exchange.
REASON-R Lenticel checks excessive evaporation of water.
Answer:
b) Both A and R are true but R is not the correct explanation of A
![]()
Question 6.
ASSERTION:-A Heartwood is more durable
REASON – R Heartwood contains organic compounds like tannins, resins, oil, gums, aromatic substances and essential oils help to resist microbial and termites attack.
Answer:
a) Both A and R are true and R is the correct explanation of A
V. 2 Marks Questions
Question 1.
Distinguish between Primary and Secondary growth.
Answer:
Primary growth
- The roots and stem grow in length with the help of Apical meristem
- It is known as longitudinal growth
- Eg. Angiosperms & Gymnosperms
Secondary growth
- The roots and stem show an increase in thickness or width with the help of Lateral meristem
- It is also known as latitudinal growth or growth in girth
- Eg. Most Angiosperms, including some Monocots and Gymnosperms
Question 2.
Mention the two Lateral meristems responsible for secondary growth.
Answer:
The secondary growth in dicots and gymnosperms is brought about by two lateral meristems.
- Vascular Cambium and
- Cork Cambium
![]()
Question 3.
Define interfascicular cambium?
Answer:
In between the vascular bundles, a few parenchymatous cells of the medullary rays that are in line with the fascicular cambium become meristematic and form strips of the vascular cambium. It is called interfascicular cambium.
Question 4.
Fill in the blanks
| The botanical name of the plant | The common name of the product | Use |
| 1. Abies balsamea | Canada balsam | ……………………………….. |
| 2. Acacia Senegal (meska) | ………………………………………… | Natural gum used as a bind to water colour painting |
| 3. ………………………………… | Cork | Hydrophobic, impermeable, used as a bottle stopper. |
| 4. ……………………………………………… | Hematoxylin | Dye from the heartwood to stain plant materials view under a microscope |
Answer:
- The resin used as a mounting medium for microscopic slide preparation.
- Gum Arabic
- Quercus suber
- Haematoxylon campechianum
Question 5.
Distinguish between the stratified cambium and Non-stratified cambium.
Answer:
Stratified cambium: Plants with short fusiform initials, produced, storied, cambium in horizontal tiers known as
stratified cambium
Non-stratified cambium: Plants with long fusiform initials produced non-storied cambium, strongly overlap at the ends, known as Non-stratified cambium
Question 6.
Why does porous wood be harder than non-porous wood?
Answer:
- Porous wood is wood with xylem vessels which appear as a pore in cross-section.
- When a tree stem become old, most of its vessels are blocked by tyloses with deposition of gum, resin, tannin, oils, etc. (Heartwood)
- So porous wood is harder and commercially important.
![]()
Question 7.
What is the source of turpentine? and What is its use?
Answer:
- Turpentine is a resin obtained from the bark of conifers Eg. pinus
- It is also used as a thinner for oil-based paints.
- It is also used as an organic solvent.
- It is also used as a balm to relieve muscular pain.
Question 8.
Distinguish between Periderm and Polydor
Answer:
Periderm:
- The secondary growth replaces the epidermis and primary cortex and forms the Periderm
- It consists of
- Phellem
- Phellogen
- Phelloderm
- Eg. Dicot stem & roots
Polyderm:
- It is a special type of protective tissue consisting of a miserable suberized layer, alternating with multiseriate nonsuberized cells in periderm
- Eg. Roots and underground stems of Rosaceae plants
Question 9.
Define Rhytidome?
Answer:
Rhytidome is a technical term used for the outer dead bark which consists of periderm and isolated cortical or phloem tissues ? formed during successive secondary growth, eg: Quercus.
![]()
Question 10.
What is the use of Canada balsam?
Answer:
From the resin ducts, the Abies balsamea plant produces an organic gum-like substance, used as a permanent mounting medium for microscopic slide preparation.
Eg. A slide of 60 years old holotype specimen of a flatworm is permanently mounted in Canada balsam.
VI. 3 Mark Questions
Question 1.
Distinguish between primary and secondary growth.
Answer:
1. Primary growth: The plant organs originating from the apical meristems pass through a period of expansion in length and width. The roots and stems grow in length with the help of apical meristems. This is tailed primary growth or longitudinal growth.
2. Secondary growth: The gymnosperms and most angiosperms, including some monocots, show an increase in the thickness of stems and roots by means of secondary growth or latitudinal growth.
![]()
Question 2.
Notes on Lenticels.
Answer:
- The aerating pores are seen as raised opening on the surface of bark as scars on old stems and roots.
- It is fonned during secondary growth in stems.
- In this portion phellogen activity is more than elsewhere, a filling tissue known as complementary tissue (loosely arranged parenchyma) is formed.
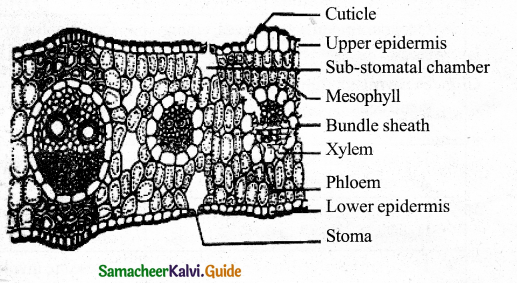
- Lenticel is helpful in the exchange of gases and also facilitate the little amount of transpiration
Question 3.
Explain briefly about false annual rings.
Answer:
Additional growth rings are developed within a year due to adverse natural calamities like drought, frost, defoliation, flood, mechanical injury and biotic factors during the middle of a growing season, which results in the formation of more than one annual ring. Such rings are called pseudo – or false – annual rings.
Question 4.
Differences between Diffuse porous wood and Ring porous wood
Answer:
Diffuse porous wood:
- This type of wood is formed where the climatic conditions are uniform
- The vessels are more or less equal in diameter in any annual ring
- The vessels are uniformly distributed throughout the wood
Ring porous wood:
- This type of wood is formed where the climatic conditions are not uniform
- The vessels are wide and narrow within an annual ring
- The vessels are not uniformly distributed throughout the wood
Question 5.
Differences between Porous Wood and Non – porous wood
Answer:
Porous wood or Hardwood, Ex: Morus:
- Common in Angiosperms
- Porous because it contains vessels
Non-porous wood or softwood Ex: Pinus
- Common in Gymnosperms
- Non – porous because it does not contain vessels
Question 6.
Differences between Sapwood (alburnum) and Heart Wood (duramen)
Answer:
| Sapwood (Alburnum) | Heartwood (Duramen) |
| 1. Living part of the wood | 1. Dead part of the wood |
| 2. It is situated on the outer side of wood | 2. It is situated in the centre part of wood |
| 3. It is lighter in colour | 3. It is dark coloured |
| 4. Very soft in nature | 4. Hard in nature |
| 5. Tyloses are absent | 5. Tyloses are present |
| 6. It is not durable and not resistant to microorganisms | 6. It is more durable and resists microorganisms insects and termites |
Question 7.
Differences Between Phellem and Phelloderm
Answer:
| Phellem (Cork) | Phelloderm (secondary cortex) |
| 1. It is formed on the outer side of phellogen1 | 1. It is formed on the inner side of the phellogen |
| 2. Cells are compactly arranged in regular tires and rows without intercellular spaces. | 2. Cells are loosely arranged with intercellular spaces. |
| 3. Protective in function. | 3. As it contains chloroplasts, it synthesizes and stores food |
| 4. Consists of non-living cells with suberized walls | 4. Consists of living cells, parenchymatous in nature and does not have suberin |
| 5. Lenticels are present | 5. Lenticels are absent |
Question 8.
Explain the term lenticel.
Answer:
Lenticel is raised opening or pore on the epidermis or bark of stems and roots. It is formed during secondary growth in stems. When phellogen is more active in the region of lenticels, a mass of loosely arranged thin-walled parenchyma cells is formed. It is called complementary tissue or filling tissue. Lenticel is helpful in the exchange of gases and transpiration called lenticular transpiration.
Question 9.
Mention the benefits of bark in a tree.
Answer:
Bark protects the plant from parasitic fungi and insects, prevents water loss by evaporation, and guards against variations of external temperature. It is insect repellent, decay proof, fireproof, and is used in obtaining drugs or spices. The phloem cells of the bark are involved in the conduction of food while secondary cortical cells involved in storage.
Question 10.
Annual rings are not clear and distinct in desert trees and seashore plants – Justify.
Answer:
- In the desert, as well seashore regions the climatic condition remain the same throughout the year.
- Secondary growth in plants is influenced by seasonal changes since in these areas seasonal changes are not significant enough to bring in distinct Annual rings with early and latewood formation alternatively.
![]()
Question 11.
A plant dies if the sapwood is damaged, but not with that of Heartwood – Give factual justification.
Answer:
- Sapwood is a living part of the wood, perform water conduction, that’s why it is known as Sapwood.
- Heartwood is a dead part of the wood, do not perform water conduction so if destroyed, no vital function of the plant is affected.
- If sapwood is damaged, or exposed conduction of water will be blocked, water loss is rapid leading to decay and decomposition of tissues and leads to death of the plant.
Question 12.
What is Dendrochronology? Add a note on the significance of studying the growth rings.
Answer:
- The annual ring of a tree corresponds to one year’s growth.
- If we count the rings we can determine the very age of the plant.
- This method of calculating the age of a tree by counting the annual ring is known as dendrochronology.
Significance of studying growth rings:
- Age of wood – calculated
- Age verified by Radioactive carbon dating
- Provides evidence in Forensic investigation.
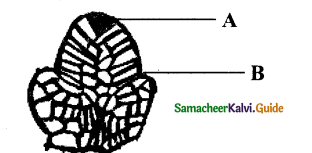
Question 13.
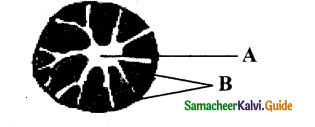
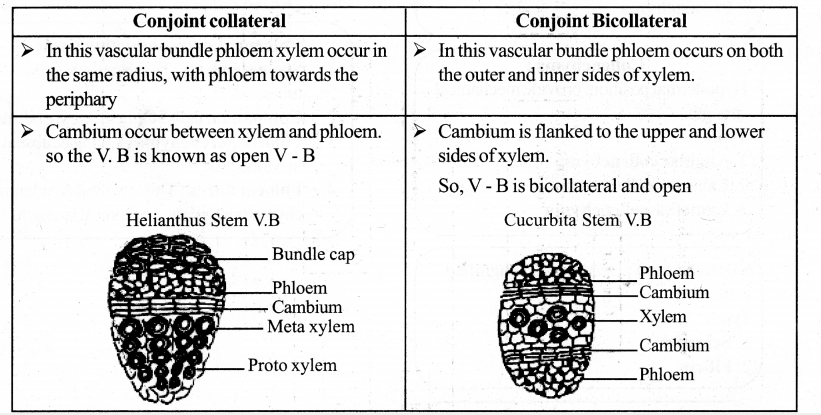
If ‘A’ is vascular cambium, then label other parts with reference to cambial activity.
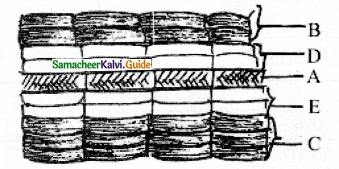
Answer:
A – Vascular cambium
B – First formed phloem – (Primary phloem)
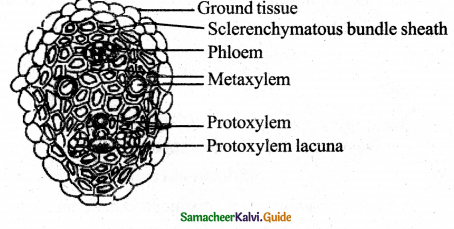
C – First formed xylem – (Primary xylem)
D – Second formed phloem – (Secondary phloem)
E – Second formed xylem – (Secondary xylem)
![]()
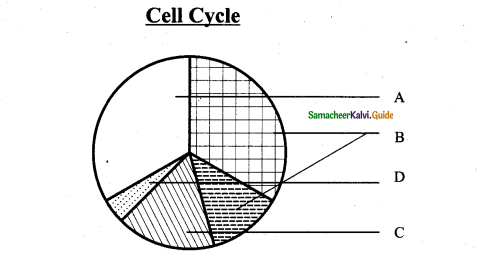
Question 14.
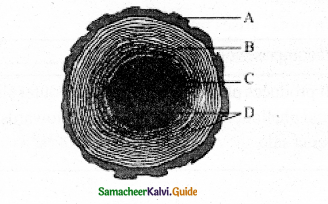
Answer:
Cross-section of wood showing Annual rings.
A-Bark
B-Sapwood
C – heartwood
D -Annual rings
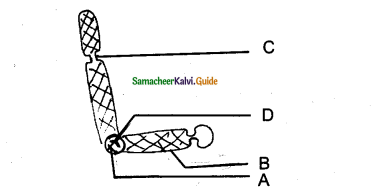
Question 15.
Identify the diagram & Label the parts.
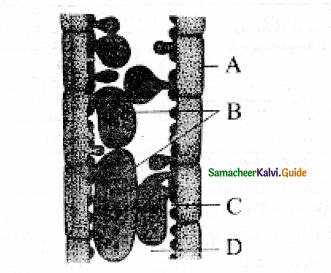
Answer:
Structure of Tyloses A – Parenchyma cell
B – Tyloses
C – Vessel wall
D – Vessel Lumen
![]()
Question 16.
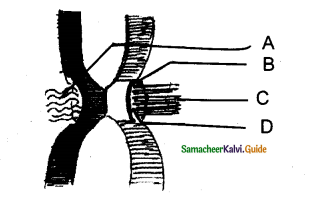
In the given diagram, the parts labelled are A, B, C, D identify the part correctly with respect to its function.
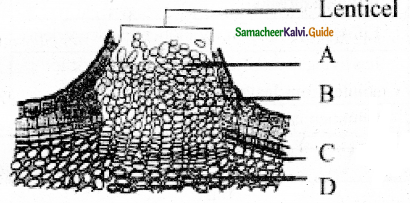
a) A – Periderm for gaseous exchange
b) C – Secondary cortex for protection
c) B – Complementary tissues for gaseous exchange
d) D – Phellogen for xylem and phloem formation
Answer:
c) B – Complementary tissues for gaseous exchange
Question 17.
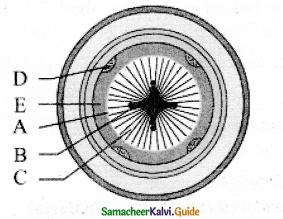
Answer:
Different stages of secondary growth in Dicot root
A – Cambial ring
B – Primary’ xylem
C – Secondary xylem
D – Primary phloem
E – Secondary phloem
VII. 5 Mark Questions
Question 1.
Distinguish between Phellem and Phelloderm.
Answer:
Phellem (Cork):
- It is formed on the outer side of phellogen.
- Cells are compactly arranged in regular tires and rows without intercellular spaces.
- Protective in function.
- Consists of nonliving cells with suberized walls.
- Lenticels are present.
Phelloderm (Secondary cortex):
- It is formed on the inner side of phellogen.
- Cells are loosely arranged with intercellular spaces.
- As it contains chloroplast, it synthesises and stores food.
- Consists of living cells, parenchymatous in nature and does not have suberin.
- Lenticels are absent.
Question 2.
Distinguish the significance of Cork and Bark
Answer:
Cork:
- It includes only phellem layer of bark
- It is composed of suberin a hydrophobic substance
- It has impermeable buoyant, elastic and fibre retartant properties
- Used in making bottle stoppers Eg. Bark of Quercus suber
Bark:
- It includes all tissues outside vascular cambium (Periderm, Cortex, Primary and secondary phloem)
- It has insect repellent, decay proof, fire proof properties.
- Used as Drugs or spices.
- Eg. Bark of Chichona – (AntimalariaJ drug), Bark of Cinnamomum (Used as spice)
![]()
Question 3.
Differentiate between, the vascular cambial components Fusiform initials and Ray initials.
Answer:
Fusiform initials:
- Vertically elongated cells
- Give rise to axial system of secondary tissues, xylem and phloem
- Secondary xylem includes tracheary elements, fibres and axial parenchyma
- Secondary phloem includes sieve elements
- Based on arrangement of fusiform initials 2 types of vascular cambium recognised
a – stratified cambium
b – Nonstratified cambium
Ray initials
- Horizontally elongated cells
- Give rise to radial system of secondary xylem and phloem
- Radial system consists of rows of parenchymatous cells oriented at right angles to the longitudinal axis of xylem elements
- Secondary phloem include phloem rays fibres and axial parenchyma
Question 4.
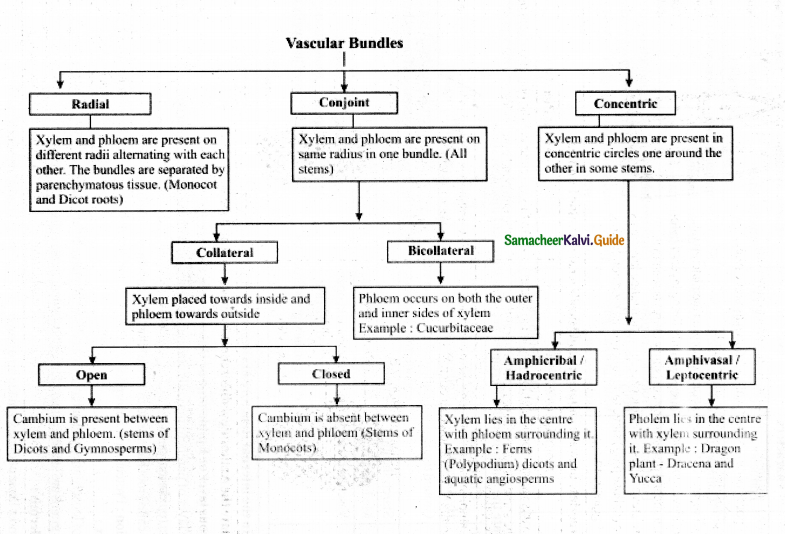
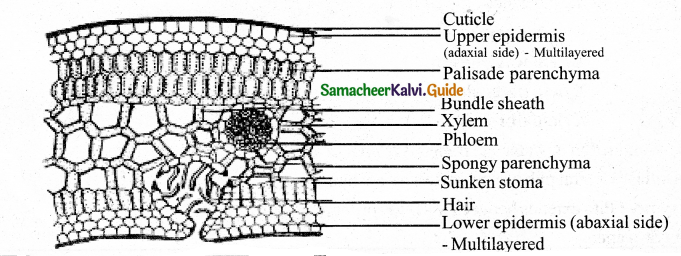
Give an account of Primary & Secondary structure of Dicto Stern. (Flow chart)
Answer:
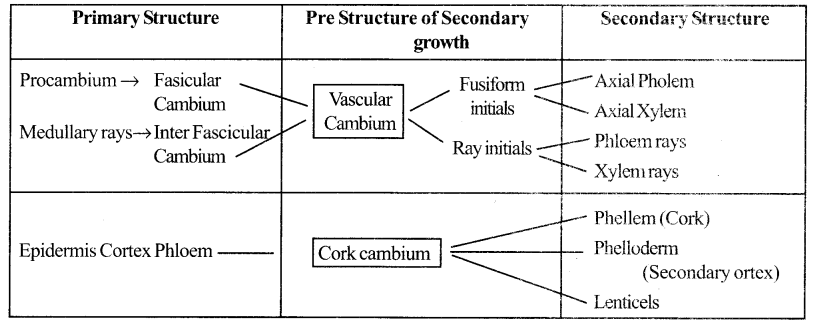
![]()
Question 5.
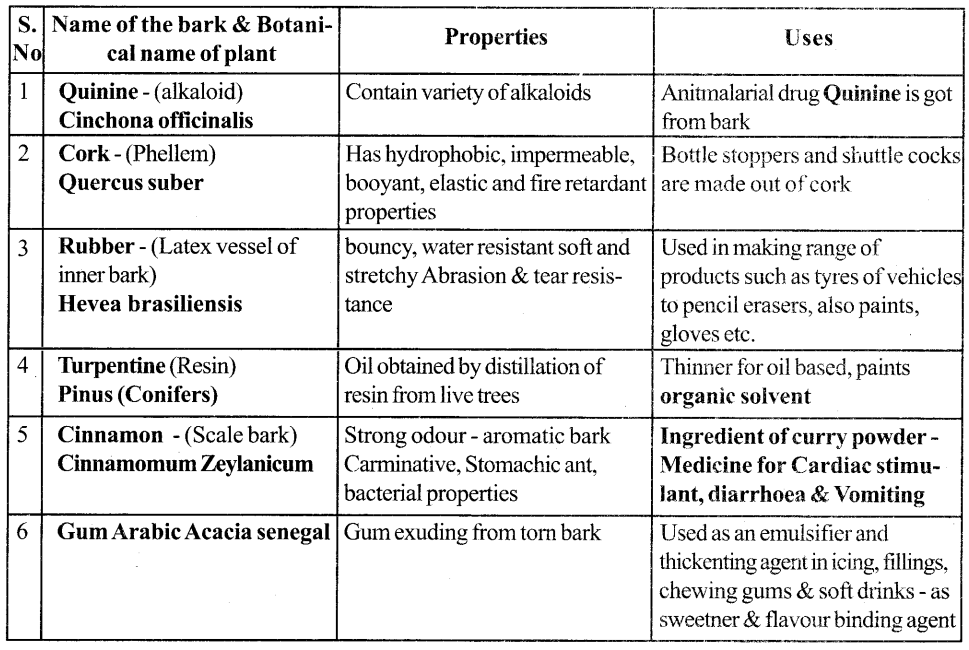
Give an account of Any 5 Commercial barks their, properties and uses
Answer:























 Nuclear envelope Nuclear space (nucleoplasm)
Nuclear envelope Nuclear space (nucleoplasm)