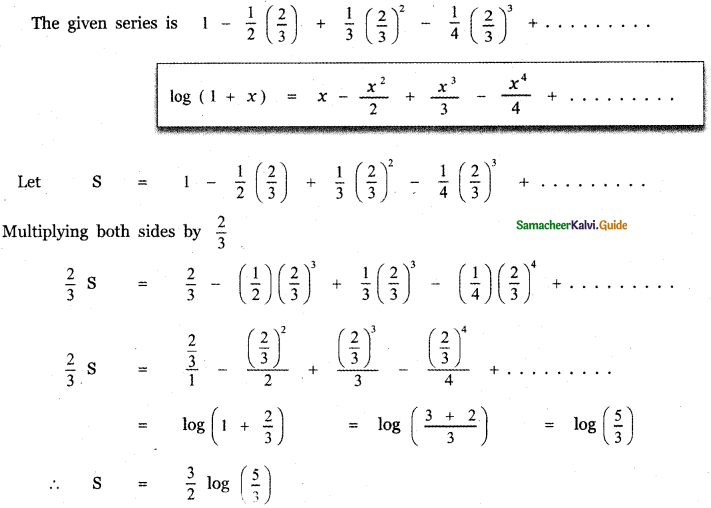
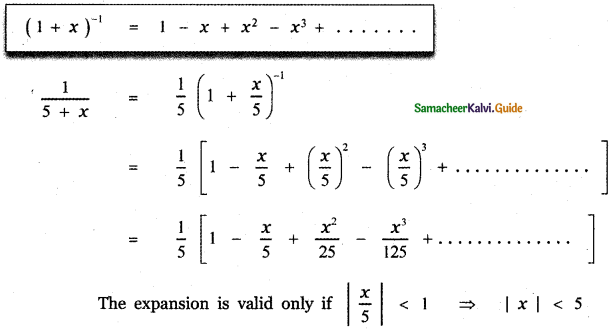
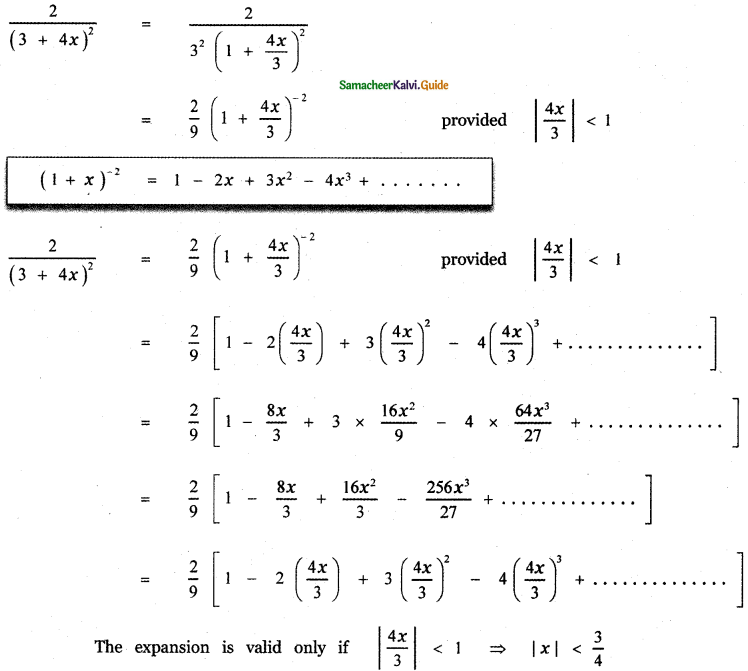
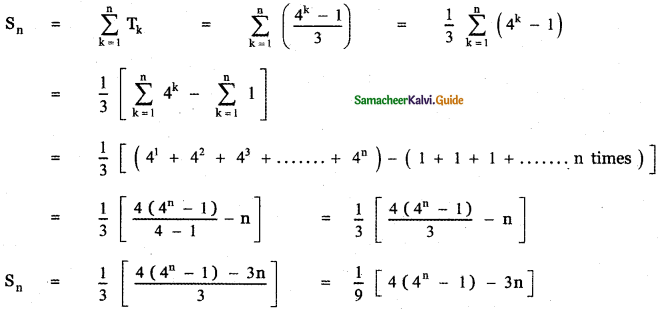
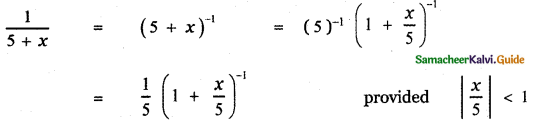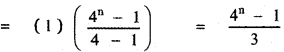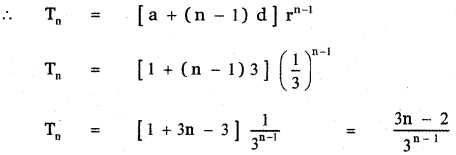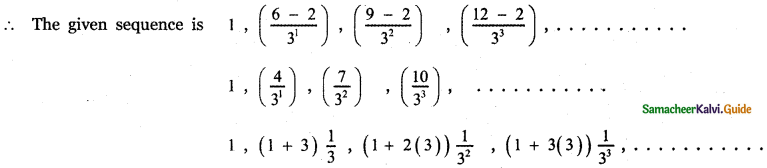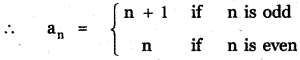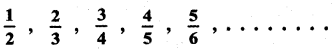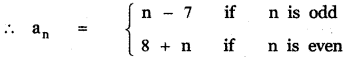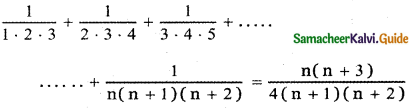Tamilnadu State Board New Syllabus Samacheer Kalvi 12th English Guide Pdf Prose Chapter 5 The Chair Text Book Back Questions and Answers, Summary, Notes.
Tamilnadu Samacheer Kalvi 12th English Solutions Prose Chapter 5 The Chair
12th English Guide The Chair Text Book Back Questions and Answers
Textual Questions:
1. Answer the following questions in one or two sentences each based on your understanding of the story: (Text Book Page No. 148) (Note: IQ – Important Questions)
Question a.
What was put on the family agenda?
Answer:
The idea of buying a chair for the house was put on the family agenda.
Question b.
Who visited the family?
Answer:
Their family friend who was a sub-judge visited the family.
Question c.
Describe the stool that the narrator’s family hpd.
Answer:
The three legged stool measured a mere three – fourth foot. It would topple over if the weight vvps not placed exactly above the legs.
Question d.
What was Pedanna suggested to their father?
Answer:
Pedanna suggested that they could buy a chair from the town.
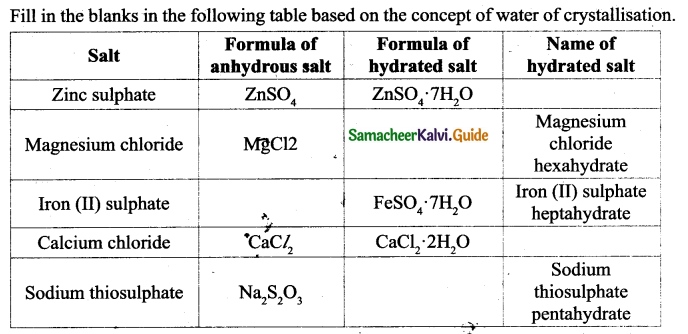
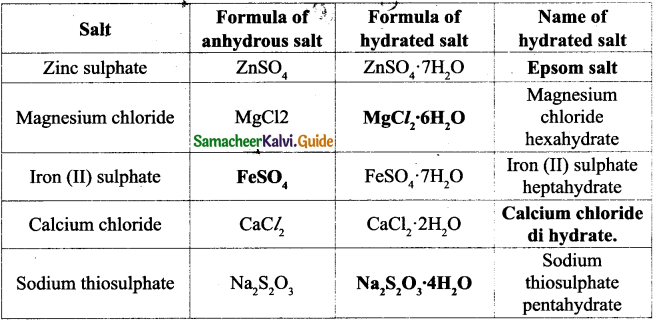
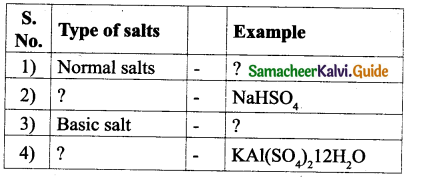
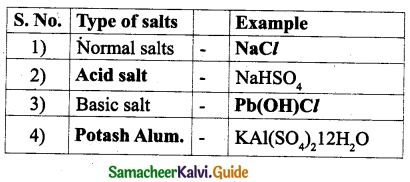
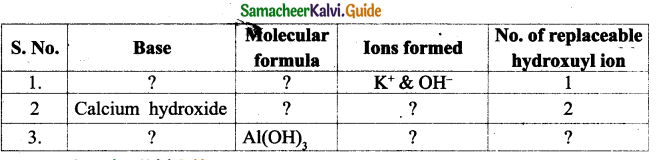
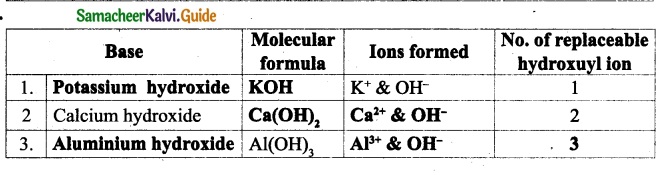
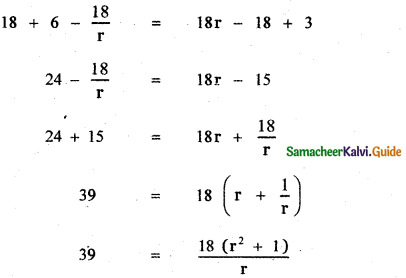
![]()
Question e.
What was offered to Maamanaar by their mother?
Answer:
Their mother offered buttermilk seasoned with asafoetida to Maamanaar.
Question f.
Why were the two chairs compared to Rama-Lakshmana?
Answer:
Rama and Lakshmana were the brothers who were identical in their character. In the same manner the two chairs were identical.
Question g.
When did the children shy away from the chair?
Answer:
The children shied away from the chair as it was used to propup a deadbody in their village.
Question h.
How did Maamanaar handle the chair at home?
Answer:
Maamanaar used to wipe it every morning. While shifting the chair he himself carried it and put it down carefully as if placing down gently a mud pot brimming with waiter.
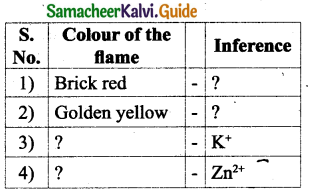
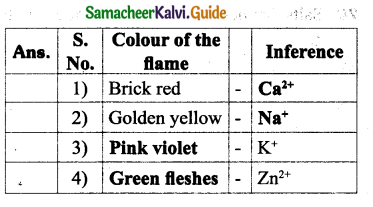
![]()
2. Answer the following questions in three or four sentences each: (Text Book Page No. 149)
Question a.
What happened to the visitor when he sat on the stool?
Answer:
The stool would topple over if one didn’t place one’s weight exactly above the legs. Before the family members tried to caution the sub judge, he sat on it fell down with a thud and rolled over.
Question b.
Why did the family find it difficult to make a chair?
Answer:
The family found it difficult to make a chair because there was no model of a chair in their village. Moreover there was not a single carpenter who knew how to make one.
Question c.
What was grandmother’s suggestion of wood ? Why ?
Answer:
Grandmother suggested to make use of teak wood as it would be of light in weight and could be carried around easily. Moreover it would be strong.
Question d.
How was the chair made and how did the villagers react to it?
Answer:
The chair was made of black wood with a mirror like gleam. It had perfectly shaped front legs and curved back legs. The villagers arrived in groups to see the chair. A few touched it gently. An old man lifted it and fall it to be heavy and strong.
Question e.
When did the children get over the fear of sitting on the chair?
Answer:
One of their neighbour Suganthi came to their home with her baby brother. She put her baby brother on the chair. It was only after that incident the children got over the fear of sitting on the chair.
Question f.
Why did Maamanaar hand over the chair to the villagers to retain it?
Answer:
Maamanaar handed over the chair to the villagers to retain it as he thought that it could be used to prop up dead bodies in the village whenever needed.
![]()
3. Answer the following in a paragraph of 100-150 words each: (Text Book Page No. 149)
Question a.
Narrate the humorous incidents that happened in the author’s home before and after the arrival of the chair.
Answer:
There was only a three legged stool in the narrator’s home when the story begins. The woeful thing about the stool was that if one didn’t place one’s weight exactly above the legs, the stool would topple over. Their family friend, a sub-judge paid a visit to their home one day. He was provided with the stool to sit on. Before he was given a caution, he sat on it and fell down with a thud and rolled over.
This incident made the family members giggle for a long time. After the arrival of the chair there arose a different scenario. The chair was asked by the villagers whenever there was a death in the village. It was used to prop up the corpse. Whenever the mourners came for the chair, the family members felt very sad which was misinterpreted by the mourners. The family members had fear to sit on the chair after that incident. They persuaded their visitors to sit on it and made fun that they were rehearsing for their death. When they started to sit on the chair again it would be asked for by the mourners. Thus the witty incidents continued.
Question b.
Write character sketches of Maamanaar and Pedanna.
Answer:
Maamanaar is considered to be a person who is very affectionate and calm. Though his sister’s children tease him to a great extend he remains mum With a smile. He never scolds them for their action. He is the one who is well-mannered and tidy. The way in which he takes care of his betel box and the newly brought chair reveals his perfection in handling things at home.
His cleanliness also comes to lime light when one has a close look at his lime paste dabba. Though he considers and maintains the things in his home as treasures, he is ready to lend his chair to the villagers. He even asks them to retain it for funeral purpose. This act of him highlights his generosity.
Pedanna seems to be a person who disrespects elders. The way in which he ridicules his Maamanaar when the latter pays a visit to his home proves this statement. He is also very good in making tricks. When the mourners come for the chair he diverts them to his Maamanaar’s house, which shows his deceitful act. In other sense, based on the above said qualities we can also consider Pedanna to be a person who likes to lead his life with fun and cheer.
![]()
Paragraph:
Introduction:
In the story “The Chair” the narrator, Rajanarayanan amusingly narrates the humorous incidents that took place in his home before and after the arrival of the chair.
Fun with stool:
There was only a three legged stool in the narrator’s home. It would topple over if one didnot place his weight exactly above its legs. Their family friend, a sub judge paid a visit to their home on6 day. He was provided with the. stool to sit on-. Without knowing the wretched thing about the stool, he sat on it and fell down with a thud and rolled over. The family members could not control their giggles.
The arrival of chair:
The narrator’s family decided to make a chair for their home. They had a lengthy discussion about it. Finally they decided to make two chairs. One for them and the other for their maamanaar. The chair arrived. The narrator and his siblings took turns to sit on it and even fought for it often.
Ordeal of the chair:
The village people came in groups to have a look at the chair as it was the first chair in their village. Unfortunately, there was a death of an eminent person in the village and the chair was asked for the propup the corpse of that eminent person. From then the chair was asked for whenever there occurred a death in the village. This act disturbed the sleep of the family members as the chair was asked for mostly during late night hours.
Pedanna’s trick:
The narrator and his siblings were afraid to sit on the chair, once after it was got back after the funeral ceremony. They washed it with many buckets of water. They made each and every visitor who came to their home to sit on it. Only after a long time they started to sit on the chair again. By that time the mourners came for the chair again. In order to overcome it Pedanna diverted the mourners to go to his maamanaar’s house and get the chair.
Maamanaar’s generosity:
Pedanna thought that his Maamanaar would not give the chair to the mourners. Pedanna v and his siblings were of the opinion that their Maamanaar was stingy. But things happened viceversa. Maamanaar was so generous to lend the chair to the villagers and asked them to retain it for funeral purpose.
Conclusion:
Thus the narrator elucidates the incidents that took place because of the chair in a humorous way.
![]()
Vocabulary:
a) Find out the synonym of the underlined word in each of the following sentences: (Text Book Page No. 149)
Question 1.
Just as we thought our chuckles had subsided.
a) diminished
b) increased
c) completed
d) submerged.
Answer:
a) diminished
Question 2.
Our father rejected it, saying it wouldn’t be sturdy.
a) weak
b) strong
c) tall
d) good
Answer:
b) strong
Question 3.
Anna would say with an impudent smile.
a) innocent
b) fake
c) disrespectful
d) decent
Answer:
c) disrespectful
Question 4.
A silver tumbler of buttermilk seasoned with asafoetida.
a) mixed
b) garnished
c) filled
d) loosened
Answer:
b) garnished
Question 5.
A few people from the house of bereavement stood outside.
a) rejoice
b) celebration
c) grief
d)war
Answer:
c) grief
![]()
b. Find out the antonym of the underlined word in each of the following sentences: (Text Book Page No. 149)
Question 1.
The anticipation of this imagined separation only increases their fondness for the calf.
a) expectancy
b) contemplation
c) outlook
d) ignorance
Answer:
d) ignorance
Question 2.
Fortuitously, a quest visited our house.
a) luckily
b) peacefully
c) unfortunately
d) happily
Answer:
c) unfortunately
Question 3.
There he was sitting in splendour on his chair.
a) magnificence
b) pomp
c) effulgence
d) simplicity
Answer:
d) simplicity
Question 4.
Maamanaar was unmatched.
a) inferior
b) incomparable
c) excellent
d) supreme
Answer:
a) inferior
Question 5.
He greeted me with his usual smile and banter.
a) flattery
b) small talk
c) chitchat
d) repartee.
Answer:
a) flattery
![]()
c. Identify the correct combination of the following compound words: (Text Book Page No. 150)
- Haircut – Noun + Verb
- waterfall – Noun + Verb
- drawback – Verb + Adverb (verb+noun)
- output – Preposition + Verb
- show case – Verb + Noun
- headmaster – Noun + Noun
- swimming pool – Gerund + Verb
- public speaking – Adjective + Gerund
- software – Adjective + Noun
- world famous – Noun + Adjective
![]()
d. Blend the following words: (Text Book Page No. 150)
Question 1.
binary + digit
Answer:
bit
Question 2.
electronic + mail
Answer:
email
Question 3.
foreign + exchange
Answer:
forex
Question 4.
motor + pedal
Answer:
moped
Question 5.
parachute + troop
Answer:
paratroop
![]()
e. Replace the underlined word/ expressions with possibly polite forms: (Text Book Page No. 150)
Question a.
The culprit was sent to jail.
Answer:
Jail – Penitentiary
Question b.
Dheeraj is unemployed.
Answer:
unemployed – between jobs
Question c.
I saw a disabled man.
Answer:
disabled – physically challenged
Question d.
Subsidies are given to the poor.
Answer:
poor – needy
Question e.
Elambrathi has a second-hand car.
Answer:
second hand – hand me down
![]()
Listening:
After listening to the pledge played on the tape recorder carefully, fill in the following statements with the right options given: (Text Book Page No. 153)
Question a.
The medical graduates take oath to dedicate their _______ to the service of humanity.
i) money
ii) talent
iii) life
iv) nation
Answer:
iii) life
Question b.
The _______ of the patient should be the doctor’s greatest concern.
i) dignity
ii) gratitude
iii) health
iv) honour
Answer:
iii) health
Question c.
The would-be graduates promise to practise their profession with _______ and dignity.
i) conscience
ii) knowledge
iii) understanding
iv) respect
Answer:
i) conscience
Question d.
They should respect the _______ of the patients.
i) age
ii) wealth
iii) background
iv) secrets
Answer:
iv) secrets
Question e.
The pledge is also to treat the patients without any _______.
i) fee
ii) discrimination
iii) interest
iv) hatred
Answer:
ii) discrimination
![]()
a. Read the following passage carefully and answer the following questions: (Text Book Page No. 153)
Questions and answer: (for passage please refer Text Book Page No. 152)
Question 1.
What is meant by pollution? Mention the different kinds of pollution.
Answer:
Pollution is the introduction of contaminants into the environment that cause harm to the ecosystem. The different kinds of pollution are air pollution, water pollution, and land pollution.
Question 2.
How does Particulate Matter cause air pollution?
Answer:
Particulate matter (PM), also known as particle pollution, is a complex mixture of extremely small particles and liquid droplets that get into the air. These particles if inhaled can affect health.
Question 3.
Identify three major causes of pollution in air.
Answer:
The release of various gases, finely divided solid particles, or liquid droplets that escape into the atmosphere to disperse and dilute in the environment are the major causes of air pollution.
Question 4.
Name the types of pollution we encounter now.
Answer:
Noise pollution, light pollution, and plastic pollution.
Question 5.
What sort of health issues do people face due to air pollution?
Answer:
Several studies have shown that poor air quality is a cause for many health issues among people with lower respiratory disorders with symptoms like dry cough, breathlessness, wheezing, chest discomfort, serious lung infections, and cardiovascular diseases. Some studies throw light on the fact that about 16 percent of the deaths worldwide in 2015 were due to pollution.
Question 6.
How can we protect ourselves outdoor from air pollution?
Answer:
Air Masks are an option to protect oneself outdoor. Air masks can be used while commuting or while one is exposed to a polluted area.
Question 7.
Suggest a suitable title to the passage.
Answer:
‘Air pollution’ is a suitable title for the passage.
Question 8.
Identify the meaning of the word similar to the one used in the fourth para:
Answer:
a) emerging
b) filtering
c) floating
d) falling.
![]()
b) Read the following information given in the table below and answer the questions: (Text Book Page No. 154)
| A nice choice from Chennai to the National capital | |||
| RAJADHANI EXPRESS TIMETABLE | |||
|
Shortest Route between Chennai and Hazrat Nizamuddin 8 Halts & 324 intermediate stations in between |
|||
| Station Name | Departs | Day | Speed |
| Chennai Central | 06.05 | 1 | 75 |
| Vijayawada | 11.55 | 1 | 76 |
| Warangal | 14.40 | 1 | 77 |
| Balharshah | 18.00 | 1 | 78 |
| Nagpur | 20.45 | 1 | 74 |
| Bhopal | 02.10 | 2 | 89 |
| Jhansi | 05.31 | 2 | 99 |
| Gwalior | 06.32 | 2 | 85 |
| Agra Cantt | 07.57 | 2 | 76 |
| Hazrat Nizamuddin | 10.25 | 2 | – |
![]()
Question a.
The number of stations between Chennai Central and Hazrat Nizamuddin is _______.
i) five
ii) ten
iii) eight
iv) eleven
Answer:
iii) eight
Question b.
The train is expected to reach ______ around 8.45 PM.
i) Warangal
ii) Vijayawada
iii) Bhopal
iv) Nagpur
Answer:
iv) Nagpur
Question c.
Between ____________ the train runs at its maximum speed.
i) Bhopal and Gwalior
ii) Bhopal and Jhansi
iii) Bhopal and Hazarat Nizamudin
iv) Bhopal and Agra
Answer:
ii) Bhopal and Jhansi
Question d.
Almost ____________ the train reaches Vijayawada.
i) the day after
ii) around early morning
iii) late night
iv) around noon
Answer:
iv) around noon
Question e.
People prefer the Rajadhani Express to travel from Chennai to reach the capital because ______.
i) it reaches the destination on the same day
ii) the charge is reasonable
iii) the train halts at ten stations
iv) it is the shortest route from Chennai to New Delhi
Answer:
iv) it is the shortest route from Chennai to New Delhi
Question f.
The destination of Rajadhani express is ______.
i) Hazarat Nizamuddin
ii) New Delhi junction
iii) Old Delhi
iv) Rajkot
Answer:
i) Hazarat Nizamuddin
![]()
Grammar:
Non-finite Verbs:
Task 1:
Underline the gerunds in the following sentences: (Text Book Page No. 155)
Question 1.
Boys love playing cricket.
Answer:
playing
Question 2.
I love eating ice creams.
Answer:
eating
Question 3.
Jessie enjoys bothering others.
Answer:
bothering
Question 4.
Painting is an interesting hobby.
Answer:
interesting
Question 5.
Dancing gives me joy.
Answer:
Dancing
![]()
Task 2:
Use the gerundial form of the verb in the brackets and fill in the blanks: (Text Book Page No. 156)
Question 1.
______ (exercise) is good for health.
Answer:
Exercising
Question 2.
______ (fly) a kite is fun.
Answer:
Flying
Question 3.
______ (shop) is my favourite hobby.
Answer:
Shopping
Question 4.
My friend waited for the ______ (meet).
Answer:
meeting
Question 5.
Huckleberry Finn was responsible for _______ (signal).
Answer:
signaling
![]()
Task 3:
Fill in the blanks with the correct infinitives: (Text Book Page No. 156)
Question 1.
Deva forgot ______ the letter.
Answer:
to post
Question 2.
The doctor advised the patient ______ his medicines without fail.
Answer:
to take
Question 3.
Rajesh went to the airport ______ his friend.
Answer:
to receive
Question 4.
The bear climbed up the tree ______ the honey.
Answer:
to drink
Question 5.
The boys went to the forest ______ birds.
Answer:
to watch
![]()
Question 6.
I tried hard ______ both ends meet.
Answer:
to make
Question 7.
The archaeologists are trying ______ the ruins of Keelady.
Answer:
to study
Question 8.
Solar energy is used ______electricity.
Answer:
to generate
Question 9.
______ concession, you have to apply well in advance.
Answer:
To get
Question 10.
We have plans ______ to London during summer vacation.
Answer:
to go
![]()
Task 4:
Combine each of the following pairs of sentences using participles. The first one is done for you: (Text Book Page No. 156)
Example: I didn’t know what to do. I phoned the police.
Not knowing what to do, I phoned the police.
Question 1.
The baby cried. She was feeling sleepy.
Answer:
Feeling sleepy, the baby cried.
Question 2.
He lived alone. He had forgotten everybody.
Answer:
Having forgotten everybody, he lived alone.
Question 3.
She walked out. She was smiling.
Answer:
She walked out smiling.
Question 4.
The child says he needs attention. He shouts loudly.
Answer:
Shouting loudly the child says she needs attention.
Question 5.
I threw the pen. It was broken.
Answer:
I threw the broken pen.
![]()
Question 6.
His coat is tattered. It needs mending.
Answer:
His tattered coat needs mending.
Question 7.
I heard the noise. I turned around.
Answer:
Hearing the noise I turned around.
Question 8.
He was dissatisfied. He quit his job.
Answer:
Being dissatisfied he quit the job.
Question 9.
The politician entered the campus. He was accompanied by many comrades.
Answer:
Accompanied by many comrades the politician entered the campus.
Question 10.
The girl entered the room. She was singing a song.
Answer:
Singing a song the girl entered the room.
![]()
Articles and Determiners:
Articles:
Task 1:
Complete the following exercise using a / an / the / ‘o’ (no article) in the underlined space where appropriate. Change capital letters to lower case letters at the beginning of a sentence if necessary: (Text Book Page No. 156)
According to (1) ______ National Weather Report, cyclones are winds circulating (2) ______ counter clockwise in (3) ______ Northern Hemisphere and clockwise in (4) ______ southern Hemisphere. Cyclones are usually accompanied by (5) ______ stormy weather. Tornadoes and hurricanes are types of cyclones. (6) ______ hurricane is (7) ______ cyclone that forms over (8) ______ tropical oceans and seas. (9) ______ hurricane rotates in (10) ______ shape of (11) ______ oval or a circle. (12) ______ Hurricane Andrew, which hit (13) ______ coasts of Louisiana and Southern Florida in August 1992, caused (14) ______ extreme devastation. It was one of (15) ______ most devasting hurricanes ever to hit (16) ______ U.S.. Fourteen people died of (17) ______ Andrew’s effect.
Answer:
- the
- O
- the
- the
- a
- A
- a
- the
- A
- the
- an
- The
- the
- an
- the
- the
- O
![]()
Task 2:
Complete the following sentences using appropriate determines: (Text Book Page No. 157)
Question 1.
Only ______ people can afford to buy a flat in Chennai.
Answer:
a few
Question 2.
She earns so ______ that she could not make a decent living.
Answer:
little
Question 3.
______ information that she gave proved false.
Answer:
Every
Question 4.
How ______ sugar do you want?
Answer:
much
Question 5.
I am very tired today, as I had ______ guests today.
Answer:
some
![]()
Question 6.
______ of my students have become doctors.
Answer:
Most
Question 7.
______ do I know about his personal life.
Answer:
Little
Question 8.
How ______ pages did you read?
Answer:
many
Question 9.
______ fertilizer used these days spoils the soil.
Answer:
Each
Question 10.
During my student life I used to give ______ trouble to my teachers.
Answer:
much
![]()
Degrees of Comparison – Transformation:
Task 1:
Transform each of the following sentences using the comparative degree without changing the meaning: (Text Book Page No. 159)
Question 1.
Very few Indian languages are as ancient as Tamil.
Answer:
Tamil is more ancient than many other Indian languages.
Question 2.
Hurricanes are as dangerous as tornadoes.
Answer:
Tornadoes are not more dangerous than hurricanes.
Question 3.
This is the most challenging task I have ever undertaken.
Answer:
This task is more challenging than any other task, I have ever undertaken.
Question 4.
E-mail is the fastest means of communication.
Answer:
E-mail is faster than any other means of communications.
Question 5.
Compulsive gambling is the worst habit a man can develop.
Answer:
Compulsive gambling is worse than any other gambling a man can develop.
![]()
Task 2:
Rewrite each of the following sentences using the superlative degree retaining the meaning: (Text Book Page No. 159)
Question 1.
Shakespeare is greater than many other dramatists of the world.
Answer:
Shakespeare is one of the greatest dramatists in the world.
Question 2.
Some people think that nothing is as important as money in life.
Answer:
Some people think that money is the most important in life.
Question 3.
The peacock is more colourful than any other bird found in India.
Answer:
The peacock is the most colourful bird found in India.
Question 4.
Very few people in this town are as generous as Mr. Mohan.
Answer:
Mr.Mohan is one of the most generous persons in this town.
Question 5.
No other planet in our solar system is as cold as Neptune.
Answer:
Neptune is the coldest planet in the solar system.
Question 6.
I cannot do anything better for you than this.
Answer:
This is the best thing, I can do for you.
![]()
Task 3:
Replace the comparative adjectives in the following sentences with their positive forms: (Text Book Page No. 159)
Question 1.
Rural life is certainly more peaceful than urban life.
Answer:
Urban life is certainly not as peaceful as rural life.
Question 2.
The pen is mightier than the sword.
Answer:
The sword is not as mighty as the pen.
Question 3.
Train journey is more comfortable than bus journey.
Answer:
Bus journey is not as comfortable as train journey.
Question 4.
My mother can speak more sweetly than anyone else.
Answer:
No one speaks as sweet as my mother.
Question 5.
Gold is not more useful than Iron.
Answer:
Iron is as useful as Gold.
![]()
Writing:
Slogan writing:
Look at the pictures given below, and write slogans to advertise the products. Suggest your own brand name for each of the products. (Text Book Page No. 159)
1. Toothpaste → “Smile with strength”, Everyday protection for sensitive teeth.
2. Water purifier → “Purifying agent”, Perfect quality for perfect living.
3. Camera → “Candid camera”, we capture your memories.
4. Apples → “Orchard apples”, An apple a day keeps the doctor away.
Write slogans to create awareness of the following topics using the tips given above: (Text Book Page No.159)
1. Junk food →“Maintain your weight to just feel great”
2. Labour Day → “There is no success without hard work’’, “No work for labours to labour day”
3. Save Water → “Conserve water, Conserve life”, “Thousand lived without love, but not without water”
4. Yoga → “A boon to people on earth”, “A secret to retaining the beauty of body and mind”
5. Blood Donation → “Bring a life back to power”, “Every blood donor is a lifesaver’.
![]()
Write a paragraph of about 150 words, on the following topics: (Text Book Page No. 160)
a) The teacher I like the most:
A teacher is a person who educates a group of students known as his pupils. The teacher instills in his students, the values of discipline, responsibility, honesty, courage, and truthfulness. The teacher guides his students in the right direction and prevents them from falling prey to bad intentions and influences. My favourite teacher is my English teacher. She is an elderly lady with great knowledge. Her class is my favourite of the day.
She is a well-known face in our school. In her class, almost every student pays attention to what she says. She uses the help of charts and new methodology to make us understand even better. She makes sure that her students are comfortable with her and not threatened by her. My English teacher was like a mother to me.
Her affection towards me was like a mother’s towards her child. She is the person who pushed me to try everything in life. Her constant dedication towards my interests and hobbies motivated me to pursue the same with more enthusiasm. It is because of her that I complete my work in time and with sincerity even today.
b) The value of discipline:
Discipline is important in our everyday life. It is also important for the better development of a society. It helps in time management. When one is disciplined, they are better able to manage their time at school. Work and at home. Discipline also helps to build respect. It also helps one to achieve better grades in school.
One is able to concentrate more in their studies and not on other things. Discipline is a virtue that every person should have. There are many benefits associated with discipline. One is able to live a better life when they are disciplined. The absence of discipline brings disorder and chaos. If we do not obey our parents at home, our teachers at school, we can imagine what will happen. So both our parents and the teachers are very anxious to make us see the need for disciplined soldiers.
![]()
c) Need for Moral Education in schools:
Education is only complete when it leads to all-round development of the individual, not only mental but also moral development. Moral Education influences the social thinking of the individual and makes him or her distinguish between what is right and what is wrong. Moral Education is important as it teaches diversity, tolerance mutual respect, and moral values.
Moral values are values that express ideas about the good life. Honesty, responsibility, and respect for others is the domain of moral education. The study of morality is vital because we live in a world of rapid change. Education should make children to learn moral values such as truthfulness, honesty, charity, hospitality, tolerance, love, kindness, and simplicity. Moral education should be taught as a separate subject like moral science. Thus moral education is important in schools as it teaches many good things.
d) The importance of Good Health:
Good health is important because a man of health can enjoy great happiness during his lifetime. Without health, we can not do anything in this world. Health is wealth is a common proverb which reveals a very simple meaning by comparing the value of health with wealth. Everyone knows that nothing is important in life than good health. No one can be happy and peaceful without good health.
There is no success in people’s life if they suffer with bad health. In reality, health is more valuable for a person than money because money can not buy good health and happiness intellectually and financially. Maintaining health is not so simple. Good or bad health depends upon several factors including healthy food, environment, lifestyle, sleeping habits, social status, psychological condition, financial condition, and many more things.
e) The importance of Reading:
Reading is one of the most important and priceless activities. If you have ever read a book in life, you will know the pleasure of reading. Reading is the kind of exercise that keeps your mind active and healthy. It is important to develop the habit of reading not only for knowledge but also for personal growth and development. It develops positive thinking and gives you a better perspective of life.
Reading habits develop imagination, knowledge, and vocabulary. The most important reason for reading is that we gain knowledge. Books are a rich source of information and knowledge. It also develops the creative mind of a person. It is the best way to relieve your stress and enhance your mood. It builds your confidence and leads to higher self-esteem. So it is very important to develop a good reading habit.
![]()
ஆசிரியரைப் பற்றி:
ராஜநாராயணன் என்பவர் எல்லோராலும் தமிழிலில் கி.ரா. என்று அழைக்கப்படுபவர். இவர் ஒரு தமிழ் நாட்டார் வழக்காற்றியலாளர் (Folklorist) மற்றும் ஏராளமாகப் படைத்தளித்த (prolific) ஆசிரியர். “தி சேர்” என்ற சிறுகதை, 1969ல் நாற்காலி என்ற தலைப்பில் எழுதப்பட்டது. நாவல்கள் கோபால கிராமம் மற்றும் கோபாலபுரத்து மக்கள் அனைவராலும் மிகவும் பாராட்டப்பட்டது (acclaimed). இதற்காக 1991ல் அவருக்கு சாகித்ய அகாதமி விருது வழங்கப்பட்டது. நாட்டார் வழக்காற்றியல் துறையில் இருந்ததால், கி.ரா. 10 ஆண்டுகளில் (decade) நாட்டுப்புற கதைகளை கரிசல் காடுகளில் இருந்து சேகரித்து, இதனை மிகப் பிரபலமான இதழ்களில் வெளியிட்டார்.
2007ல் தஞ்சாவூரில் இருக்கும் புத்தக வெளியீட்டு நிறுவனம் (publishing house) “அன்னம்” இந்த நாட்டுப்புறக் கதைகளை, 944 பக்கங்களைக் கொண்ட ஒரு புத்தகமாகத் தொகுத்து “நாட்டுப்புறக் கதைக் களஞ்சியம்” என்ற தலைப்பில் வெளியிடப்பட்டது. 2009ல் கிட்டத்தட்ட அவர் 30 புத்தகங்களை வெளியிட்டார். அதிலிருந்து ஒரு சில புத்தகங்களைத் தேர்ந்தெடுத்து பிரித்தம் K. சக்கரவர்த்தி என்பவர் ஆங்கிலத்தில் மொழிபெயர்த்து, அதனை வேர் ஆர் யு கோயுங், யு மங்கீஸ்? என்ற தலைப்பில் தமிழ்நாட்டு நாட்டுப்புறக் கதைகளாக, புத்தக வடிவில் 2009ல் வெளியிட்டார்.
பாடத்தைப் பற்றி:
கீழே உள்ள கதையில் ஒரு நாற்காலி இல்லாத குடும்பத்தைப் பற்றி பார்ப்போம். (இருப்பினும் அந்த ஒட்டுமொத்த (entire) கிராமமே நாற்காலியைப் பார்த்ததில்லை ) இங்கு கதை சொல்பவர் (narrator) கிராம மக்கள் அந்த திட்டங்களை செயல்படுத்தும் விதம் பற்றியும் மற்றும் நாற்காலி வந்த பிறகு ஏற்படும் பின்விளைவு (aftermath) பற்றியும் ஒரு நகைச்சுவை சம்பவமாக மிகவும் வேடிக்கையாகக் கூறுகிறார். இதைப் பற்றி விரிவாக கீழே காண்போம்.
![]()
The Chair Summary in Tamil
தமிழாக்கம் நாற்காலி இல்லாத வீடா?
திடீரென எங்கள் வீட்டில் இருந்த அனைவரும் இவ்வாறு நினைக்கத் தொடங்கினர். அது என்னவெனில் குடும்ப நிகழ்ச்சி நிரலில் போடப்பட்டிருந்தது (agenda) மற்றும் விவாதங்கள் ஆரம்பிக்கப்பட்டன.
ஒரு நாள் முன்பு, ஒரு குடும்ப நண்பர், எங்கள் வீட்டுக்கு விஜயம் செய்தார். அவர் ஒரு துணை நீதிபதி. சட்டை அணிந்து வரவில்லை. மாறாக அவர் கோட்டும், சூட்டும், காலணியும் அணிந்து வந்திருந்தார். எங்கள் வீட்டில் ஒரு முக்காலி மட்டுமே இருந்தது. அது ஒன்றே முக்கால் மீட்டர் அடி அளவு கொண்டதாக இருக்கும். பாட்டி தயிர் சலிக்கும் போது அந்த முக்காலியில் தான் அமர்ந்திருப்பார். பாட்டி கொஞ்சம் எடை கொண்டவர் அதனால், தாத்தா அதனை தச்சரிடம் கொடுத்து கொஞ்சம் பரந்துபட்ட அளவு உடையதாக மாற்றிக் கேட்டார்.
வீட்டுக்கு வந்திருந்த துணை நீதிபதியும் சிறிய உருவம் கொண்டவர் (portly). எங்கள் வீட்டில் எந்த ஒரு இருக்கைகளும் இல்லாத காரணத்தால், அந்த முக்காலியை அவர் உட்கார கொண்டு வந்தோம். அதன் விளிம்பில் ஒருகையைவைத்தவாறு, அவரை அமரச் செய்தோம். அந்த முக்காலியைப் பற்றி ஒரு மோசமான (wretched)
செய்தி: சரியாக அந்த முக்காலியின் கால்கள் மேல் எடையை (உடலை) அமர்த்தவில்லையென்றால் அது கீழே கவிழ்ந்து (topple) விடும். நாங்கள் இது போன்று பலமுறை கவனக்குறைவினால் கீழே விழுந்திருக்கிறோம். அதன் மீது ஏறி, உறியில் இருந்த நெய்யைத் திருடும் போது (rope net). இந்த செய்தியைச் சொல்வதற்கு முன்னால், துணை நீதிபதி கீழே விழுந்தார்.
கீழே “தொப்” (thud) என்ற மெல்லிய சத்தத்தோடு விழுந்த அவர் உருண்டோடினார். நான் எனது தம்பி மற்றும் என் குட்டித் தங்கை, இதனைக் கண்டு இளித்தவர்களாய் (giggles) பின்புறம் உள்ள தோட்டத்துக்கு ஒடினோம். இந்த உள்ளூர சிரிப்பு தணிந்தது என்று தான் நினைத்தோம். என் தங்கை பல குரலில் நன்றாக பேசுவாள். அவள் அந்த துணை நீதிபதி விழுந்தது போன்று கைகளை சாய்த்தும், தரையில் உருண்டும் செய்து காண்பித்தாள். இதைப் பார்த்து எங்களுக்குச் சிரிப்பு நீளத்தான் செய்தது.
![]()
இந்த இளிப்புக்கு இன்னொரு காரணமும் உண்டு. இது போன்று மற்ற விருந்தினர்கள் தரையில் உருண்டு விழும்போது, எங்களின் பெற்றோர் சிரிப்பை அடக்கிக் கொண்டு (suppressing) அவர்கள் முன்னே நிற்பது எங்களின் நினைவுக்கு வந்தது.
அதனால், எல்லோருடைய சார்பாகவும் சிரிப்பை முடிவுக்கு கொண்டு வந்து, நாங்கள் மெல்ல வீட்டுக்குள் அடியெடுத்து வைத்தோம் (pussy footed). ஆனால் அந்த துணை நீதிபதி அங்கு இல்லை . அந்த முக்காலியும் அங்கு இல்லை. அவரோடு அதனை எடுத்துச் சென்று விட்டாரா? என்று என் சகோதரி என்னிடம் கேட்டாள்.
இந்த சம்பவத்திற்குப் பிறகு, எங்கள் வீட்டுக்கு ஒரு நாற்காலி செய்ய வேண்டும் என்று முடிவு எடுக்கப்பட்டது. இதில் ஒரு பிரச்சனை என்னவென்றால், அந்த நாற்காலியை செய்வதற்கு, அந்த கிராமத்தில், மாதிரி காண்பிக்க ஒரு நாற்காலி கூட இல்லை. ஒரு தச்சர் கூட அதனை செய்வதற்கு இல்லை.
“பரவாயில்லை. ஒரு நாற்காலியை நகரத்தில் இருந்து வாங்கி விடலாம்” என்று தம்பி பெடன்னா (Pedanna) பரிந்துரைத்தான். ஆனால் என் தந்தை அதனை நிராகரித்தார். காரணம் அது சாத்தியமிக்கது அல்ல (sturdy) என்று கூறினார்.
எங்கள் அத்தை, பக்கத்து கிராமத்தில் ஒரு கை தேர்ந்த (skilled) தச்சர் இருப்பதாகக் கூறினார். ஆனால், அவர் எந்த ஒரு நாற்காலியும் செய்ததில்லை. அந்த ஆளுநர் தாமே அவருக்கு பரிசாகக் கொடுத்திருந்தார்.
அம்மா, அத்தையின் இரண்டாவது வாக்கியத்தைச் கேட்ட போது, தலையைத் திருப்பி, “அவள் எல்லாவற்றையும் பார்த்திருப்பாள்” என்று கூறுவதைப் போல இருந்தது.
![]()
அப்பா வேலைக்காரர் ஒருவரை அழைத்து, அந்த தச்சன் இருக்கும் கிராமத்துக்கு அனுப்பி (despatched) அழைத்து வரச் சொன்னார். அவர் அருகில் வந்து அமர்ந்தார். இப்போது, என்ன வகையான மரத்தைப் பயன்படுத்தலாம் என்ற விவாதம் நடந்தது.
“தேக்குதான் சிறந்தது. அது தான் தூக்குவதற்கும், பிற இடங்களுக்கு நகர்த்தி செல்வதற்கும் சாத்தியம் உள்ளதாக இருக்கும்” என்று தன் பக்கவாத கால்களைத் தடவியவாறே கூறினார். பாட்டி (பாட்டி எப்போதும் தன் கால்களை பக்கவாதத்தின் காரணமாக, ஆட்டிக் கொண்டே இருப்பார்)
அந்நேரம், மாமனார் (தாய் வழி மாமன்) உள்ளே நுழைகிறார். பெடன்னா, ஓடிச்சென்று முக்காலியை எடுத்து வருகிறான். சற்று நேரம், ஒட்டுமொத்த குடும்பமே, உணர்ச்சி மிகுதியினால் (spluttered) இளித்தது. எல்லா நிகழ்வுகளும் சீராவதற்கு முன்.
மாமனார், அவரே, தனக்கென ஒரு இருப்பிடத்தை வீட்டுக்கு வரும்போதெல்லாம் தேர்ந்தெடுத்து கொள்வார். தலையை சாய்த்தவாறு (chop off) அந்த இடத்தைத் தவிர வேறு எங்கும் அமர மாட்டார். அந்த தூணில் அவர் சாய்ந்து கொண்டு இருப்பார். அந்த தூண் பொருட்கள் வைக்கும் அறைக்கு தெற்கே உள்ள சுவருக்கும் அடுத்து இருக்கும், முதல் காரியம் என்ன செய்வார் என்றால் உட்கார்ந்த உடனே, குடுமியை அவிழ்த்துவிட்டு, நன்கு குலுக்கி, தலையைத் நன்கு தேய்த்து, பின்பு இறுகலாக கட்டி விடுவார். இந்த சடங்குகளை (ritual) தவறாமல் அவர் செய்வார். பின்பு அந்த தரையினை ஆய்வு செய்வார். “ஏதாவது பணம் தலையில் இருந்து விழுந்ததா என்று பார்த்தீர்களா? அண்ணா ஒரு அடக்கமற்ற சிரிப்போடு சொல்வார்.
அவர் காகித அம்புகள் போல எப்போதும் துளைத்து கொண்டே இருப்பார். எங்களைப் பார்க்கும் போதெல்லாம் ஒரு புன்னகையுடன்கல்லுப்பிள்ளையார்போல இருப்பார். நீங்கள் என் உறவினர்கள். நீங்கள் கிண்டல் செய்யவில்லையென்றால் வேறு யார் செய்வார்கள்? நாங்கள் செய்யும் ஏளனம் எல்லை மீறி போனால், அம்மா எங்களிடம் கோபித்துக் கொண்டு சென்று விடுவார். கடைசியாக வரும் வார்த்தை, “நீங்கள் கழுதைகள்.
![]()
மாமனார் உட்கார்ந்த உடன், அம்மா சமையலறைக்குச் சென்றுவிடுவார். ஒரு ஆட்டுக்குட்டியைப் போல அப்பாவும் அவளைப் பின்தொடர்ந்தார். சிறிது நேரத்தில், அம்மா ஒரு வெள்ளி டம்ளரில் மோர் எடுத்து வந்தாள். பெருங்காயமும் கையில் எடுத்து வந்தாள். அப்பா பின்னால் வந்தது அவளுக்கு தெரியாது. நாங்கள் அவளை, “பார், அண்ணா வந்தவுடன், எவ்வாறு அக்கறையோடு கையில் மோர் கொண்டு கொடுக்கிறாள்”. மோரின் வாசனையும், பெருங்காயத்தின் வாசனையும் எங்களையும் குடிக்கத் (asafoetida) தூண்டியது.
நாங்கள் மாமனார் மோர் குடிப்பதற்காகவே வந்திருக்கிறார் என்று நினைத்தோம். எங்கள் பசுவிடம் இருந்து கிடைக்கும் மோர் மிகவும் தெய்வீகம் உடையதாக இருக்கும். இதற்கிடையில் எங்கள் மாமனார், அந்த கிராமத்தில் மிகப்பெரிய கஞ்சன் (stingiest) என்று கேள்விப்பட்டோம். அவரை எதுவும் யாருக்கும் கொடுக்க மாட்டார் என்று நம்பினோம், அவர் தானே கண்ணாவரத்திற்குச் சென்று புகழ்பெற்ற கருப்பு நாக்கு கறவை மாடு, அவரின் தங்கைக்காக வாங்கச் சென்றார். என் தம்பியும் தங்கையும் அந்த கன்றுக்குட்டியுடன் பேரன்பு கொண்டு இருந்தனர்.
மாமனார் இங்கு வரும் போதெல்லாம், அந்த மாட்டின் அருகில் சென்று தட்டிக் கொடுப்பார். (pat) (யாருடைய கண்களும் பட்டுவிடக் கூடாது என நினைப்பார்) சிக்கனமாக நடந்து கொள்வார். இதனால் அந்த குட்டிகள் எப்போதும் பயத்துடனே இருந்தன. மாட்டில் பால் வற்றி விட்டது என்றால் அவர் அந்த மாட்டை, கன்றுகளோடு அவருடைய வீட்டுக்கு அழைத்துச் சென்றுவிடுவார்.
இது நிகழ இருப்பதைக் கண்டு, மாமனாரின் மேல் ஒரு கசப்புத் தன்மையை உருவாக்கியது. அவர் எப்போதெல்லாம் மோரை மிக்க மகிழ்ச்சியோடு (relish) குடிக்கிறாரோ, சிறு பிள்ளைகள் அவரை முறைத்துப் பார்ப்பர்.
மாமனார் அந்த நாற்காலி பற்றி விவாதத்தில் பங்கு கொள்ள ஆர்வம் காட்டினார். தனக்கும் ஒன்று வேண்டும் என்று கூறினார். இதனால் எங்களுக்கும், அவர் உடன் வருவது மகிழ்ச்சி அளித்தது.
மாமனார் வேப்ப மர கட்டையில் நாற்காலி செய்தால் நல்லது என்றார். அது எப்போதும் உடலை குளிர்ச்சியாக வைத்திருக்கும் என்று கூறினார். – அவர் வேப்ப மர கட்டை பற்றி விளக்கும் போது (expounded) அப்பா, வட்டக் கண்களோடு அவரைப் பார்த்து ஆச்சரியப்பட்டார்.
(astonished) ஒரு நாளைக்கு முன்பு தான், அப்பா ஒரு பழமையான வைரம் பொருந்திய வேப்ப மரத்தை வெட்டுவதற்கு ஒரு கூலியாளிடம் (farmhand) பேசிக் கொண்டிருந்தார். பூவரச மரத்தில் நாற்காலி செய்தால் நல்லது. அது ஒரு பளபளப்பான (glossy), நன்றாக தானியங்கள் முடிச்சுகள் இல்லாத கட்டை மற்றும் பலத்திலும் சிறந்தது என்று பெடன்னா கூறினான்.
எங்கள் மூத்த சகோதரி, “இதெல்லாம் மெல்லிய நிற கட்டைகள், பார்ப்பதற்கு அழகாக இருக்காது என்று கூறினாள். சிறிது நேரத்தில் நாம் அவற்றை வெறுத்துவிடுவோம் (detest). பழுத்த கரும்பு அல்லது எள் எண்ணெய் கலரில் இருந்தால் நல்லது அப்புறம் உங்கள் இஷ்டம்” என்று கூறினாள். கருப்பு மரத்தில் கண்ணாடி போன்ற தோற்றத்துடன், நன்றாக வடிவமைக்கப்பட்ட கால்கள், வளைந்த பின் கால்கள், சோர்வு இல்லாத தோற்றம் தான் சாய்வு ஏற்படுத்தக்கூடிய ஒரு நல்ல – நாற்காலி. அந்த ஆடம்பர நாற்காலி தான் எங்கள் கண்களில் முன் நிழலாடியது.
![]()
எல்லோரும் அவள் சொல்வது சரி என்று ஆமோதித்தனர். எனவே, இரண்டு நாற்காலிகள் உடனடியாக செய்ய முடிவு. – எடுக்கப்பட்டது. ஒன்று எங்களுக்கு, மற்றொன்று மாமனாருக்கும்.
இரண்டு நாற்காலிகளும் வந்து சேர்ந்த போது, எந்த நாற்காலியை நாங்கள் வைத்துக் கொள்வது என்றும், எதை மாமனாருக்கு அனுப்புவது என்று தெரியவில்லை. ஒன்றைப் பார்த்தால், மற்றொன்றைப் பார்க்க அவசியமில்லை. இரண்டுமே ராமன் லெட்சுமணன் போல உருவத்தில் ஒத்து இருந்தது. நாங்கள் ஒன்றை வைத்துக் கொண்டு மற்றொன்றை. – மாமனாருக்கு அனுப்பிவிட்டோம். ஆனால் ஒரு சந்தேகம். நல்லதை அவருக்கு அனுப்பிவிட்டோமோ என்று?
ஒருவர் பின் ஒருவராக, அந்த புதிய நாற்காலியில் உட்கார நாங்கள் வரிசை அமைத்துக் கொண்டோம். அதிலிருந்து. எழுந்து செல்ல எங்களுக்கு மனமில்லை. ஆனால் மற்றவருக்கும் வாய்ப்புக் கிடைக்க வேண்டும் என்ற நியதிக்காகத் தான் எழ வேண்டியிருந்தது. பெடன்னா உட்கார்ந்து பார்த்துக் கொண்டு, ஆச்சரியத்தோடு ஆஹா ஹா என்று பாராட்டினான்.. இரு கைகளையும் அந்த நாற்காலியின் மேல் தூக்கினான். கால் மேல் கால் போட்டு அமர்ந்தான். “இதற்கு ஒரு உறை தைக்க வேண்டும். இல்லையெனில் அது மண்ணாகி விடும்” என்று அத்தை கூறினார்.
என்னுடைய இளைய சகோதரியும், தம்பியும் அந்நாற்காலிக்காக அடிக்கடி சண்டையிட்டனர். அதனால் அவள் நீ”, ரொம்ப நேரமாக அதில் உட்கார்ந்து கொண்டு இருக்கிறாய். எழுந்திரு. நானும் இப்போது அமர வேண்டும் என்று கத்தினாள்.
![]()
‘அய்யோ நான் இப்போது தான் அமர்ந்தேன். அம்மா அவளைப் பாருங்கள்’, என்று கூறுவான். அம்மாவைப் பார்த்து திரைப்புள்ள முகத்தோடு அழுவது போன்று கத்துவான்.
நாற்காலி வீடு வந்து சேர்ந்த விஷயம் காட்டுத்தீ போல் அக்கிராமம் முழுவதும் பரவியது. பெரியவர்கள், குழந்தைகள், முதியவர்கள் என அனைவரும் கூட்டமாக வந்து (hordes) பார்த்துச் சென்றனர். ஒரு சிலர் அதனை பக்கவாட்டில் தட்டிப் பார்த்தனர். ஒரு முதியவர் அதனை தூக்கி, கொஞ்சம் பாரமாகத்தான் இருக்கிறது. நல்ல துணிச்சலோடு செய்து இருக்கிறார்.” என்று அந்த தச்சரைப் பாராட்டினார் (commended).
சில நாட்கள் கழித்தன.
ஓர் இரவு யாரோ ஒருவர் வந்து கதவைத் தட்டினார். உள் திண்ணையில் தூங்கிக் கொண்டிருந்த பெடன்னா, எழுந்து கதவைத் திறந்தான். கிராமத்தில் உள்ள முக்கியமான நபர் ஒருவர் இறந்து விட்டதாகவும், அதற்கு நாற்காலி தேவைப்படுகிறது. என்று வந்தவர் கூறினார்.
இறந்தவர் யார் என்று எங்களுக்கும் தெரியுமாதலால் நாங்களும் அந்த இறுதிச்சடங்கில் கலந்து கொண்டோம். அங்கே எங்கள் நாற்காலியில் தான் இறந்தவரை உட்கார வைத்து முட்டுக்கொடுத்திருந்தனர் (propped).
ஆனால் இதுவரை எங்கள் கிராமத்தில் இறந்தவரை தரையில் தான் படுக்க வைத்திருப்பது வழக்கம். ஒரு பெரிய ஆட்டுக்கல்லை, தரையில் வைத்து, உருண்டு விடாமல் இருக்க முட்டுக் கொடுத்திருப்பர். ஒரு கோணிப்பையை எடுத்துக்கொண்டு அதனுள் தினை வைக்கோலை அடைத்து வைத்திருப்பர். அதன் மீது உயர்த்தி, அந்தப் பிணத்தைச் சாய்த்து வைத்திருப்பர் (bolster).
![]()
இதிலிருந்து தான் நகரத்துவாசிகளும் இந்த புதிய முறைக்கு மாறினார் (fad) அவர்களும் பிணத்தை நாற்காலியில் சாய்த்து வைக்கத் தொடங்கினர். எங்களுக்கு எந்த துப்பும் இல்லை. ஆனால் அன்றிலிருந்து தான் எங்கள் நாற்காலிக்கு ஒரு புதிய பிரச்சனை வரத் தொடங்கியது. (தரையில் படுக்க வைத்தவர்கள் இப்போது நாற்காலியில் சாய்த்து வைக்க முன்னேறினர்.
எல்லா நிகழ்வுகளும் அந்த வீட்டில் (துக்க வீட்டில்) முடிந்த பின்னர், அவர்கள் அந்த நாற்காலியினை எங்கள் முன் முற்றத்தில் வைத்துவிட்டனர். வீட்டில் உள்ள குழந்தைகள் யாவரும் அந்த நாற்காலியை பார்க்கப் பயந்தனர். நாங்கள் ஒரு வேலைக்காரனை அமர்த்தி, அதனை கிணற்றுக்குத் தூக்கிக் கொண்டு போய், நன்றாக வைக்கோலினால் தேய்த்து (scrub) அதை மீண்டும் 15 பெரிய வாளிகள் தண்ணீர் கொண்டு தேய்த்து கழுவினோம்.
வெகு நாட்களுக்கு பின்பு கூட, அந்த நாற்காலியில் அமர யாருக்கும் தைரியம் இல்லை (guts) எங்களுக்கு மீண்டும் அந்த நாற்காலியை எப்படி புழக்கத்திற்கு கொண்டு வர வேண்டும் என்று தெரியவில்லை.
ஒரு நாள் அதிர்ஷ்டவசமாக (fortuitously) ஒரு உறவினர் ஒருவர் வீட்டுக்கு வந்தார். அவருக்கு அந்த நாற்காலியை அமருவதற்குக் கொண்டு வந்தோம். இல்லை தம்பி வேண்டாம். நான் இதில் உட்கார்ந்து கொள்கிறேன் என்று அந்த நாற்காலியில் உட்காராமல், அங்கே கிடந்த துணி பாயை (cloth mat) நோக்கி நகர்ந்தார். நாங்கள் அவர் தரையில் உட்கார்ந்து விடுவார் என நினைத்தோம், பயந்தோம். அவரை நாற்காலியில் அமர சம்மதித்தோம்.
அவர் உட்கார்ந்த உடனே சின்னத் தம்பியும், தங்கையும் பின்புறத்தில் உள்ள தோட்டத்திற்கு தப்பி ஒடினர் (fled). சில சமயங்களில் அவர்கள் அங்கிருந்து வந்து நாற்காலியில் உட்கார்ந்து இருப்பவருக்கு எதுவும் நடந்துவிட்டதா என்று உற்றுப் பார்த்தனர்.
மறுநாள், எங்கள் ஊரில் உள்ள ஒரு மூத்தவர் நடந்து செல்லும் போது, தானாகவே, அந்த நாற்காலியில் வந்து அமர நினைத்தார். அது எங்களுக்கு மிகுந்த ஆறுதலைத் தந்தது. (இப்போதே அந்த நாற்காலியில் அமர ஒத்திகை பார்க்கிறார் என்று பெடன்னா என் காதுகளில் ஓதினான்). இப்படித்தான் நாங்கள் அந்த நாற்காலியைப் பயன்படுத்தினோம் (seasoned).
முதலில் அந்த நாற்காலியில் மூத்தவர்கள் அமர்ந்து பார்த்தனர். ஆனால் குழந்தைகள் பயந்து கொண்டு இருந்தனர். என்னுடைய சகோதரி, நீ ஏன் முதலில் போய் அதில் அமரக் கூடாது என்று என் தம்பியை நோக்கி கெஞ்சினாள். அதற்கு அவன், நீ ஏன் முதலில் போய் உட்காரக் கூடாது என எதிருரைத்தான்.
![]()
பக்கத்துத் தெருவைச் சேர்ந்தவர் சுகந்தி, அவர் வந்து தனது குழந்தைத் தம்பியை நாற்காலியில் வைத்தார். அன்றிலிருந்து தான், எங்கள் வீட்டில் உள்ள குழந்தைகளும் எவ்வித பயமும் இல்லாமல், அந்த நாற்காலியில் அமரத் தொடங்கினர்.
மீண்டும், ஓர் இரவு, இன்னொருவர் இறந்து போகவே, அந்த நாற்காலியைத் தூக்கிச் சென்றனர். இது அடிக்கடி நிகழ்ந்தது. நாங்கள் ஒவ்வொரு முறையும் சோகத்தில் அனுப்பினோம். துக்க வீட்டினர், எங்கள் சோகத்தை வேறு வழியில்லாமல் புரிந்து கொண்டனர். நாங்கள் இறந்து போனவர்களுக்காக வருந்துறோம் என்று தவறாக நினைத்துக் கொண்டனர்.
ஒவ்வொரு முறையும், எங்கள் தூக்கம் கெடுக்கப்படுவதை நினைத்து வருந்தினோம். அக்காள் ஒரு நாள், எதற்கு இந்த மோசமான (wretched) மனிதர்கள் நிலவுலகில் உரியதாக இயலும் போது, அந்நேரத்தில் ஏன் இறக்க வேண்டும் என்று கடவுளுக்குத் தெரியும் என்று குறிப்பிட்டாள். அண்ணா உற்சாகமாக (exasperatedly) நாம் நல்ல ஒரு நாற்காலியை உருவாக்கினோம். பிணங்களை (corpses) உட்கார வைக்க வேண்டிய நிலை வந்து விட்டதே …. சீ என்றான்.
இதெல்லாம் அந்த நாற்காலியை அமங்கலமான நேரத்தில் செய்ய சொன்னதே தான் காரணம் என்றாள் அத்தை.
பெடன்னா இறுதியாக ஒரு யோசனையை நினைத்தான். ஆனால் நாங்கள் இருவருக்குள்ளும் அதை மூடி மறைத்து வைத்துக் கொண்டோம்.
அம்மா என்னை ஒரு முறை மாமனாரின் வீட்டுக்கு தூது அனுப்பினார் (errand) நான் நுழைந்த வேளை, அங்கே மாமனார், அற்புதமாக நாற்காலியில் அமர்ந்திருந்தார். வெற்றிலையை மடித்து வாயில் போட்டு மென்று கொண்டிருந்தார். இது பார்ப்பதற்கு ஒரு இனிமையான தருணமாக இருந்தது. வெற்றிலை பெட்டியை, அவர் மிகவும் கவனமாக கையாள்வதும், அதற்கு வலிக்காதவாறும் அதனைத் திறந்தார்.
பரந்த அளவில் (span wide) முழங்கை நீளமான, நான்கு விரல்கள் உயரம் கொண்ட அந்த வெற்றிலையை, தினமும் பொன்னைப் போன்று மின்னும் வரை துடைத்தார். அந்த சாதனங்களை பயபக்தியோடு பூஜைப்பெட்டியில் இருந்து எடுப்பதைப் போன்று எடுப்பார்.
அவர் வெற்றிலையைத் தூய்மையாகத் துடைத்தாலும் அதன் தண்டினை (stalk) கிள்ளி எறிய மாட்டார். மிகவும் சிக்கனமானவர். அவர் ஒரு கரடுமுரடான (coarse leaf) இலையைக் கண்டாலும், அதன் நரம்புகளை துண்டு துண்டாக எடுத்துவிடுவார். இது வெற்றிலை பற்றிய ஒரு பழைய புதிர் மந்திரத்தை நினைவூட்டுகிறது.
முத்தப்பனை பிடித்துக் கொண்டு
அவன் முதுகெலும்பை நீக்கு
தூய்மையான வெண்ணெய் கொண்டு பூசு …..
அவர் உடைந்த பாக்கினை நுகர்ந்து பார்ப்பார். இது நுகர்ந்து பார்த்தலின் போது போதை ஏறுவதை விரட்டுகிறது (ward off) பாக்கில் இருக்கும் சின்னச் சின்ன பூச்சிகளை விரட்ட இவ்வாறு செய்யலாம். ஊதுதல் மூலம் ஒளிந்திருக்கும் பூச்சிகளை விரட்டலாம். முதலில் மெதுவாக ஆரம்பித்து பின்பு வேகம் எடுக்கும் பின்பு வாயில் போட்டு மென்று விடுவார்.
ஒருவர் எவ்வாறு சுத்தமாக இருக்கிறார் என்பதை அறிய, அவர் வைத்திருக்கும் சுண்ணாம்பு டப்பாவை பார்த்தாலே போதும். மாமனார் இதற்கு சற்றும் பொருந்தாதவர். விரலிலிருக்கும் மிஞ்சிய சுண்ணாம்பு பசையைக் கூட அடுத்த பொருட்களின் மீது துடைக்க மாட்டார். பயபக்தியோடு அவரின் சுண்ணாம்பு டப்பாவை கண்ணில் ஒற்றி எடுத்துக் கொள்ளலாம். அவரின் எவெரெடி டார்ச் விளக்கு 15 வருடங்களுக்கு முன் வாங்கப்பட்டது.
![]()
இன்னும் உபயோகத்தில் உள்ளது. ஆயினும் பளிச் என இருப்பதோடு இப்போது தான் வாங்கியது போன்று இருக்கும். அதோடு எங்கள் குடும்பத்துக்கும் சேர்த்து வாங்கிய டார்ச் லைட், துவாரம் உடையதாகவும், மஞ்சள் நிறத்தில், பரிதாபமிக்க நிலைக்குத் தள்ளப்பட்டு, நாட்பட்ட (chronic) நோயாளி சாகும் தருவாயில் இருப்பதைப் போல் இருக்கிறது.
அவரது வீட்டில் அவரைத் தவிர அந்த நாற்காலியை யாரும் உபயோகப்படுத்த மாட்டார்கள். காலை எழுந்தவுடன் ஒவ்வொரு நாளும் முதல் வேளையாக அதனை நன்கு துடைத்துவிடுவார். ஒரு மண் பானையில் தண்ணீர் உள்ளதைப் போல நினைத்து, அவரே அதனை கவனமாக தூக்குவதும், ஓரிடத்திலிருந்து மற்றொரு இடத்திற்கு மாற்றுவதும் அவரே செய்வார்.
என்னைப் பார்த்து, மாமனார் வாழ்த்து தெரிவித்து வரவேற்றார். மருமகனே, வாருங்கள். வெற்றிலை சாப்பிடுகிறீர்களா? பின்பு அவரே அதற்குப் பதிலளித்தார். பவுளிப்பருவத்து மாணவர்கள் இப்போதே வெற்றிலை. மென்றால், சிறு கோழிக்குஞ்சுகள் கூட பீப்பாய் போல உருமாறிவிடும் (butting).
நான், அம்மா கூறியவற்றை அவரிடம் சொல்லிவிட்டு வீடு திரும்பினேன்.
பின்பு, அன்று ஒரு தேவ பக்தியற்ற இரவில், கதவை யாரோ தட்டினர். எல்லோரும் ஆழ்ந்த தூக்கத்தில் இருந்தனர். நான் பெட்டனாவை எழுப்பினேன். வீட்டுக்கு வெளியே ஒரு சிலர் ஒரு துயரமடைந்த வீட்டில் இருந்து வந்து நின்று கொண்டிருந்தனர். அவர் நாற்காலி வாங்குவதற்காக நின்று கொண்டிருந்தனர். பெடன்னா அவர்களை தெருவுக்குள் அழைத்துச் செல்ல நானும் அவர்களைப் பின்தொடர்ந்தேன். அவர்கள் எதற்காக வந்திருக்கிறார்கள் என்பதை எங்களிடம் சொல்லி முடித்த பின்பு, பொறுமையாக பெடன்னா பதிலளித்தான். நாற்காலி தானே. அது என்னுடைய மாமனார் வீட்டில் உள்ளது. நீங்கள் போய் அவரிடம் கேளுங்கள். தருவார். அவர்களை அனுப்பிய பின் நாங்கள் வீட்டுக்குத் திரும்பினோம். சத்தமில்லாமல் யார் அது? தூக்கத்தில் அப்பா கேட்டார்.
“ஒன்னும் இல்லை ….” சிலர் நமது காளைகளை இதிரடிப்பதற்கு (threshing) கேட்டனர்.
![]()
போர்வையை இழுத்துப் போர்த்தியவராய் அப்பா மீண்டும் படுக்கைக்குச் சென்றார். இப்போது மாமனாரின் காட்டில் மழை. சில நாட்கள் கழித்து, மாமனாரை சந்தித்த போது அவர் தரையில் உட்கார்ந்திருந்தார். வெற்றிலை மெல்ல தயார் படுத்திக் கொண்டிருந்தார். அவர் வழக்கமான புன்னகையை உதிர்த்து என்னை வரவேற்றார்.
“இது என்ன? தரையில் அமர்ந்து இருக்கிறீர்கள், நாற்காலி எங்கே?” நான் தேடினேன். சுண்ணாம்பு பசையை வெற்றிலையில் பின்புறத்தில் தடவியவராய் என்னைப் பார்த்து புன்னகைத்தார். பின்பு மெல்லிய குரலில் கூறினார். நான் அவர்களை, அந்த நாற்காலியை அந்த ஒன்றுக்காக மட்டும் பயன்படுத்திக் கொள்ளுங்கள் என்று கூறினேன். இருப்பினும் உனக்கு இன்னொன்று தேவைப்படுகிறது என்றார்.
எனக்கு என்ன சொல்வது என்று தெரியவில்லை வீட்டுக்கு விரைந்தேன். இந்த செய்தியை சொல்வதற்காக பெடன்னாவைத் தேடிச் சென்றேன். ஆனால் என்னுடைய நகர்வுகள் அனைத்தும் சாதாரண நடையாக மாறியது (gait).